Platelet α-granules constitute the major rapidly releasable reservoir of thrombospondin-1 in higher animals. Although some fragments and peptides derived from thrombospondin-1 stimulate or inhibit platelet aggregation, its physiologic function in platelets has remained elusive. We now show that endogenous thrombospondin-1 is necessary for platelet aggregation in vitro in the presence of physiologic levels of nitric oxide (NO). Exogenous NO or elevation of cGMP delays thrombin-induced platelet aggregation under high shear and static conditions, and exogenous thrombospondin-1 reverses this delay. Thrombospondin-1–null murine platelets fail to aggregate in response to thrombin in the presence of exogenous NO or 8Br-cGMP. At physiologic concentrations of the NO synthase substrate arginine, thrombospondin-1–null platelets have elevated basal cGMP. Ligation of CD36 or CD47 is sufficient to block NO-induced cGMP accumulation and mimic the effect of thrombospondin-1 on aggregation. Exogenous thrombospondin-1 also reverses the suppression by NO of αIIb/β3 integrin–mediated platelet adhesion on immobilized fibrinogen, mediated in part by increased GTP loading of Rap1. Thrombospondin-1 also inhibits cGMP-mediated activation of cGMP-dependent protein kinase and thereby prevents phosphorylation of VASP. Thus, release of thrombospondin-1 from α-granules during activation provides positive feedback to promote efficient platelet aggregation and adhesion by overcoming the antithrombotic activity of physiologic NO.
Introduction
Platelets play important roles in hemostasis and cancer metastasis and were the first source from which thrombospondin-1 (TSP1) was isolated.1,2 TSP1 is a major protein component of platelet α-granules, from which it is rapidly released during platelet activation. The physiologic function of TSP1 in platelets, however, remains controversial. Platelets from TSP1-null mice show normal aggregation in vitro.3,4 However, exogenous TSP1 enhances thrombin-stimulated aggregation,5 and some monoclonal antibodies recognizing TSP1 inhibit thrombin- and ionophore-stimulated platelet aggregation.6,–8 Certain fragments of TSP1 inhibit platelet aggregation,9 yet some monovalent TSP1 peptides promote aggregation.10,–12 Because TSP1 interacts with fibrinogen, TSP1 was proposed to bridge platelets via binding to fibrinogen bound to the platelet integrin αIIbβ3 or by binding directly to this integrin.13 Alternatively, TSP1 may regulate degradation of von Willebrand factor by ADAMTS13,4 which is consistent with the increased collagen- and von Willebrand factor–mediated aggregation of TSP1-null platelets.14
Functions of the several TSP1 receptors expressed on platelets have also been controversial. CD36 was the first such receptor identified,15 but subsequent studies showed that TSP1 binding is normal to activated platelets from Naka− individuals who lack CD36.16,17 A proposed role for the platelet integrin αIIbβ3 as a TSP1 receptor was similarly put in doubt by normal TSP1 binding to thrombin-activated platelets from patients with Glanzmann thrombasthenia who lack αIIbβ3.18,19
The TSP1 receptor CD47 is highly expressed on platelets.10 Despite some controversy about the role of CD47 as platelet receptor for native TSP1, several groups have confirmed that CD47-binding peptides derived from TSP1 stimulate platelet aggregation.10,–12 Similarly, the TSP1 antibody C6.7, which inhibits TSP1 binding to CD47, inhibits platelet aggregation.6 The relevance of the peptide data, however, has been questioned because some CD47-binding peptides appear to signal in platelets through FcRγ rather than CD47.20 Furthermore, the VVM sequences implicated in their binding to CD47 may not be accessible to mediate binding of native TSP1 to this receptor.21
Nitric oxide (NO) is a well-defined inhibitor of platelet activation,22 although its effector cGMP also exerts some stimulatory effects on the early phases of activation.23 Recently, we demonstrated that TSP1 potently inhibits NO-driven responses in vascular smooth muscle and endothelial cells.24,25 This activity of TSP1 involves inhibition of NO-stimulated synthesis of cGMP by soluble guanylyl cyclase (sGC) as well as inhibition of an unknown target downstream of cGMP.
Engaging either CD36 or CD47 mimics the inhibitory actions of TSP1 on NO/cGMP signaling in vascular cells, although only CD47 is necessary for inhibition by TSP1.26 Expression of CD36 and CD47 on platelets led us to propose that the potent antagonism of NO signaling we identified in vascular cells could extend to platelets and might clarify the role TSP1 plays in platelet aggregation. We report here that TSP1 is a physiologic antagonist of NO to regulate platelet aggregation and adhesion. In the absence of TSP1, NO/cGMP signaling precludes thrombin-induced platelet aggregation.
Methods
Animals
Wild-type (WT) and TSP1-null C57BL/6 mice were housed under pathogen-free conditions with ad libitum access to filtered water and standard chow. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committees of the National Cancer Institute (NCI) and Washington University.
Reagents
Thrombin was kindly provided by Dr Jules Gladner (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). The nitric oxide donor diethylamine NONOate (DEA/NO) was kindly provided by Dr Larry Keefer (NCI, Frederick, MD). TSP1 was purified from fresh human platelets as described.27 TSP1-based peptides were synthesized as described28 or purchased from Peptides International (Louisville, KY). Oxadiazole-[4,3-a]quinoxalin-1-one (ODQ) was from Sigma-Aldrich (St Louis, MO). Recombinant domains of TSP1 were kindly provided by Dr Deane Mosher (University of Wisconsin, Madison, WI) and Jack Lawler (Harvard University, Boston, MA). Platelet-rich plasma (PRP) was provided by the blood bank of the Clinical Center of the National Institutes of Health (NIH). Fibronectin was purified from human plasma (Clinical Center of the NIH) as described.29 Type I collagen was purchased by Inamed (Fremont, CA). Fibrinogen was obtained from Calbiochem (La Jolla, CA).
Preparation of human platelets
Platelets were pelleted from platelet-rich plasma (PRP) by centrifugation for 10 minutes at 200g. They were then washed with acid citrate dextrose (ACD; 85 mM citric acid, 65 mM sodium citrate, 100 mM glucose, pH 5.1) at a ratio of 1:7 at room temperature. After pelleting the platelets again and removing the supernatant, the platelets were resuspended in 5 mL Tyrode buffer (137 mM NaCl, 3 mM KCl, 12 mM NaHCO3, 0.3 mM NaHPO4, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM glucose, 5 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), 3.5 mg/mL BSA, pH 7.4). The final platelet number was adjusted to 200 platelets/μL in 500 μL/cuvette of Tyrode buffer.
Preparation of murine platelets for cGMP assay
After induction of general anesthesia with isoflurane 2%, age- and sex-matched C57BL/6 WT and TSP1-null mice underwent cardiac puncture. Blood was aspirated into 1-cc syringes with a 25-gauge needle containing 100 μL heparin (Heparin Lock Flush; Abbott, Chicago, IL), mixed with 100 μL 3% ACD and centrifuged in 1.2-mL S-Monovette separation tubes (Sarstedt, Nümbrecht, Germany). PRP was aspirated off and centrifuged, and the resulting platelet pellet was resuspended in 200 μL Tyrode buffer.
Platelet aggregation assay
Aggregation of human platelets under high shear conditions was assessed using a standard optical aggregometer (Lumi-Dual Aggregometer; Chrono-Log, Havertown, PA) at 37°C and 1200 rpm in a volume of 500 μL buffer with a final platelet concentration of 2 × 105 platelets/μL over a 5-minute interval. Preincubation with TSP1 and TSP1-based agents was for 15 minutes prior to addition of thrombin and/or the rapidly releasing nitric oxide donor DEA/NO. In some experiments, platelets were preincubated with the Rap1 inhibitor GGTI-298 (10 μM; Calbiochem) for 30 minutes prior to initiating aggregation. In other high shear aggregation experiments, murine platelets were prepared with the following modifications. Mouse blood was collected by retro-orbital bleeding of anesthetized mice using heparinized tubes. PRP was prepared and then diluted with 4 vol Tyrode containing 5 mM EDTA to prevent activation. After collection by centrifugation, platelets were resuspended in 2 times initial PRP volume and held at room temperature for less than 1 hour before use. Aggregation conditions consisted of 250 μL washed platelets stirred at 1200 rpm in a Chronolog Optical aggregometer at 37°C. DEA/NO (10 μM) was added 30 seconds prior to activation and 8-Br-cGMP added 15 minutes prior to activation with 0.2 U/mL human thrombin. Data were collected using Chronolog Aggrolink software.
Platelet aggregation under static conditions was assessed using a spectrophotometer (Beckman DU 640; Beckman Coulter, Fullerton, CA) and determined as a change in absorbance at 400 nm with continuous observation over a 5-minute interval. The cuvette was inverted once every 60 seconds. Preincubation with TSP1 and TSP1-based agents was for 15 minutes prior to addition of thrombin and/or DEA/NO to minimize the formation of thrombin-serpin-thrombospondin complexes,30 which interfere with thrombin-platelet interactions.
Platelet adhesion assay
Bacteriologic Petri dishes (35 × 10 mm; Becton Dickinson Labware, Lincoln Park, NJ) were precoated with collagen (3 μg/mL) or fibrinogen (15 μg/mL) overnight. Following aspiration of nonadherent matrix, dishes were blocked with 1% BSA. Fresh platelets were washed and suspended in Tyrode buffer and allowed to adhere for 30 minutes. Plates were washed with PBS, fixed with 0.5% glutaraldehyde, and stained with 0.02% toluidine blue. Adherent platelets were counted microscopically. In some experiments, platelets were preincubated with the Rap1 geranylgeranyltransferase inhibitor GGTI-298 (10 μM) for 30 minutes prior to initiating adhesion.
Intracellular cGMP assay
Fresh human platelets at 2 × 105 platelets/μL in Tyrode buffer or PRP were preincubated with the indicated agents for 15 minutes and then challenged with DEA/NO for the indicated time interval at room temperature, and total cGMP was determined via immunoassay (Amersham/GE Healthcare, Amersham, United Kingdom). Murine WT and TSP1-null platelets were treated with DEA/NO and cGMP flux was measured.
Rap1 pull-down assay
Platelets were diluted to 5 × 108 platelets/mL in Tyrode buffer and treated with various reagents for the indicated times. Immediately following treatment, platelets were pelleted at 13 000g for 30 seconds at 4°C and resuspended in ice-cold lysis buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 10 mM EGTA, 20 mM MgCl2, 8 mM α-glycerophosphate, 1% Triton X-100, 1 mM phenylmethylsulfonylfluoride, 40 mM NaF, 1 mM Na3VO4, 10 μg/mL aprotinin, and 10 μg/mL leupeptin. Lysates were centrifuged at 13 000g for 5 minutes at 4°C, and the supernatants were incubated for 15 minutes on a tumbler at 4°C with glutathione Sepharose 4B beads (Amersham, GE Healthcare) prebound with a GST-RalGDS RBD fusion protein (kind gift of J. Silvio Gutkind, NIH, Bethesda, MD). Bead complexes were washed by centrifugation at 6000g for 30 seconds at 4°C followed by resuspension in 0.5 mL lysis buffer; 2 washes were performed for each sample. After the second wash, beads were resuspended in an equal volume of 2 times SDS sample buffer and stored at − 20°C. For Western analysis, bead-bound proteins in sample buffer were boiled for 10 minutes and subjected to electrophoresis on NuPAGE 10% Bis-Tris gels (Invitrogen, Carlsbad, CA), transferred to Immobilon-P PVDF membranes (Millipore, Billerica, MA), and blotted with rabbit polyclonal anti-Rap1 (sc-65; Santa Cruz Biotechnology, Santa Cruz, CA).
Phospho-Ser239 vasodilator-stimulated phosphoprotein (VASP) Western blotting
Washed human platelets (resuspended in Tyrode buffer to 3 × 108 platelets/mL to a final volume of 0.5 mL) were incubated at 37°C for 30 minutes before treatment. TSP1 was added 15 minutes prior to treatment. Immediately following treatment, platelets were pelleted at 13 000g at 4°C for 15 seconds and resuspended in 1 mL of a lysis buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 40 mM NaF, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 mM phenylmethysulfonylfluoride, and 1% Triton X-10. This suspension was placed at 4°C with gentle agitation for 5 minutes before centrifugation at 13 000g for 5 minutes at 4°C. Lysates (5 μg) were electrophoresed on 4% to 12% Bis-Tris NuPAGE gels, transferred to Immobilon PVDF membranes, and probed with a rabbit polyclonal antiserum against Serine 239–phosphorylated VASP (Santa Cruz Biotechnology).
cGK in vitro kinase assay
Platelets were washed as described above and resuspended in Tyrode buffer (3 × 108 platelets/mL). Platelets were preincubated with TSP1 (2.2 nM) or Rp-8-pCPT-cGMP for 15 minutes prior to treatment with 8-Br-cGMP (100 μM) or NO (DEA/NO 10 μM) for 2 minutes. Treatment was stopped by placing the platelets on ice. Immediately following treatment, platelets were pelleted by centrifugation at 13 000g at 4°C for 15 seconds and resuspended in a lysis buffer containing 10 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM 3-isobutyl-1-methylxanthine, 125 mM KCl, 1 mM phenylmethysulfonylfluoride, 1 μg/mL aprotinin, and 1 μg/mL leupeptin. Resuspended platelets were sonicated on ice 3 times for 10 seconds each before centrifugation at 13 000g for 5 minutes at 4°C. Lysate (100 μg from each sample) was incubated at 25°C for 20 minutes with kinase buffer (150 μM Arg-Lys-Arg-Ser-Arg-Ala-Glu peptide substrate [Bachem, King of Prussia, PA], 10 mM HEPES [pH 7.4], 35 mM β-glycerophosphate, 4 mM MgCl2, 5 μM Rp-8pCPT-cAMPS, 0.5 mM EDTA, 200 mM [γ-32P]ATPS [Sigma-Aldrich]). Kinase assay reactions were terminated by spotting 50 μL reaction mixture onto nitrocellulose. Nitrocellulose spots were washed 5 times each with 100 μL 0.5% o-phosphoric acid before analysis with a scintillation counter.
Statistics
All assays were repeated at least in triplicate and are presented as the mean plus or minus SD with significance determined by the Student t test for a P value less than .05. Where appropriate, significance was assessed with one-way ANOVA for an F value of 0.95.
Results
TSP1 blocks the ability of NO to delay thrombin-stimulated platelet aggregation
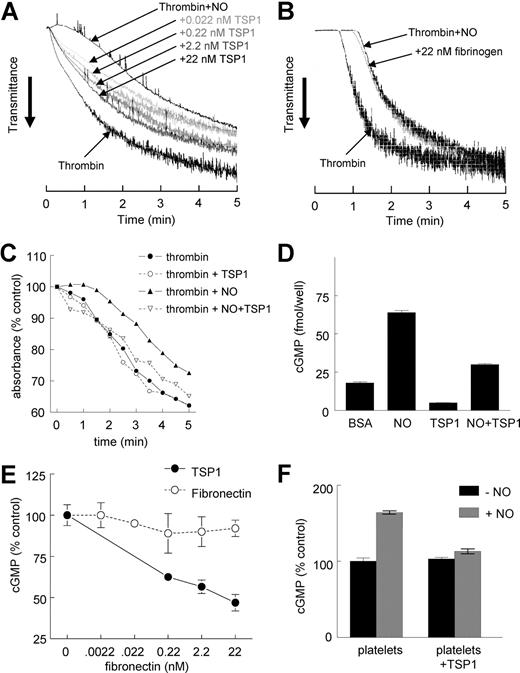
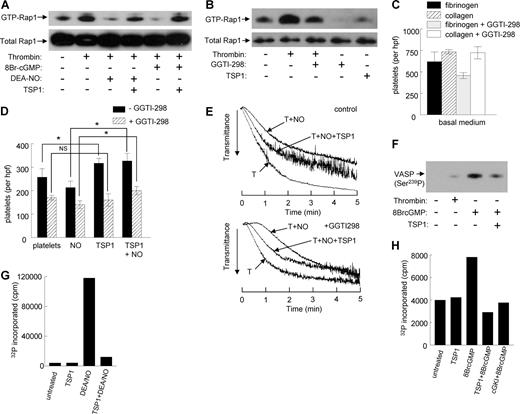
Thrombin-induced aggregation of human platelets was significantly delayed in the presence of exogenous NO (10 μM DEA/NO) under high shear conditions, and this delay was reversed in a dose-dependent manner by TSP1 (Figure 1A). The lowest concentration of TSP1 tested, 0.022 nM, was sufficient to accelerate platelet aggregation in the presence of NO, indicating that the physiologic levels of TSP1 in normal plasma (0.1-0.2 nM) are sufficient to tonically regulate this response. This response was specific for TSP1 in that fibrinogen or fibronectin had no significant effect on the NO delay at the same concentrations (Figure 1B and results not shown).
Exogenous TSP1 reverses the delay of platelet aggregation by NO. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes under high shear (1200 rpm, A,B) or static (C) conditions and absorbance was recorded. In other experiments, fresh, washed human platelets in Tyrode buffer (500 μL) were treated with TSP1 (2.2 nM) (D) or the indicated concentrations of TSP1 or fibronectin (E) and DEA/NO (10 μM) for 5 minutes and lysed, and cGMP was determined via immunoassay. Platelets in PRP were treated with TSP1 (2.2 nM) for 15 minutes followed by NO (DEA/NO 10 μM) for 5 minutes and lysed, and cGMP was determined via immunoassay (F). Data presented are representative of at least 3 experiments (A-C). Results are the mean (± SD) of at least 3 experiments (D,F).
Exogenous TSP1 reverses the delay of platelet aggregation by NO. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes under high shear (1200 rpm, A,B) or static (C) conditions and absorbance was recorded. In other experiments, fresh, washed human platelets in Tyrode buffer (500 μL) were treated with TSP1 (2.2 nM) (D) or the indicated concentrations of TSP1 or fibronectin (E) and DEA/NO (10 μM) for 5 minutes and lysed, and cGMP was determined via immunoassay. Platelets in PRP were treated with TSP1 (2.2 nM) for 15 minutes followed by NO (DEA/NO 10 μM) for 5 minutes and lysed, and cGMP was determined via immunoassay (F). Data presented are representative of at least 3 experiments (A-C). Results are the mean (± SD) of at least 3 experiments (D,F).
Because TSP1 has been reported to differentially affect platelet aggregation at high and low shear,8,31,32 we also examined the effect of TSP1 on static platelet aggregation in the presence of NO. Preincubation of platelets with exogenous TSP1 (2.2 nM) did not significantly alter aggregation in the presence of thrombin alone under these conditions, but TSP1 completely abrogated the NO-stimulated delay in aggregation (Figure 1C). Therefore, TSP1 stimulates aggregation independent of shear rate.
TSP1 prevents activation by NO of sGC in platelets
sGC is the primary intracellular target of NO in platelets.33 Increased synthesis of cGMP induced by binding of NO to the heme of sGC activates cGMP-dependent protein kinase Iβ (cGK-I), which in turn phosphorylates several targets to delay platelet aggregation.34 As previously demonstrated in vascular cells,24,25 sGC is a target of TSP1 signaling in platelets. Both basal and NO-stimulated cGMP levels were decreased following pretreatment with TSP1 (Figure 1D). The dose dependence for TSP1 to inhibit NO-mediated sGC activation is consistent with that for accelerating platelet aggregation (Figure 1E). Inhibition of cGMP accumulation is specific for TSP1 in that platelets pretreated with fibronectin demonstrated no inhibition of an NO-stimulated cGMP flux.
Even in PRP, which contains physiologic levels of both fibrinogen and fibronectin, TSP1 continued to block NO-stimulated cGMP flux in platelets (Figure 1F). Thus physiologic levels of these known TSP1 ligands do not interfere with its activity.
Endogenous TSP1 inhibits NO-driven cGMP accumulation in murine platelets
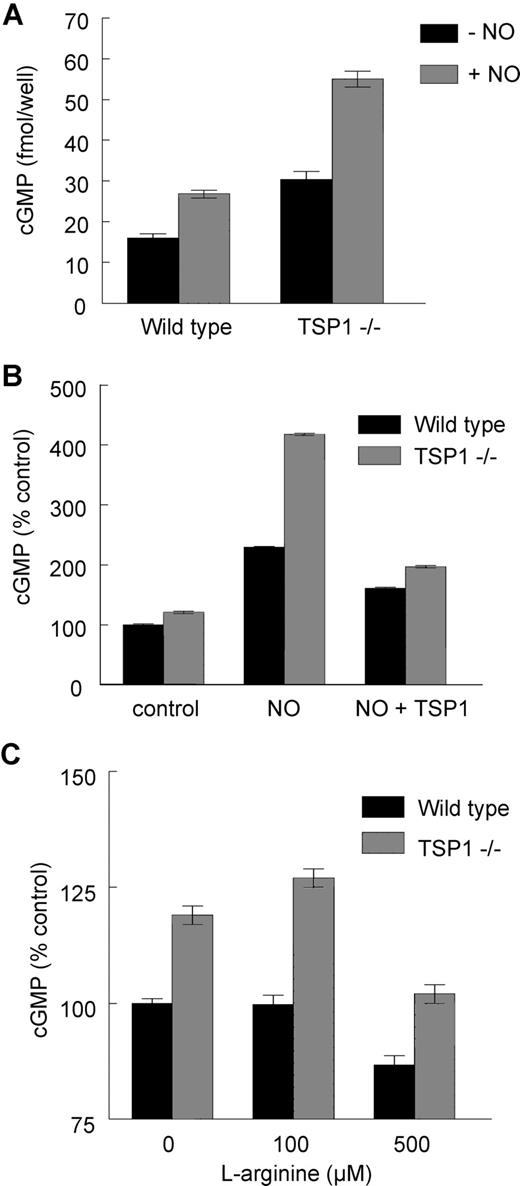
The preceding results establish that exogenous TSP1 can limit NO/cGMP signaling in human platelets but do not reveal whether endogenous TSP1 significantly influences this pathway. Based on Figure 1A, basal plasma TSP1 levels in the WT mouse should be sufficient to limit NO/cGMP signaling. Using platelets from WT and TSP1-null mice, we found that both basal and NO-stimulated platelet cGMP levels were elevated in TSP1-null compared with WT platelets (Figure 2A). Exogenous TSP1 decreased the NO-driven flux in platelet cGMP in both WT and null platelets, though inhibition was greater in TSP1-null platelets (Figure 2B).
Endogenous TSP1 limits NO/cGMP signaling in murine platelets. Equal numbers of murine C57BL/6 WT and TSP1-null platelets were incubated in Tyrode buffer in the presence of 10 μM DEA/NO for 5 minutes, and cGMP was determined by immunoassay (A). In other experiments WT and TSP1-null platelets were preincubated with exogenous TSP1 (2.2 nM) for 15 minutes and then treated with NO (10 μM DEA/NO) (B) or treated with l-arginine at the indicated doses for 20 minutes and cGMP levels determined via immunoassay (C). Results are the mean (± SD) of at least 3 experiments.
Endogenous TSP1 limits NO/cGMP signaling in murine platelets. Equal numbers of murine C57BL/6 WT and TSP1-null platelets were incubated in Tyrode buffer in the presence of 10 μM DEA/NO for 5 minutes, and cGMP was determined by immunoassay (A). In other experiments WT and TSP1-null platelets were preincubated with exogenous TSP1 (2.2 nM) for 15 minutes and then treated with NO (10 μM DEA/NO) (B) or treated with l-arginine at the indicated doses for 20 minutes and cGMP levels determined via immunoassay (C). Results are the mean (± SD) of at least 3 experiments.
Platelets are known to express eNOS, the absence of which in platelets decreases bleeding times,35,36 but the Tyrode buffer typically used to study platelet function lacks the eNOS substrate l-arginine. Addition of 100 μM l-arginine to Tyrode elevated cGMP levels in TSP1-null but not in WT platelets (Figure 2C). Thus, endogenous TSP1 limits cGMP flux driven by both exogenous and endogenous NO.
Endogenous TSP1 is necessary for platelet aggregation in the presence of NO
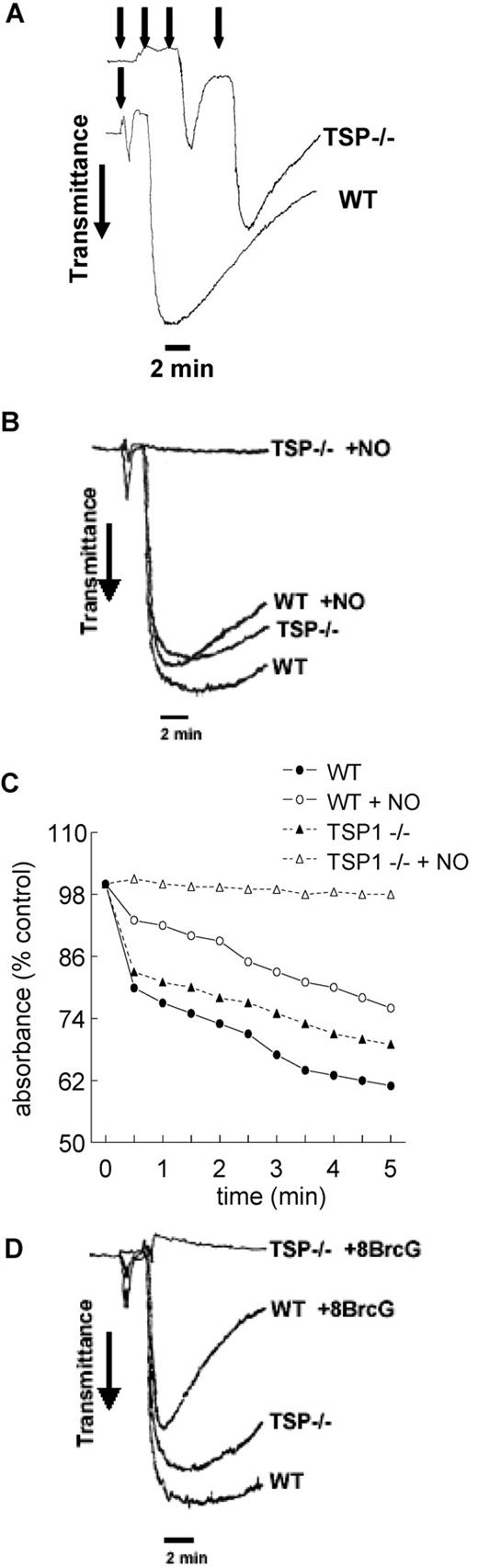
To determine the relevance of endogenous TSP1 to platelet aggregation, we compared thrombin-induced aggregation at high shear using platelets from WT and TSP1-null mice. At low thrombin concentrations, null platelets demonstrated resistance to thrombin-driven aggregation compared with WT platelets as determined by titration with multiple additions of 0.1 U/mL thrombin (Figure 3A). At sufficiently high thrombin levels, null and WT platelets aggregated at the same rate and to nearly the same extent (Figure 3B). However, even at this thrombin dose, null platelets became completely refractory to thrombin in the presence of exogenous NO, while WT platelets demonstrated only a modest decrement in the extent and stability of aggregation in response to NO (Figure 3B).
Endogenous TSP1 is necessary for platelet aggregation in the presence of NO. Equal numbers of murine C57BL/6 WT and TSP1-null platelets were incubated under standard high shear (A,B) or static aggregation (C) conditions. Aggregation profiles were determined in the presence of a titrated dose of thrombin (0.1 U/mL added at time points indicated by arrows, A) or a fixed thrombin dose (0.2 U/mL, B,C) plus or minus DEA/NO (10 μM). Alternatively, equal numbers of WT and TSP1-null platelets were treated with a fixed dose of thrombin (0.2 U/mL) and 8-Br-cGMP (10 μM) under high shear conditions and aggregation was determined (D). Data presented are representative of at least 3 experiments.
Endogenous TSP1 is necessary for platelet aggregation in the presence of NO. Equal numbers of murine C57BL/6 WT and TSP1-null platelets were incubated under standard high shear (A,B) or static aggregation (C) conditions. Aggregation profiles were determined in the presence of a titrated dose of thrombin (0.1 U/mL added at time points indicated by arrows, A) or a fixed thrombin dose (0.2 U/mL, B,C) plus or minus DEA/NO (10 μM). Alternatively, equal numbers of WT and TSP1-null platelets were treated with a fixed dose of thrombin (0.2 U/mL) and 8-Br-cGMP (10 μM) under high shear conditions and aggregation was determined (D). Data presented are representative of at least 3 experiments.
Under static conditions, similar patterns of response were found in murine platelets. Null platelets demonstrated aggregation in the presence of thrombin, though the lack of endogenous TSP1 in these platelets led to decreased aggregation compared with WT (Figure 3C). As with high shear conditions, exogenous NO delayed aggregation of WT murine platelets, but TSP1-null platelets were completely inhibited.
Endogenous TSP1 also limits aggregation downstream of cGMP
In addition to limiting cGMP synthesis by sGC, we previously showed that TSP1 inhibits signaling downstream of cGMP in endothelial cells.24 This second mechanism is also conserved in platelets. The membrane-permeable cGMP analog 8Br-cGMP substantially inhibited aggregation of WT murine platelets. As with NO, the cGMP analog rendered TSP1-null platelets resistant to thrombin-induced aggregation (Figure 3D).
TSP1 limits the antiadhesive activity of NO in platelets
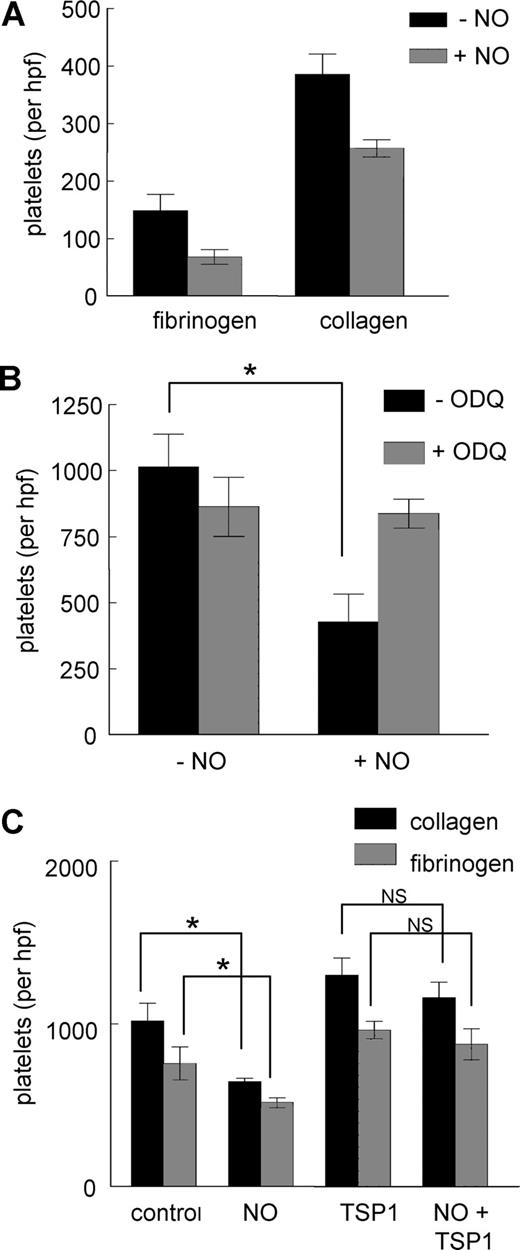
NO is known to inhibit activation of the platelet integrin αIIbβ3.37 Human platelet adhesion to the αIIbβ3 ligand fibrinogen (5 μg/mL) was partially inhibited by exogenous NO (Figure 4A). Similar inhibition was observed for adhesion on type I collagen (3 μg/mL). The effect of NO on adhesion to fibrinogen is mediated by cGMP signaling because the sGC inhibitor ODQ prevented the decrease in platelet adhesion in response to exogenous NO (Figure 4B). Treatment with TSP1 alone moderately increased basal adhesion of platelets on type I collagen and fibrinogen, but the ability of NO to significantly decrease platelet adhesion to fibrinogen or collagen was lost in the presence of TSP1 (Figure 4C).
Regulation of platelet adhesion by NO is blocked by TSP1. Fresh, washed human platelets were added to 35 × 10-mm plastic dishes precoated with either type I collagen (3 μg/mL) or fibrinogen (5 μg/mL) (A) and incubated in Tyrode buffer and the indicated treatment agents for 1 hour at 37°C. Wells were washed and platelets fixed, stained, and counted. Human platelets were incubated on collagen-coated plates in the presence of DEA/NO (10 μM) plus or minus ODQ (10 μM) (B) or collagen and fibrinogen coated plates plus or minus exogenous TSP1 (2.2 nM) (C), and adhesion was determined. Results are the mean (± SD) of at least 3 experiments.
Regulation of platelet adhesion by NO is blocked by TSP1. Fresh, washed human platelets were added to 35 × 10-mm plastic dishes precoated with either type I collagen (3 μg/mL) or fibrinogen (5 μg/mL) (A) and incubated in Tyrode buffer and the indicated treatment agents for 1 hour at 37°C. Wells were washed and platelets fixed, stained, and counted. Human platelets were incubated on collagen-coated plates in the presence of DEA/NO (10 μM) plus or minus ODQ (10 μM) (B) or collagen and fibrinogen coated plates plus or minus exogenous TSP1 (2.2 nM) (C), and adhesion was determined. Results are the mean (± SD) of at least 3 experiments.
TSP1 regulates platelet adhesion via Rap1
The GTPase Rap1 plays important roles in promoting platelet aggregation and adhesion.38,39 cGMP activates cGK-I to phosphorylate Rap1GAP2 on Ser,7 which in turn limits thrombin-mediated activation of Rap1.40,41 GTP-bound Rap1 activates platelet αIIbβ3 integrin by binding to the adapter protein RIAM.42 To determine whether TSP1 regulates Rap1 activation in platelets, we assessed GTP loading by pull down using the Rap1-binding domain of RalGDS (Figure 5A). As expected, thrombin stimulated Rap1 activation, and either NO or 8Br-cGMP inhibited this activation. Addition of 2.2 nM TSP1 reversed the inhibition of Rap1 activation in the presence of NO or 8Br-cGMP, indicating that TSP1 blocks this response both at the level of sGC and downstream of cGMP. TSP1 alone did not increase Rap1 activation (Figure 5B).
TSP-1 enhances platelet adhesion by antagonizing NO and 8Br-cGMP signaling via Rap1 and blocks VASP-Ser239 phosphorylation by inhibiting cGK. Platelets preincubated in the presence or absence of TSP-1 (2.2 nM) were treated with DEA/NO (10 μM) or 8Br-cGMP (100 μM) 2 minutes prior to stimulation with 0.5 U/mL thrombin (A). Rap1 activation was analyzed by affinity purification using GST-RalGDS-RBD fusion protein immobilized on Glutathione-Sepharose beads. Platelets incubated at 37°C in the presence or absence of GGTI-298 (10 μM for 30 minutes) before addition of 1 U/mL thrombin for 1 minute, or incubated with TSP1 (2.2 nM for 15 minutes), were lysed and subjected to a Rap activation assay (B). Fresh, washed human platelets were used directly or preincubated in the presence of GGTI-298 for 30 minutes, added to 35-mm plastic dishes precoated with either type I collagen (3 μg/mL) or fibrinogen (5 μg/mL) (C,D), and incubated in Tyrode buffer and the indicated treatment agents for 1 hour at 37°C. Wells were washed and platelets fixed, stained, and counted. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) plus or minus TSP1 (2.2 nM) for 5 minutes or preincubated with GGTI-298 for 30 minutes and treated as described earlier for 5 minutes; aggregation was determined under high shear (E). Washed human platelets, either untreated or treated with thrombin (0.1 U/mL), 8Br-cGMP (100 μM for 2 minutes), or 2.2 nM TSP1 followed by 8Br-cGMP, were lysed, resolved on SDS gels, blotted, and probed with a polyclonal antiserum against Ser239-phosphorylated VASP (F). Washed human platelets were preincubated with TSP1 (2.2 nM) or Rp-8pCPT-cGMP (5 μM) for 15 minutes prior to treatment with NO (DEA-NO 10 μM) for 5 minutes (G) or 8Br-cGMP (100 μM) for 1 minute (H). The platelets were chilled to terminate the reaction, washed, lysed, and centrifuged. Lysate supernatants containing equal amounts of protein (100 μg) were assayed for phosphorylation of the cGK-I–selective substrate Arg-Lys-Arg-Ser-Arg-Ala-Glu. Data are representative of at least 3 experiments (A,B, E-H). Results are the mean (± SD) of at least 3 experiments (C,D).
TSP-1 enhances platelet adhesion by antagonizing NO and 8Br-cGMP signaling via Rap1 and blocks VASP-Ser239 phosphorylation by inhibiting cGK. Platelets preincubated in the presence or absence of TSP-1 (2.2 nM) were treated with DEA/NO (10 μM) or 8Br-cGMP (100 μM) 2 minutes prior to stimulation with 0.5 U/mL thrombin (A). Rap1 activation was analyzed by affinity purification using GST-RalGDS-RBD fusion protein immobilized on Glutathione-Sepharose beads. Platelets incubated at 37°C in the presence or absence of GGTI-298 (10 μM for 30 minutes) before addition of 1 U/mL thrombin for 1 minute, or incubated with TSP1 (2.2 nM for 15 minutes), were lysed and subjected to a Rap activation assay (B). Fresh, washed human platelets were used directly or preincubated in the presence of GGTI-298 for 30 minutes, added to 35-mm plastic dishes precoated with either type I collagen (3 μg/mL) or fibrinogen (5 μg/mL) (C,D), and incubated in Tyrode buffer and the indicated treatment agents for 1 hour at 37°C. Wells were washed and platelets fixed, stained, and counted. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) plus or minus TSP1 (2.2 nM) for 5 minutes or preincubated with GGTI-298 for 30 minutes and treated as described earlier for 5 minutes; aggregation was determined under high shear (E). Washed human platelets, either untreated or treated with thrombin (0.1 U/mL), 8Br-cGMP (100 μM for 2 minutes), or 2.2 nM TSP1 followed by 8Br-cGMP, were lysed, resolved on SDS gels, blotted, and probed with a polyclonal antiserum against Ser239-phosphorylated VASP (F). Washed human platelets were preincubated with TSP1 (2.2 nM) or Rp-8pCPT-cGMP (5 μM) for 15 minutes prior to treatment with NO (DEA-NO 10 μM) for 5 minutes (G) or 8Br-cGMP (100 μM) for 1 minute (H). The platelets were chilled to terminate the reaction, washed, lysed, and centrifuged. Lysate supernatants containing equal amounts of protein (100 μg) were assayed for phosphorylation of the cGK-I–selective substrate Arg-Lys-Arg-Ser-Arg-Ala-Glu. Data are representative of at least 3 experiments (A,B, E-H). Results are the mean (± SD) of at least 3 experiments (C,D).
The geranylgeranyl transferase inhibitor GGTI-298 inhibits membrane translocation of Rap1,43 and we verified that it inhibits basal and partially inhibits thrombin-stimulated Rap1 activation in platelets (Figure 5B). Although other pathways may also be affected by this inhibitor, GGTI-298 was selective in adhesion assays for blocking thrombin-stimulated adhesion of platelets on fibrinogen but not on the α2β1 ligand collagen (Figure 5C).
The ability of TSP1 to stimulate thrombin-activated platelet adhesion to a substrate coated with the αIIbβ3 integrin ligand fibrinogen was lost following preincubation of platelets with GGTI-298 (Figure 5D). Partial reversal of the TSP1 stimulation of adhesion was also seen in the presence of NO. In the presence of NO, the 55% plus or minus 9% stimulation of adhesion by TSP1 decreased to 25% plus or minus 8% following Rap1 blockade with GGTI-298. Thus, promotion of αIIbβ3-mediated platelet adhesion by TSP1 is at least partially Rap1 dependent.
GGTI-298 preferentially delayed platelet aggregation at high shear in the presence of NO, consistent with the known stimulatory role of Rap1 signaling in platelet aggregation (Figure 5E). However, TSP1 maintained its ability to accelerate platelet aggregation in the presence of both NO and GGTI-298. Thus, the positive effects of TSP1 on aggregation must require additional signaling pathways.
cGK-I is regulated by TSP1 signaling
VASP is another target of cGK, and in mice VASP is required for NO to delay aggregation.44 We first confirmed that treatment of platelets with 8Br-cGMP stimulates VASP phosphorylation at Ser239 and found that this was inhibited by pretreating the platelets with TSP1 (Figure 5F). The ability of TSP1 to prevent phosphorylation of a direct cGK target stimulated by a cell-permeable cGMP analog implied that cGK itself is negatively regulated by TSP1 signaling. We tested this hypothesis using an in vitro kinase assay with a defined cGK-I–selective peptide substrate.45 Activation of cGK via sGC using DEA/NO in intact platelets strongly induced 32P incorporation into the peptide, and this was prevented in platelets treated with TSP1 (Figure 5G). This inhibition by TSP1 could occur at the level of sGC and/or cGK, so to specifically assess inhibition of cGK we activated intact platelets by treating with 100 μM 8Br-cGMP for 1 minute prior to washing and lysis for the kinase assay. Treatment with 2.2 nM TSP1 inhibited 32P incorporation stimulated by 8Br-cGMP to the same extent as the well-characterized cGK-I inhibitor Rp-8pCPT-cGMP (Figure 5H). Therefore, cGK is a second target for TSP1 signaling in platelets.
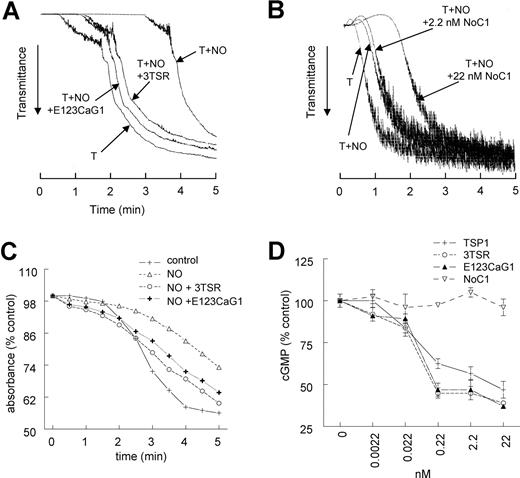
Two domains of TSP1 block the NO-driven delay in platelet aggregation
To define the functional domains of TSP1 that block an NO-stimulated delay in platelet aggregation, we treated platelets with recombinant type 1 repeats (3TSR) and the C-terminal domains of TSP1 (E123CaG-1). Both TSP1 fragments blocked the ability of NO to delay thrombin-induced aggregation at high shear (Figure 6A), whereas a trimeric recombinant construct containing the N-terminal region of TSP1 (NoC1) was inactive at the same concentration and further delayed aggregation at a higher dose (Figure 6B). 3TSR and E123CaG1 also reversed the NO delay of aggregation under static conditions and even tended to enhance aggregation beyond control conditions (Figure 6C). 3TSR and E123CaG1 inhibited NO-stimulated cGMP flux in platelets with similar dose dependencies as full-length TSP, whereas NoC1 was also inactive in this assay (Figure 6D). The type 1 repeats mediate TSP1 interactions with its receptor CD36, and the G module mediates CD47 binding. Thus, consistent with antagonism of NO signaling in vascular cells,26 engaging either TSP1 receptor on platelets appears to be sufficient to overcome the NO-driven delay in aggregation.
CD36- and CD47-binding domains of TSP1 block NO-driven delay of platelet aggregation. Washed human platelets (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes in the presence of recombinant TSP1 constructs 3TSR and E123CaG-1 (2.2 nM) or NoC1 (2.2–22 nM) and aggregation was determined under high shear (A,B) or low shear (C) conditions. In other experiments, washed platelet were preincubated with the indicated concentrations of recombinant fragments and treated with DEA/NO (1 μM) for 60 seconds and lysed, and cGMP levels were determined by immunoassay (D). Data are representative of at least 3 experiments (A-C). Results are the mean (± SD) of at least 3 experiments (D).
CD36- and CD47-binding domains of TSP1 block NO-driven delay of platelet aggregation. Washed human platelets (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes in the presence of recombinant TSP1 constructs 3TSR and E123CaG-1 (2.2 nM) or NoC1 (2.2–22 nM) and aggregation was determined under high shear (A,B) or low shear (C) conditions. In other experiments, washed platelet were preincubated with the indicated concentrations of recombinant fragments and treated with DEA/NO (1 μM) for 60 seconds and lysed, and cGMP levels were determined by immunoassay (D). Data are representative of at least 3 experiments (A-C). Results are the mean (± SD) of at least 3 experiments (D).
CD36- and CD47-binding peptides mimic TSP1 antagonism of NO in platelet aggregation
Two CD47-binding sequences have been identified in the G module of TSP1.46 The NO-driven delay in platelet aggregation at high shear was blocked by a peptide from this region of TSP1 (7N3, 1102FIRVVMYEGKK1112). Two control peptides in which the VVM sequence required for CD47 binding was mutated (604, FIRGGMYEGKK, and 605, FIRVAIYEGKK) had no effect at 10 μM (Figure 7A). Remarkably, 10 nM 7N3 was sufficient to significantly inhibit the NO delay (Figure 7B). Peptide 4N1-1 (1016RFYVVMWK1023), comprising the first VVM sequence in TSP1, and a derivative of the 4N1-1 peptide with terminal lysines to increase its solubility (4N1K47 ) similarly prevented an NO-stimulated delay in platelet aggregation under static conditions (Figure 7C,D).
CD47- and CD36-binding peptides antagonize the NO delay in platelet aggregation. Washed human platelets (2 × 105 platelets/μL) were incubated in Tyrode buffer in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from relevant domains of TSP1 including 7N3 (FIRVVMYEGKK, 10 μM) and control peptides p604 (FIRGGMYEGKK, 10 μM) and p605 (FIRVAIYEGKK, 10 μM, A), and p7N3 (0.1-10 μM, B), and absorbance was determined under high shear, or p459 (4N1–1, RFYVVMWK, 10 μM, C) and 4N1K (KRFYVVMWKK, 10 μM, D), and absorbance was determined under low shear conditions. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from TSP1, including p7N3 and p907 (GDGV[D-I]TRIR, E) (10 μM) and aggregation was determined under high shear, and p906 (VTAGGGVQKRSRL, F; 10 μM) and p246 (KRFKQDGGWSHWSPWSS, G; 10 μM), and absorbance was measured under low shear. Human platelets were pretreated with TSP1-based peptides p907 and p7N3 (10 μM) before adding DEA/NO (10 μM), and cGMP levels were determined (H). Data are representative of at least 3 experiments (A-G). Results are the mean (± SD) of at least 3 experiments (H).
CD47- and CD36-binding peptides antagonize the NO delay in platelet aggregation. Washed human platelets (2 × 105 platelets/μL) were incubated in Tyrode buffer in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from relevant domains of TSP1 including 7N3 (FIRVVMYEGKK, 10 μM) and control peptides p604 (FIRGGMYEGKK, 10 μM) and p605 (FIRVAIYEGKK, 10 μM, A), and p7N3 (0.1-10 μM, B), and absorbance was determined under high shear, or p459 (4N1–1, RFYVVMWK, 10 μM, C) and 4N1K (KRFYVVMWKK, 10 μM, D), and absorbance was determined under low shear conditions. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from TSP1, including p7N3 and p907 (GDGV[D-I]TRIR, E) (10 μM) and aggregation was determined under high shear, and p906 (VTAGGGVQKRSRL, F; 10 μM) and p246 (KRFKQDGGWSHWSPWSS, G; 10 μM), and absorbance was measured under low shear. Human platelets were pretreated with TSP1-based peptides p907 and p7N3 (10 μM) before adding DEA/NO (10 μM), and cGMP levels were determined (H). Data are representative of at least 3 experiments (A-G). Results are the mean (± SD) of at least 3 experiments (H).
A modified CD36-binding peptide derived from the second type 1 repeat (907, 434GDGV(d-I)TRIR442) accelerated platelet aggregation in the presence of NO to a similar degree as the CD47-binding peptide 7N3 in high shear conditions (Figure 7E). A second CD36-binding peptide derived from the third type 1 repeats of TSP1 (906, 488VTAGGGVQKRSRL500) also reversed an NO-stimulated delay of platelet aggregation (Figure 7F), but a heparin-binding peptide derived from the same region (246, 412KRFKQDGGWSHWSPWSS428) was inactive (Figure 7G). The CD47 (7N3)– and CD36 (907)–binding peptides partially inhibited NO-stimulated increases in platelet cGMP (Figure 7H), but the respective control peptides did not (data not shown). These peptide data support the results using the recombinant TSP1 domains: engaging either CD36 or CD47 is sufficient to inhibit cGMP accumulation in platelets.
Discussion
Tonic NO/cGMP signaling acutely regulates vascular tone and tissue perfusion and, if sustained, can induce angiogenesis and vascular remodeling.48,,–51 TSP1 is a major endogenous antagonist of NO-dependent vasodilation24,25,52 and restricts blood flow by vasoconstriction at sites of injury when released from platelets.52 Here, we demonstrate that the hemostatic role of TSP1 extends to regulation of platelet function. Under both high and low shear conditions, NO significantly delays thrombin-stimulated aggregation and decreases adhesion of human and murine platelets. The differential effects of NO on aggregation of WT versus TSP1-null platelets demonstrate that endogenous TSP1 released from platelets in response to thrombin plays an important role to facilitate hemostasis by overcoming the tonic antithrombotic activity of NO. Thus, local release of TSP1 from activated platelets can simultaneously stimulate local vasoconstriction, platelet adhesion, platelet activation, recruitment of additional platelets, and stabilization of the thrombus. Unlike small molecule platelet agonists such as ADP, TSP1 is tethered to both platelets and the fibrin clot, ensuring its localization and persistence in controlling hemorrhage.
TSP1 orthologs occur in all chordates examined and are presumed to have evolved by gene duplication and divergence from a single primordial thrombospondin gene whose present-day descendants are found in lower animals.53,54 Therefore, this potent antagonist of NO signaling has been present throughout vertebrate evolution, supporting a central role of TSP1 in regulating vascular NO signaling.
This function of TSP1 was not previously appreciated because NO is a volatile gas that is lost during the isolation of platelets for in vitro functional assays. In vivo, endothelial eNOS provides a tonic inhibitory level of NO. Platelets also contain eNOS and use circulating l-arginine to generate NO (Figure 8).35,36,56 However, the buffers used in standard aggregation assays do not provide the l-arginine required for NO synthesis by endogenous platelet eNOS. Consequently, endogenous cGMP levels decay as platelets are “rested” before testing, explaining why washed TSP1-null and WT platelets exhibit identical responses to thrombin activation.3,4 The lack of a phenotype for TSP1-null mice in a tail snip bleeding assay may also be explained by the limited dependence of this assay on NO as evidenced by the similar lack of a phenotype for eNOS-null mice.57
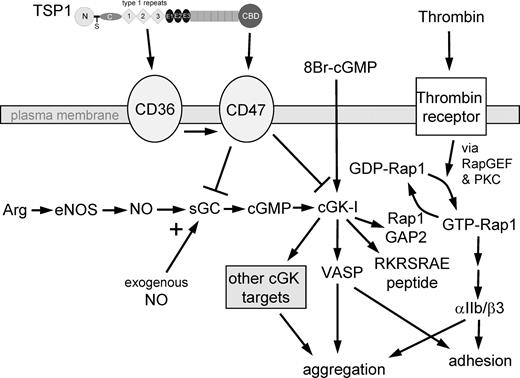
Proposed mechanism for TSP1 antagonism of NO/cGMP signaling in platelets. Using recombinant domains and peptides of TSP1, we show that ligation of CD36 or CD47 is sufficient to block an NO-mediated delay in platelet aggregation. TSP1 blocks a delay mediated by either exogenous NO or NO synthesized by endogenous eNOS using Arg as substrate. The ability of TSP1 to prevent cGMP synthesis stimulated by exogenous NO identifies sGC as one target of TSP1 signaling. The ability of TSP1 to inhibit cGK-I–mediated phosphorylation of VASP and a cGK-I–selective peptide (RKRSRAE) stimulated by a cell-permeable cGMP analog (8Br-cGMP) identifies cGK-I as a second target of TSP1 signaling in platelets. VASP is required for NO/cGMP-mediated inhibition of agonist-induced platelet aggregation44 as well as platelet adhesion.55 TSP1 prevents cGK-I–mediated phosphorylation of VASP at Ser239. NO also stimulates phosphorylation of the cGK-I target Rap1GAP2,40 so TSP1 inhibition of sGC and cGK-I also controls GTP loading of Rap1, which is required for thrombin-stimulated activation of the adhesion receptor αIIb/β3.
Proposed mechanism for TSP1 antagonism of NO/cGMP signaling in platelets. Using recombinant domains and peptides of TSP1, we show that ligation of CD36 or CD47 is sufficient to block an NO-mediated delay in platelet aggregation. TSP1 blocks a delay mediated by either exogenous NO or NO synthesized by endogenous eNOS using Arg as substrate. The ability of TSP1 to prevent cGMP synthesis stimulated by exogenous NO identifies sGC as one target of TSP1 signaling. The ability of TSP1 to inhibit cGK-I–mediated phosphorylation of VASP and a cGK-I–selective peptide (RKRSRAE) stimulated by a cell-permeable cGMP analog (8Br-cGMP) identifies cGK-I as a second target of TSP1 signaling in platelets. VASP is required for NO/cGMP-mediated inhibition of agonist-induced platelet aggregation44 as well as platelet adhesion.55 TSP1 prevents cGK-I–mediated phosphorylation of VASP at Ser239. NO also stimulates phosphorylation of the cGK-I target Rap1GAP2,40 so TSP1 inhibition of sGC and cGK-I also controls GTP loading of Rap1, which is required for thrombin-stimulated activation of the adhesion receptor αIIb/β3.
Our results further suggest that the magnitude of the antithrombotic activity of NO may have been underestimated due to tonic antagonism by endogenous platelet TSP1. In normal platelets at high or low shear, exogenous NO typically delays but does not prevent aggregation22,58,59 (and our data). This implies that NO/cGMP signaling modulates but does not prevent the signal transduction downstream of thrombin that initiates platelet aggregation. One consequence of thrombin signaling is α-granule release, which rapidly makes TSP1 available to bind to its receptors CD36 and CD47. Signaling through these receptors limits NO signaling in platelets at the level of sGC and cGK, thereby promoting aggregation and adhesion (Figure 8). By examining TSP1-null platelets, the potent inhibitory activity of NO in the absence of this positive feedback is revealed.
Our data provide a new interpretation of the previously described effects of TSP1 on αIIbβ3-fibrinogen interactions.7,13,60 Independent of any direct interaction with fibrinogen or αIIbβ3, we propose that TSP1 promotes fibrinogen binding and platelet adhesion by activating αIIbβ3 via Rap1 (Figure 8). We found that NO inhibits platelet adhesion on fibrinogen, whereas TSP1 increases basal adhesion and reverses the inhibition by NO. This probably occurs via blocking the tonic inhibitory effect of cGMP/cGK signaling on activation of this integrin via Rap1GAP2 phosphorylation and consequent Rap1 activation,34,40,41 which may mediate or occur downstream of CD47-mediated activation of αIIbβ3.61 By these mechanisms, TSP1 can increase platelet adhesion and incorporation into fibrin clots. A different CD47 ligand, SHPS1 (also called SIRPα), was also shown to regulate αIIbβ3 function.62 However, the activity of SHPS1 was to inhibit rather than stimulate the integrin. These results implicate either cGK or its substrate Rap1GAP2 as the downstream target through which TSP1 inhibits 8Br-cGMP responses in platelets. However, the ability of TSP1 treatment to prevent phosphorylation of a second cGK substrate VASP and to inhibit cGK activity in an in vitro kinase assay clearly establishes that cGK is a downstream target of TSP1 signaling. Based on studies in VASP-null mice, VASP plays a critical role in the modulation of platelet aggregation by NO,44 and phosphorylation of Ser239 is both a direct and indirect target of NO/cGMP signaling to delay aggregation.23
Collagen is also an important physiologic agonist for platelet activation. Platelet adhesion on collagen and activation in response to this adhesion is mediated by the platelet integrin α2β1 and the collagen signaling receptor glycoprotein VI.63 Platelet adhesion to and aggregation induced by collagen are stimulated by the TSP1-derived peptide 4N1K (but not by the control 4NGG) and by TSP1 in WT but not in CD47-null platelets.10 Here we found that NO inhibits platelet adhesion to type I collagen via a sGC-dependent mechanism, and TSP1 reverses this inhibition. Thus, in addition to enhancing platelet aggregation, TSP1 can promote adhesive interactions with matrix collagen that induce platelet activation. The reported changes in von Willebrand factor processing in TSP1-null mice provide an additional mechanism by which platelet adhesion on collagen can be regulated, and the potential role of NO in this pathway merits further investigation.4,14 The role of endogenous TSP1 in the process of thrombus formation is less clear in severe vessel injury such as those created by guide wire endothelial stripping of arterial segments.64
Consistent with previous reports that CD47-binding peptides increase platelet aggregation,10,–12 we found that CD47-binding peptides potently antagonize NO-stimulated delays in aggregation. This occurred under high shear and static conditions. Both conditions are physiologically relevant since some agonist receptor signaling pathways are initiated only under low shear conditions.65,66 Thus, CD47 may be a useful pharmacological target for controlling platelet activation in diseases associated with decreased blood flow and shear such as peripheral vascular disease. Vascular thrombus frequently complicates this process. Drugs that act as antagonists of CD47 may be useful for treating prothrombotic disorders, and agonists of CD47 could benefit bleeding disorders where thrombin activation is limited. CD47-binding peptides derived from TSP1 could provide leads for creating such mimetics. Such drugs might also be useful for individuals at risk for cardiovascular disease associated with TSP1 polymorphisms.67,68
Our data suggest that CD36 could also be a useful target to control platelet activation. CD36-null platelets aggregate normally, but this was also assessed without NO.69 CD36-null mice and Naka− humans, who lack CD36, may have no phenotype with respect to NO signaling because CD47 can mediate this activity of TSP1 independent of CD36.26 However, because we show that engaging CD36 is sufficient to perturb NO signaling in platelets, CD36-directed drugs such as those currently in clinical testing as antitumor agents might be useful to modulate platelet function.70
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Deane Mosher, Jack Lawler, Larry Keefer, Jules Gladner, and Silvio Gutkind for providing reagents and Mr Atul Kannan for technical assistance with the Rap1 pull downs.
This work was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research (D.D.R., D.A.W.) and NIH grants HL54390 and GM57573 (W.A.F.).
National Institutes of Health
Authorship
Contribution: J.S.I., W.A.F., and M.J.R. designed, performed, and interpreted experiments and wrote the paper; C.Y. and C.K.Y. performed experiments and collected data; K.N., J.M., and M.E.R. assisted with experiments; D.A.W. interpreted experiments; and D.D.R. designed and interpreted experiments and wrote the paper.
Conflict-of-interest disclosure: W.A.F. is president of Vasculox. All other authors declare no competing financial interests.
Correspondence: David D. Roberts, NIH, Bldg 10, Rm 2A33, 10 Center Dr MSC1500, Bethesda, MD 20892; e-mail droberts@helix.nih.gov.

![Figure 7. CD47- and CD36-binding peptides antagonize the NO delay in platelet aggregation. Washed human platelets (2 × 105 platelets/μL) were incubated in Tyrode buffer in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from relevant domains of TSP1 including 7N3 (FIRVVMYEGKK, 10 μM) and control peptides p604 (FIRGGMYEGKK, 10 μM) and p605 (FIRVAIYEGKK, 10 μM, A), and p7N3 (0.1-10 μM, B), and absorbance was determined under high shear, or p459 (4N1–1, RFYVVMWK, 10 μM, C) and 4N1K (KRFYVVMWKK, 10 μM, D), and absorbance was determined under low shear conditions. Washed human platelets in Tyrode buffer (2 × 105 platelets/μL) were incubated in the presence of thrombin (0.2 U/mL) and exogenous NO (DEA/NO 10 μM) for 5 minutes. Peptide sequences were derived from TSP1, including p7N3 and p907 (GDGV[D-I]TRIR, E) (10 μM) and aggregation was determined under high shear, and p906 (VTAGGGVQKRSRL, F; 10 μM) and p246 (KRFKQDGGWSHWSPWSS, G; 10 μM), and absorbance was measured under low shear. Human platelets were pretreated with TSP1-based peptides p907 and p7N3 (10 μM) before adding DEA/NO (10 μM), and cGMP levels were determined (H). Data are representative of at least 3 experiments (A-G). Results are the mean (± SD) of at least 3 experiments (H).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/2/10.1182_blood-2007-06-098392/4/m_zh80020811540007.jpeg?Expires=1769112146&Signature=oB-aY2nr4vD5UIMXL86H392C3UXHTmAjq2rXf1gRd84XKH4RYoEoW6Y3sjw5WgrLpkKHTYq23qLFrN3mOMq0gFTftLNbkmT0fCBi00I9~DrtrlYwqQdyhQYAads7puz9QiO4OoFGGugt6iGIQuS-9RBLsg-j7Sp7OWmMyotuqf~5B9fixiY9GlQsxmn0iitkDhKQqeLAEh5~QzQ5juymjkxjI0kxL-5Rzm5jWS4B5EuPeaHAkAfZVyItRXJXMqAsX2b9u7I0mVcvkkRbpHMnzeIdNTmqguXxD5w0DGKkYQYhgcyHoBN8vzFXcZmrNsBLQud6AUllvbeuutPct7EReA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal