In this issue of Blood, Isenberg and colleagues describe a novel function for thrombospondin-1 (THBS-1) in the α-granules of platelets: THBS-1 opposes nitric oxide (NO) and allows platelet activation when NO is being generated. This insight may complete the solution of an intriguing puzzle. α-granules are loaded with THBS-1, but mice with knock-out of the THBS-1 gene have normal tail bleeding times and platelets that aggregate normally after washing and activation with thrombin.1 In contrast, mice with knock-out of THBS-2, which is closely related to THBS-1 but not present in α-granules, have grossly dysfunctional platelets.2,3 Part of the puzzle was solved several years ago when Kyriakides et al3 demonstrated that THBS-2 is present in the marrow microenvironment and needed by megakaryocytes for production of normal platelets. Until now, we have not known of a compelling role for THBS-1 in platelet function.
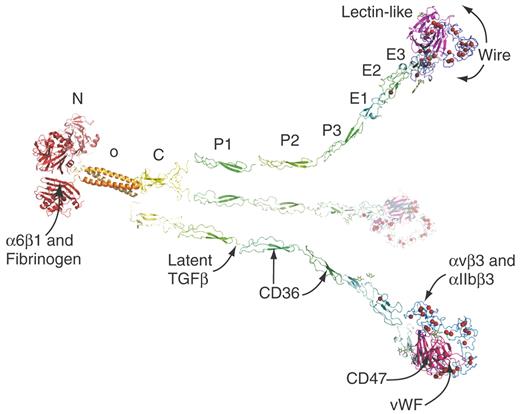
The figure depicts the ribbon structure of THBS-1 and THBS-2. The THBS subunit has an N-terminal module (N); an oligomerization sequence (o) that forms a trimeric α-helical coiled-coil; 1 von Willebrand factor-C (C), 3 properdin (P1, P2, P3), and 3 EGF-like (E1, E2, E3) modules; a THBS wire module with 13 calcium ion-binding repeats; and a C-terminal lectin-like module. Approximately 30 calcium ions (shown as red balls) bind per subunit.4 Sites where THBS-1 interacts with platelet proteins are indicated. Putative cell surface receptors include α6β1, αIIbβ3, and αVβ3 integrins; CD36, which binds to the properdin modules; and CD47, which binds to the lectin-like module. In addition, THBS-1 binds to fibrinogen associated with the platelet surface, activates latent TGF-β secreted from platelets, and disassembles VWF multimers by a disulfide exchange requiring the free cysteine in the lectin-like module. Interactions important for counteracting the effects of NO on platelet aggregation were found to involve CD36 and CD47 and to be mimicked by the appropriate segment of THBS-1 or peptides based on sequences in properdin modules (for CD36) or the lectin-like module (for CD47).
Surprisingly, ligation of either CD36 or CD47 suffices to counteract NO. Prior studies showed that THBS-1 also counteracts stimulation of endothelial or vascular smooth muscle cells by NO.5 Study of smooth muscle cells from mice null in CD36 or CD47 indicates that CD47 is necessary to counteract NO when the cells are presented with reagents that ligate CD36 or CD47 but that CD36 is not necessary for a CD47 ligand to counteract NO.5 Although such experiments are not presented for platelets, the summary mechanistic figure depicts a pathway whereby ligation of CD36 causes a signal that is funneled through CD47.
Exogenous THBS-1 counteracted the effects of exogenous NO on thrombin-induced platelet aggregation and production of cGMP with the same dose responses. THBS-1 in the range of only 22 pM to 22 nM (3.3 ng/mL to 3.3 μg/mL) was required. Importantly, comparisons of platelets from wild-type and THBS-1–null mice demonstrated that endogenously released THBS-1 can counteract NO. Decreased production of cGMP in the presence of THBS-1 indicated that soluble guanylyl cyclase is a target of ligated CD47. Evidence is also presented for signaling from ligated CD47 to inhibit cGMP-dependent protein kinase and downstream phosphorylation of VASP and activation of Rap1.
The paper from Isenberg et al does raise questions. First, what are the molecular interactions and reactions that link ligated CD36 to CD47 and allow CD47 to modulate the activities of soluble guanylyl cyclase and cGMP-dependent protein kinase? Second, why are the platelet stores of THBS-1 so large, if so little is needed to counteract NO? The serum concentration of THBS-1 is 15 to 30 μg/mL, and its calculated concentration in α-granules is 60 mg/mL.6 Third, how are CD-47–binding sequences exposed in the lectin-like module? Peptides that ligate CD47 are based on sequences that are buried in the module.4 Loss of calcium ions is associated with major rearrangements of the convoluted structure of the EGF-like modules, wire, and lectin-like module7 and may expose the CD-47 binding sequences. It is a good bet that THBS-1 stored in α-granules lacks a full complement of bound calcium because so much calcium would be required. Finally, do THBSs other than THBS-1 modulate effects of NO? Only THBS-1 and THBS-2 have properdin modules to interact with CD36, but all 5 tetrapod THBSs have lectin-like modules for possible interaction with CD-47.
Conceptual ribbon diagram of THBS-1 or THBS-2. The structures used to make the model are of recombinant constructs from 5 different proteins. Sites of interaction of THBS-1 with platelet proteins are indicated by arrows.
Conceptual ribbon diagram of THBS-1 or THBS-2. The structures used to make the model are of recombinant constructs from 5 different proteins. Sites of interaction of THBS-1 with platelet proteins are indicated by arrows.
THBS-1 has been assigned many roles in platelet and vascular biology. Will its major and lasting role be as a modulator of NO? If so, there are many pathophysiological ramifications, and we may expect some very interesting plot lines.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal