AV513 is a select fucoidan, a sulfated polysaccharide of botanical origin. It inhibits tissue factor pathway inhibitor (TFPI) activity and accelerates clotting of human hemophilia A and B plasma. In prior work, subcutaneous administration of AV513 to mice with hemophilia A improved hemostasis. The current studies were designed to evaluate potential efficacy and safety in dogs with hemophilia A (hemophilia A dogs) with minimally increased hemostasis after adenoassociated viral-FVIII gene transfer and in treatment-naive severe hemophilia A dogs. AV513 administered subcutaneously to low-FVIII dogs for multiple weeks improved hemostasis as exhibited in thromboelastography (TEG) and cuticle bleeding time (CBT) tests. Moreover, AV513 administered orally to AAV-FVIII dogs and treatment-naive severe hemophilia A dogs for a multiweek dose-escalating period yielded correction to normal ranges in both TEG and CBT end points at 5 to 15 mg/kg and 15 to 20 mg/kg dose levels, respectively. In all 3 separate studies, throughout their duration, AV513 was well tolerated by the dogs without any adverse events. Additional pharmacologic characterization of AV513 included intravenous pharmacokinetic analysis in rats. In summary, the combination of safety and efficacy in 2 global tests of hemostasis in the hemophilia A dog model indicate that further evaluation of AV513 as a hemostatic agent in hemophilia A patients is warranted.
Introduction
Hemophilia A patients have an increased bleeding tendency due to the absence or dysfunction of Factor VIII (FVIII). The current therapy for hemophilia A patients involves the intravenous administration of FVIII concentrates (either recombinant or plasma-derived). In addition to FVIII protein replacement, treatment with desmopressin1,2 will increase FVIII levels in most mild hemophilia A patients, and the antifibrinolytic agents tranexamic acid2 and epsilon-aminocaproic acid3 can be effective adjunctive therapies. However, all of these therapies have limitations; for example, the potential for the development of functionally neutralizing anti-FVIII antibodies in approximately 25% of factor-treated patients.4,,,–8 Given the high cost associated with recombinant factor therapy, there is a need to develop alternative treatments that are conveniently administered, effective, less costly, and safe. An added beneficial therapeutic characteristic would be hemostatic efficacy in multiple bleeding disorders.
AV513 is a plant polysaccharide, a fucoidan, derived from brown seaweed and described as a nonanticoagulant sulfated polysaccharide (NASP).9 Compared with other sulfated polysaccharides including heparin, pentosan polysulfate, N-acetyl heparin (NAH) and de-N-sulfated heparin (De-N-SH), AV513 demonstrated a superior nonanticoagulant activity in dilute prothrombin time (dPT) and activated partial thromboplastin time (aPTT) assays performed with human hemophilia A and B plasma. NASPs were originally conceived as a novel approach for improving coagulation based on their inhibition of physiologic anticoagulation. Specifically, AV513 reversed the tissue factor pathway inhibitor (TFPI)–induced prolonged clotting time at nanomolar concentrations and accelerated clotting in dPT assays with hemophilia A or B human plasma in the absence of added TFPI.9
Fucoidans have been recognized to have various pharmacologic activities in model systems in vitro or in vivo. In this context, several animal models of inflammation and hematopoietic progenitor cell mobilization have been used to assess the benefit of fucoidan by blocking selectin function expressed on the surface of activated circulating and vascular cells.10,,–13 Low molecular weight fucoidan (8 kDa) has been shown to regulate neo-intimal formation and endothelial cell proliferation.14,–16 The observed effect on vascular cells is suggested to be due to the enhanced half-life of fibroblast growth factor through fucoidan binding. Unfractionated fucoidans, from various sources, are heterogeneous sulfated polysaccharides and may differ substantially in size and exhibit structural diversity.17,,–20 As fucoidans have some structural similarities to heparin, anticoagulant activity has also been documented. Anticoagulation has been linked to multiple mechanisms; partially through heparin cofactor II, and by directly inhibiting thrombin activity20,21 and potentially also via inhibition of the conversion of fibrinogen to fibrin by direct binding to fibrinogen.22,23
In prior work, a fucoidan selected for minimal anticoagulant activity, AV513, was shown to modestly enhance the survival of hemophilia A mice after tail vein transection when the animals were treated by subcutaneous administration as a monotherapy. Moreover, in combination with rFVIII injection, enhanced efficacy was observed in the AV513 plus rFVIII group compared with sub-optimal dose of rFVIII alone.9 To further explore the potential clinical utility of AV513, the drug candidate has now been evaluated in the well-characterized hemophilia A dog model24 wherein both efficacy and safety endpoints could be robustly evaluated. There is significant precedent for the use of the hemophilic dog model to perform preclinical evaluation of novel hemophilia treatments25,,–28
In this report, data are presented demonstrating dose-related cuticle bleeding time improvement and TEG correction in both minimally treated and naive FVIII-deficient hemophilic dogs after either subcutaneous or oral administration of AV513. Studies were of both short-term and multiweek durations and comonitored safety endpoints showed no adverse effects of AV513 therapy.
Methods
Fucoidans prepared from Fucus vesiculosus (Sigma, St Louis, MO) and Laminaria japonica (proprietary) species of brown sea weed were used. Both preparations had similar potency in in vitro clotting studies. The molecular weight of fucoidans in the preparations range from 10 to 300 kDa in size as measured by laser light scattering (polyhydroxy methacrylate Shodex Pak-column; Thomas Scientific USA, Swedesboro, NJ; and PD2020 multidetector light scattering system, Precision Detectors, Bellingham, MA). The monosaccharide composition of AV513 preparations are mainly composed of fucose, xylose, mannose, glucose and galactose as determined by GC/MS analysis.29
Animal procedures
The dogs with hemophilia A (hemophilia A dogs) were bred and maintained at Queen's University, Kingston, ON. All animal procedures were in compliance with the Canadian Council for Animal Care, institutional animal care committees, and with Unites States Department of Agriculture (USDA) guide for the care and use of laboratory animals.
Dog cuticle bleeding time assay
This in vivo test of hemostasis has been previously validated in the canine model in studies of novel prohemostatic agents and anticoagulants.24,,–27,30 Briefly, 10 minutes after anesthetic induction, the dogs were placed in the lateral recumbency position, and all hair around the nail bed was carefully removed by clipping around the base of the claw to be used for the cuticle bleeding time (CBT) assay. Silicone grease was applied to the claw to prevent blood tracking back underneath the nail. The apex of the cuticle was visualized, and the nail severed proximal to the dorsal nail groove using a spring-loaded sliding blade guillotine clipper. All CBT assays were performed by the same experienced veterinary technologist. The animal's paw was subsequently positioned over the edge of the operating table and blood from the severed cuticle allowed to fall freely. The number of blood drops in each of the subsequent 15 minutes was recorded and converted to a cumulative CBT score for the 15 minutes (Table 1). This cumulative CBT score for the 15 minute observation period for each dog is represented as the total CBT score. After 15 minutes of observation, if the cuticle was still bleeding, the site of injury was cauterized by the topical application of silver nitrate. A single cuticle bleeding time was examined at the end of each dosing period and no cuticle was subjected to more than one injury in a single experiment in both (adeno-associated viral [AAV] vector-mediated canine FVIII gene therapy [AAV-FVIII]) dogs and severe hemophilia A dogs. The AAV-FVIII dog's cuticles had been previously subjected to bleeding time measurements at the time of their gene transfer studies, while the cuticles of the naive, severely hemophilic dogs were injured for the first time during this study. Nevertheless, given the large number of CBTs performed during this study, repeat injuries of some cuticles was inevitable. Cuticle injuries on a single paw were never performed more than once weekly (as per the approved animal care protocol).
Coagulation assays
Diluted PT (dPT) and aPTT assays were performed as described by Liu et al.9 Whole blood clotting time was measured by incubating citrated whole blood at 37°C in a glass test tube with continuous monitoring for clot formation.
TEG was performed with 340 μL of citrated whole blood from severe hemophilia A dogs or citrated, frozen platelet-poor plasma from AAV-hemophilia A dogs after prewarming to 37°C and transferring to a prewarmed disposable TEG cup containing 20 μL of 0.2 M calcium chloride. The samples were gently mixed once by reverse pipetting and the real time dynamics of clot formation was monitored using a Haemoscope thromboelastograph (Haemoscope, Niles, IL). TEG R time (latency period for initial coagulation), angle α (rapidity of clot strengthening), and MA (maximum strength of the formed clot) were determined by the TEGAnalytical Software (version 4.2.2 Haemoscope). For in vitro whole blood TEG studies, 330 μL of citrated whole blood was mixed with 10 μL of the appropriate AV513 concentration. The mixture was later added to the TEG cup containing 20 μL of 0.2 M calcium chloride.
Canine efficacy studies
Subcutaneous administration of AV513.
Three AAV-FVIII hemophilia A dogs; Angus, Gloria, and Morag, were treated several years ago with canine FVIII-expressing adeno-associated virus vector constructs expressing 1% greater than or equal to plasma FVIII levels. In a dose escalation study, the AAV-FVIII hemophilia A dogs were subcutaneously injected with 0.03, 0.1, 0.5, 1.0, and 1.5 mg/kg doses of AV513 twice daily for 5 days per dose with a 7-day washout period after each dosing period. For whole blood clotting time and to prepare citrated plasma for TEG analysis and aPTT and dPT assays, blood was drawn into 1/10 volume of 3.8% trisodium citrate 2 hours before and after the morning dose on the last day of each dose period.
Oral administration of AV513.
Angus, Gloria, and Morag were orally administered with AV513 in #0 capsules. In this continuous dose escalation study, animals were treated twice daily for 5 days at 3, 5 and 7.5 mg/kg and for 10 days at 15 mg/kg. For whole blood clotting time and to prepare citrated plasma for TEG analysis and aPTT and dPT assays, blood was drawn into 1/10 volume of 3.8% trisodium citrate 2 hours before and after the morning dose on the last day of each dose period.
Multiweek dose-escalation study.
Three treatment-naïve, FVIII-deficient hemophilia A dogs, Bertha, Darla, and Wembley, were treated orally with AV513 twice daily for 7 days at 5, 7.5, 10 mg/kg and for 10 days at 15 mg/kg. After a 3-week wash out period, animals were treated with 20 mg/kg of AV513 for 5 days twice daily. Blood was drawn into 1/10 volume of 3.8% trisodium citrate for whole blood clotting time and TEG analysis 2 hours before and after the morning dose on the last day of each dose period, except when animals were treated at 5 mg/kg, the analyses were performed on day 5, before and after the morning dose. During the 10-day treatment at 15 mg/kg, blood was collected on the fifth day 2 hours after the morning dose to perform interim TEG analysis. Citrated plasma was prepared from each blood draw and frozen for aPTT and TEG assays.
In all dog studies, baseline analyses (TEG, aPTT, dPT, and whole blood clotting time) were performed 7 days before starting the AV513 treatments. Clinical chemistry (urea, creatinine, alanine transaminase, bilirubin, creatine kinase) and hematology (hemoglobin, white blood cell [WBC], and platelet counts) assays were performed before and after the last dose of each dosing period. During the treatment period the dogs were closely monitored for 24 hours for cuticle rebleeding after the CBT procedure and inspected daily for spontaneous bleeding and signs of discomfort.
Pharmacokinetics of AV513
Fluorescence labeling of AV513 was performed as previously described.31 Briefly, AV513 was activated by cyanogen bromide and derivatized with fluoresceinamine (Sigma). Labeled AV513 (Fla-AV513) was purified on a G-50 sephadex (Sigma) gel column at a flow rate of 1.8 mL/min. Fractions were followed with a Hitachi L-2480 fluorescence detector at 490 nm. The fluorescence tag on AV513 was quantified using fluoresceinamine to derive a standard curve. The equation of this line was used to calculate the fluorescence concentration. Carbohydrate concentration was measured, using fucose as the standard, by the phenol-sulfuric acid method.32
Four Sprague-Dawley rats (Harlan, Indianapolis, IN) housed for a week for acclimatization, were injected intravenously with Fla-AV513 at 5 mg/kg. Blood samples were collected at scheduled times through the tail vein into 1/10 volume of 3.8% trisodium citrate. Plasma was prepared by centrifuging whole blood at 6000×g and read in a fluorescence reader at 490 nm. Plasma concentrations were determined from a standard curve derived from known amount of Fla-AV513. Noncompartmental pharmacokinetic analysis was performed using the WinNonlin software program (version 4.0; Pharsight, Mountain View, CA).
Statistical analyses
Statistical significance among different groups was determined by the Student t test and error bars represent the standard deviation.
Results
The first studies were performed in 3 hemophilia A dogs that had previously received AAV-FVIII.28 After AAV gene transfer, these 3 AAV-FVIII dogs had undetectable levels of plasma FVIII (< 1%) but showed shortening of their whole blood clot times and did not experience further spontaneous bleeding episodes for at least 3 years after treatment (normal frequency approximately 5/yr). We have regarded these dogs as showing partial phenotypic correction after AAV gene transfer. They best recapitulate the clinical picture documented in approximately 10% of severe hemophilia A patients (plasma FVIII < 1%) who rarely bleed. Such a profile also mirrors the outcome of a low dose FVIII prophylaxis protocol.
In a multiweek dose escalation study, AV513 was administered subcutaneously twice daily to the AAV-FVIII hemophilia A dogs. CBT, plasma TEG assay, whole blood clotting time, and dPT and aPTT assays performed on the last day of each dosing period served as efficacy endpoints. A dose-dependent decrease in cuticle bleeding time score was observed in all 3 dogs with an optimal activity at 1.0 to 1.5 mg/kg and a magnitude of efficacy within or very close to a normal dog CBT score of less than or equal to 10 (Table 2).
Effect of subcutaneous injections of AV513 on the cuticle bleeding time in AAV-FVIII hemophilia A dogs
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Angus . | Gloria . | Morag . | |
| Baseline | 36 | 54 | 30 |
| 0.03 | 29 | 48 | 54 |
| 0.1 | 45 | 27 | 48 |
| 0.5 | 33 | 31 | 19 |
| 1.0 | 27 | 7* | 10* |
| 1.5 | 17 | 30 | 30 |
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Angus . | Gloria . | Morag . | |
| Baseline | 36 | 54 | 30 |
| 0.03 | 29 | 48 | 54 |
| 0.1 | 45 | 27 | 48 |
| 0.5 | 33 | 31 | 19 |
| 1.0 | 27 | 7* | 10* |
| 1.5 | 17 | 30 | 30 |
In a dose-escalation study, three AAV-FVIII dogs received different doses of AV513 BID subcutaneously for 5 days. After each dosing period, dogs were evaluated for the cuticle bleeding time as described in “Dog cuticle bleeding time assay.” The number of blood drops/min was converted to a bleeding score and a total bleeding score was calculated for a 15 minute bleeding time evaluation at each time point.
Normal range up to 10.
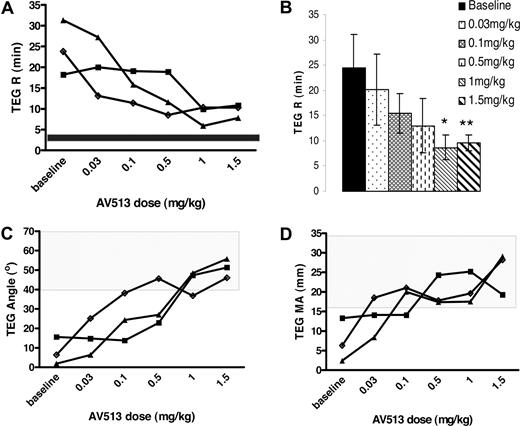
When citrated plasma from the 3 treated AAV-FVIII hemophilia A dogs was analyzed for changes in clot dynamics by TEG assay, a dose-dependent improvement in 3 important TEG parameters was observed. AV513-dependent reduction in clot initiation time (TEG R time), rate of fibrin generation (angle), and increased clot strength (MA) was evident. Significant improvement in clot initiation was observed when animals were treated with AV513 at 1.0 and 1.5 mg/kg twice daily with TEG R times of 8.7 (± 2.4) and 9.6 (± 1.6) minutes, respectively, compared with a baseline TEG R time of 24.4 (± 6.5) minutes (Figure 1A,B). Similar to the CBT scores, the slope of the TEG R time dose response curves varied between animals at low doses of AV513 (Figure 1A), although peak efficacy was observed at doses between 1.0 and 1.5 mg/kg where the TEG R time was corrected close to the normal dog plasma clot initiation time of less than 5 minutes (data not shown). Coincident with the decreased clot initiation time, a dose-dependent rapid increase in angle (Figure 1C) and clot strength (Figure 1D) was observed at doses higher than 0.5 mg/kg, with the most effect observed at 1.5 mg/kg (angle; treated 51 ± 4.9° vs baseline 8.0 ± 7.0°, P = .001 and MA; treated 25.5 ± 5.4 mm vs baseline 7.4 ± 5.5 mm, P = .015). A representative dose-dependent improvement in clot dynamics is evident in Morag's TEG tracings (Figure 2A-F).
Subcutaneous injections of AV513 improve the clot dynamics in AAV-hemophilia A dogs. In a dose-escalation study, plasma prepared from AAV-hemophilia A dogs before dosing and at the end of each dosing period were evaluated for clotting in a TEG assay. TEG R time represents the time required to initiate a 2-mm clot. (A) Progressive change in TEG R times of individual animals during the treatment period is plotted. (B) A TEG R time averaged from 3 dogs at each dose is represented as a bar graph with error bars representing SD. (*P = .017, **P = .019 compared with baseline values.) (C) TEG angle represents the rate of fibrin formation, P values at 0.5, 1.0, and 1.5 mg/kg dose are .04, .002, and .001, respectively. (D) TEG MA represents clot strength, P values at 0.5, 1.0, and 1.5 mg/kg dose are .03, .02, and .015, respectively. Gloria (■), Morag (▲), and Angus (◆). The boxed area in the graphs represents the clot dynamics values for normal plasma.
Subcutaneous injections of AV513 improve the clot dynamics in AAV-hemophilia A dogs. In a dose-escalation study, plasma prepared from AAV-hemophilia A dogs before dosing and at the end of each dosing period were evaluated for clotting in a TEG assay. TEG R time represents the time required to initiate a 2-mm clot. (A) Progressive change in TEG R times of individual animals during the treatment period is plotted. (B) A TEG R time averaged from 3 dogs at each dose is represented as a bar graph with error bars representing SD. (*P = .017, **P = .019 compared with baseline values.) (C) TEG angle represents the rate of fibrin formation, P values at 0.5, 1.0, and 1.5 mg/kg dose are .04, .002, and .001, respectively. (D) TEG MA represents clot strength, P values at 0.5, 1.0, and 1.5 mg/kg dose are .03, .02, and .015, respectively. Gloria (■), Morag (▲), and Angus (◆). The boxed area in the graphs represents the clot dynamics values for normal plasma.
Improved clot dynamics in Morag's plasma TEG tracings after subcutaneous AV513 treatment. In a dose-escalation study, plasma prepared from Morag 2 hours after the last dose was evaluated for clotting. Dose-dependent reduction in TEG R time, improved angle, and enhanced clot strength is recorded in the TEG tracings. A, baseline, B, 0.03 mg/kg, C, 0.1 mg/kg, D, 0.5 mg/kg, E, 1.0 mg/kg, F, 1.5 mg/kg.
Improved clot dynamics in Morag's plasma TEG tracings after subcutaneous AV513 treatment. In a dose-escalation study, plasma prepared from Morag 2 hours after the last dose was evaluated for clotting. Dose-dependent reduction in TEG R time, improved angle, and enhanced clot strength is recorded in the TEG tracings. A, baseline, B, 0.03 mg/kg, C, 0.1 mg/kg, D, 0.5 mg/kg, E, 1.0 mg/kg, F, 1.5 mg/kg.
AV513 treatment did not affect other end points such as the whole blood clotting time, plasma dPT, and aPTT. Moreover, during the treatment period, there were no AV513-related changes in weekly-monitored hematology and clinical chemistry tests, no spontaneous bleeding episodes, and no behavioral or body weight changes.
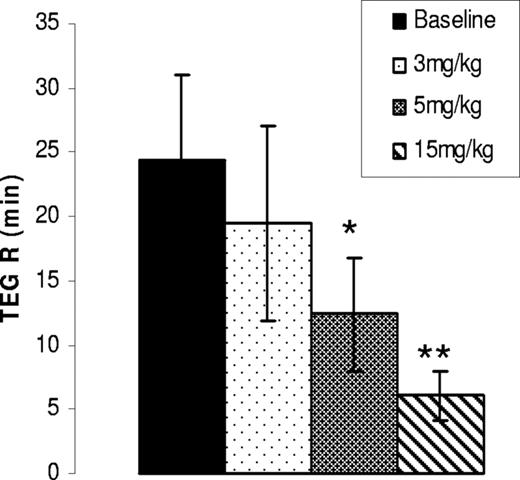
Given the positive outcome with subcutaneous dosing, AV513 was next administered orally in the 3 AAV-FVIII hemophilia A dogs. In a multiweek dose escalation study, a single CBT analysis (Table 3) at the completion of 7.5 mg/kg dose and plasma TEG assay at the end of each dosing week was performed. Angus and Gloria had substantial reductions in bleeding times (79% and 68.5% reduction in CBT scores, respectively), while Morag had a slightly elevated score. Interestingly, when citrated plasma from the treated dogs was tested for clotting by TEG assay, a dose-dependent decrease in the R time was observed with a maximum reduction in all 3 animals at 15 mg/kg (Figure 3).
Effect of orally administered AV513 on the cuticle bleeding time (CBT) in AAV-FVIII Hemophilia A dogs
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Angus . | Gloria . | Morag . | |
| Baseline | 38 | 54 | 30 |
| 7.5 | 8* | 17 | 47 |
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Angus . | Gloria . | Morag . | |
| Baseline | 38 | 54 | 30 |
| 7.5 | 8* | 17 | 47 |
Three AAV-FVIII dogs received an oral dose of 7.5 mg/kg of AV513 BID for 5 days. After the last dose, the cuticle bleeding time was measured and total CBT score was assessed.
Within normal range.
Oral administration of AV513 to AAV-FVIII hemophilia A dogs accelerates the plasma clotting time. Plasma prepared from AAV-hemophilia A dogs before and at the end of each dosing period were evaluated for clotting in a TEG assay. A TEG R time averaged from 3 dogs after each dose is represented as a bar graph with error bars representing SD. *P = .05, **P = .01 compared with baseline values.
Oral administration of AV513 to AAV-FVIII hemophilia A dogs accelerates the plasma clotting time. Plasma prepared from AAV-hemophilia A dogs before and at the end of each dosing period were evaluated for clotting in a TEG assay. A TEG R time averaged from 3 dogs after each dose is represented as a bar graph with error bars representing SD. *P = .05, **P = .01 compared with baseline values.
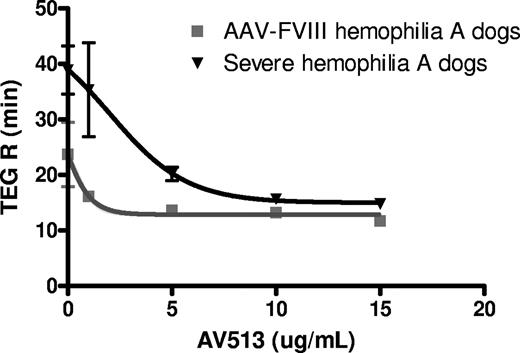
As AV513 improved hemostasis in AAV-FVIII hemophilia A dogs, we next decided to evaluate its efficacy in treatment-naive, severe hemophilia A dogs. The severe hemophilia A dogs in this colony have no detectable plasma levels of FVIII and have approximately 5 spontaneous bleeding episodes per year.33 The citrated plasma from these animals fails to clot in a TEG assay when stimulated with excess calcium. As a first step, the potency of AV513 to accelerate clot initiation was compared with citrated whole blood from AAV-FVIII hemophilia dogs and the severe hemophilia A dogs in an in vitro TEG assay. A dose-dependent reduction of TEG R time was observed in the citrated whole blood from treatment-naïve, severe hemophilia A dogs and AAV-FVIII dogs (Figure 4) with a 50% efficacy concentration (EC50) of 2.8 μg/mL and 0.9 μg/mL, respectively.
AV513 has different potency in AAV-FVIII and severe hemophilia A dog whole blood TEG assays. TEG R times were determined in citrated whole blood from dogs with low Factor VIII (AAV-FVIII) or severe, treatment-naive hemophilia A in the presence or absence of added AV513. TEG R times for each dose were averaged from 3 dogs/group, with error bars representing SD.
AV513 has different potency in AAV-FVIII and severe hemophilia A dog whole blood TEG assays. TEG R times were determined in citrated whole blood from dogs with low Factor VIII (AAV-FVIII) or severe, treatment-naive hemophilia A in the presence or absence of added AV513. TEG R times for each dose were averaged from 3 dogs/group, with error bars representing SD.
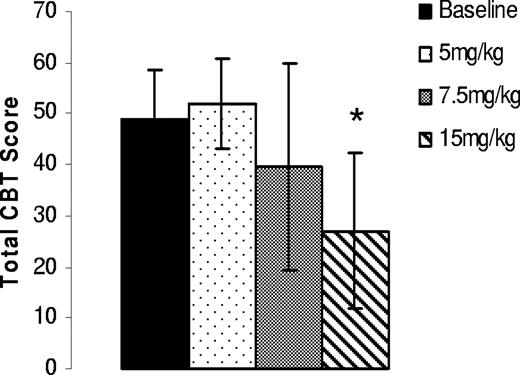
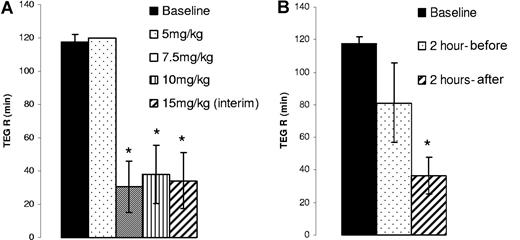
In anticipation of a higher dose requirement for optimal efficacy in the treatment-naïve, severe hemophilia A dogs, repeated 5-day oral dosing was initiated at 5 mg/kg twice daily and escalated to 20 mg/kg twice daily as the highest test dose. As end points, CBT analysis was performed at the end of 5, 7.5, and 15 mg/kg treatments, while TEG analysis was performed at the end of all dose periods with an additional interim analysis after 5 days when dogs received 15 mg/kg twice daily for 10 days. CBT scores from the treated dogs suggested a varied dose response in individual animals with a maximum efficacy observed at doses between 7.5 and 15 mg/kg (Table 4) with an optimal overall response at 15 mg/kg (Figure 5) in all 3 dogs. Interestingly, when whole blood TEG analysis was performed after each dose regimen, a 75% reduction in the R time (Figure 6A) was observed when dogs were treated with 7.5 mg/kg and 10 mg/kg and at 15 mg/kg BID for 5 days (interim). Surprisingly, when whole blood TEG analysis was performed after an additional 5 days of 15 mg/kg twice daily treatment, reduction of the TEG R time was not observed in all3 dogs even though improvement in CBT scores was recorded (Table 4) in 2 of the 3 dogs. As we have seen no indications of attenuated efficacy with repeat daily dosing and as the CBT scores were clearly improved at this dose, we suspect an error in blood sampling and/or TEG analysis at this time point.
Effect of orally administered AV513 on the cuticle bleeding time in severe hemophilia A dogs
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Bertha . | Darla . | Wembley . | |
| Baseline | 37 | 34 | 59 |
| 5.0 (5 days) | 42 | 56 | 58 |
| 7.5 (7 days) | 23 | 34 | 62 |
| 15.0 (7 days) | 29 | 11* | 44 |
| Wash out (3 weeks) | 49 | 54 | 59 |
| 20.0 (7 days) | 3* | 58 | 2* |
| AV513 dose, mg/kg . | Total CBT score . | ||
|---|---|---|---|
| Bertha . | Darla . | Wembley . | |
| Baseline | 37 | 34 | 59 |
| 5.0 (5 days) | 42 | 56 | 58 |
| 7.5 (7 days) | 23 | 34 | 62 |
| 15.0 (7 days) | 29 | 11* | 44 |
| Wash out (3 weeks) | 49 | 54 | 59 |
| 20.0 (7 days) | 3* | 58 | 2* |
Three treatment-naïve, severe hemophilia A dogs received twice daily the indicated doses of AV513 by mouth for different periods of time. After the last treatment of each regimen, dogs were evaluated for cuticle bleeding time and a total CBT score was assessed.
Within normal range.
Oral administration of AV513 improves the cuticle bleeding in treatment-naïve, severe hemophilia A dogs. Cuticle bleeding scores obtained after each dosing period were averaged from 3 dogs, with error bars representing SD. *P = .04 compared with baseline.
Oral administration of AV513 improves the cuticle bleeding in treatment-naïve, severe hemophilia A dogs. Cuticle bleeding scores obtained after each dosing period were averaged from 3 dogs, with error bars representing SD. *P = .04 compared with baseline.
Improved clotting in treatment-naive, severe hemophilia A dogs after oral AV513 administration. Citrated whole blood from severe hemophilia A dogs prepared before and at the end of each dosing period was evaluated for clot formation in the TEG assay. (A). TEG R time averaged from 3 dogs after each dose (with SD) is represented as a bar graph. (*P = .001 compared with baseline). (B) Citrated whole blood from severe hemophilia A dogs prepared before and after the last dose of 20mg/kg were evaluated for clotting in the TEG assay. TEG R times were averaged from 3 dogs and are represented as a bar graph (with SD). (*P = .002 compared with baseline). The 2-hour preanalyses were performed with blood drawn 2 hours before the last dose (ie, 15 hours after the previous dose), while the 2-hour postanalyses were performed with blood drawn 2 hours after the last dose.
Improved clotting in treatment-naive, severe hemophilia A dogs after oral AV513 administration. Citrated whole blood from severe hemophilia A dogs prepared before and at the end of each dosing period was evaluated for clot formation in the TEG assay. (A). TEG R time averaged from 3 dogs after each dose (with SD) is represented as a bar graph. (*P = .001 compared with baseline). (B) Citrated whole blood from severe hemophilia A dogs prepared before and after the last dose of 20mg/kg were evaluated for clotting in the TEG assay. TEG R times were averaged from 3 dogs and are represented as a bar graph (with SD). (*P = .002 compared with baseline). The 2-hour preanalyses were performed with blood drawn 2 hours before the last dose (ie, 15 hours after the previous dose), while the 2-hour postanalyses were performed with blood drawn 2 hours after the last dose.
Given the endpoint variability at the end of 10 days of 15 mg/kg treatment of AV513, efficacy was assessed at a higher dose. After a 3-week wash out period, the study was resumed with new baseline testing and then a 7-day twice daily regimen of 20 mg/kg of AV513. As seen in Table 4, 2 of 3 dogs had bleeding times corrected to normal and, hence, a 90% reduction from baseline CBT scores. Darla had a peak response, with 67% reduction in CBT score at 15 mg/kg and, surprisingly, did not respond at 20 mg/kg. The whole blood TEG analysis showed that all 3 dogs exhibited improved clotting dynamics 2 hours after the last treatment. The TEG analyses of blood samples drawn 2 hours before the last dose (fifteen hours after the previous dose) showed that all 3 animals clotted with an average TEG R time of 81 (± 24) minutes, while in the absence of AV513 no clotting was observed in 2 dogs (baseline > 120 minutes) and in the third dog the baseline TEG R time was 112 minutes (Figure 6B). Hence, while AV513 efficacy is more optimal 2 hours postdose, an effect 15 hours postdose is still evident. Nonetheless, in a couple of assessments of efficacy at 1 or 3 week intervals after cessation of AV513 administration (subcutaneously or orally), CBT and TEG endpoints had returned to baseline levels (data not shown), indicating the absence of a sustained, cumulative effect.
As an additional component of pharmacologic characterization, the pharmacokinetic properties of AV513, were examined with a fluorescently tagged AV513 (Fla-AV513) administered intravenously in rats. Fla-AV513, prepared with a labeling density of 2.36% (1 fluoresceine to approximately 42.4 fucose monomers), was injected intravenously into 4 rats and serial blood samples were collected at time points thereafter with analysis of plasma for Fla-AV513 levels. The plasma concentration vs time curve is presented in Figure 7. As determined by noncompartmental WIN NONLIN analysis, the elimination half-life was calculated to be 85 minutes, and other deduced parameters included a Cmax of 67 μg/mL, area under the curve (AUC∞ predicted) of 137 μg-hr/mL, Vss of 0.7 L/kg which may be indicative of low tissue penetration, and low plasma clearance of 36 mL/hr per kilogram.
AV513 plasma concentrations after intravenous administration of fluorescent labeled AV513 (Fla-AV513) in rats. Four adult rats were injected intravenously with 5 mg/kg of Fla-AV513. At indicated time intervals after dosing, blood was drawn for plasma preparation and Fla-AV513 was quantified by measuring fluorescence at 485/538 nm.
AV513 plasma concentrations after intravenous administration of fluorescent labeled AV513 (Fla-AV513) in rats. Four adult rats were injected intravenously with 5 mg/kg of Fla-AV513. At indicated time intervals after dosing, blood was drawn for plasma preparation and Fla-AV513 was quantified by measuring fluorescence at 485/538 nm.
Discussion
AV513, a fucoidan derived from brown seaweed, is a branched sulfated polysaccharide of heterogeneous size. Fucoidans, present in more than fifty different species of brown seaweed, have variable sulfation and possess fucose as the most abundant monosaccharide in the backbone but differ in the composition of other sugars such as xylose, mannose, galactose, and glucose.10,34
The current results extend prior observations of in vitro and in vivo procoagulant efficacy by AV513. We previously reported9 that AV513 inhibited exogenous TFPI activity and accelerated the clotting time of human hemophilia A and B plasma. In a murine tail transection model, fucoidan treatment improved the survival rate of hemophilia A mice. In this report, we have demonstrated, for the first time, the safety and efficacy of AV513 in minimally treated hemophilia A dogs and in treatment-naïve, severe hemophilia A dogs with undetectable levels of plasma FVIII.
Studies in FVIII-deficient mice indicated that the maximum benefit was achieved when mice received fucoidan along with low dose intravenous infusions of rFVIII. The superior activity of fucoidan in the presence of very low FVIII levels prompted us to first evaluate the efficacy of subcutaneously administered AV513 in hemophilia A dogs that had received AAV-mediated FVIII gene therapy and had less than 1% of normal FVIII levels28 but had not experienced spontaneous bleeding after gene transfer and exhibited shortened whole blood clot times. Coagulation parameters such as cuticle bleeding time, plasma and/or whole blood TEG analysis, aPTT, dPT, and whole blood clotting were measured as efficacy endpoints. In a multiweek dose escalation study, AV513 was found to be efficacious in all 3 dogs with an individualized dose response at twice daily subcutaneous doses of 0.5 to 1.5 mg/kg, with decreased CBT scores and accelerated clotting as observed in plasma TEG analysis. When AV513 was orally administered in the same dogs, plasma TEG R times were greatly improved at doses of 5 mg/kg or more. A single CBT analysis done at the end of 7.5-mg/kg treatment showed improved CBT scores in 2 of the 3 animals.
Given the demonstrated efficacy in the FVIII-AAV dogs, AV513 was then tested in treatment-naive, severe hemophilia A dogs with no detectable levels of FVIII. Oral administration of AV513 in these dogs also showed improvement in the cuticle bleeding time and TEG clotting analyses at higher doses. Improvement of the cuticle bleeding time score was observed mainly at 15 mg/kg and 20 mg/kg, while in whole blood TEG analysis, acceleration of clotting was observed at doses higher than 7.5 mg/kg. The requirement of higher doses of AV513 in the severe hemophilia A dogs for optimal activity is evident in the in vitro whole blood TEG assay (Figure 4). To achieve a similar TEG R time, a 3-fold higher concentration of AV513 is added to severe hemophilia A blood compared with that from AAV-FVIII dogs. However, concentrations higher than 5 μg/mL of AV513 have similar potency in the absence or presence of low FVIII levels. These results further substantiate that AV513 can be more effective at low concentrations in the presence of suboptimal levels of FVIII and at higher concentrations it has the potential to compensate for severe FVIII deficiency and function as a stand-alone procoagulant.
While an overall improvement from baseline was achieved at several dose levels, the individual animal optimal dose level(s) varied. AAV-FVIII dogs, Gloria and Morag, had a peak response when treated at 1.0 mg/kg and Angus at 1.5 mg/kg, as determined by the CBT score in the subcutaneous administration study. Similarly, when the treatment-naive, severe hemophilia A dogs were treated orally, Darla had a maximum reduction in CBT score at 15 mg/kg but had no response with the 20 mg/kg regimen. Considering that AV513 at high concentrations can potentially become anticoagulant,9 dogs were carefully monitored for dPT and aPTT changes or treatment-related bleeding episodes. The aPTT values of all dogs at every point in the study were slightly reduced or unchanged relative to baseline or washout values (data not shown). Platelet count and hematocrit values were likewise not affected by AV513 treatment. AAV-FVIII hemophilia A dogs, Gloria and Morag, and severe hemophilia A dog, Darla, had a maximum reduction in the TEG R time when treated orally with 1.5 mg/kg subcutaneously and 20 mg/kg orally, respectively. The ex vivo clotting of plasma and whole blood in these dogs suggests that at these treatment doses, AV513 promoted clot initiation. In contrast, the lack of CBT correction in these dogs at these AV513 doses could simply be due to low and variable circulating drug levels that can support the formation of a clot in vitro but cannot maintain the clot in vivo where other factors such as hemodynamic forces play a major role in clot stability. In addition, variability of CBT outcomes due to local vascular factors (ie, differences in the size of the injured vessel) and minor variations in the depth of incision cannot be ruled out although well controlled procedures were performed.
Loss of AV513 activity at higher doses in vivo can also be due to its potential anticoagulant activity. While no increase in aPTT was observed at higher doses in the dogs, our studies in normal rats have shown that AV513 oral doses over 100 mg/kg demonstrate little or no increase in aPTT (data not shown). In addition, the safety of fucoidan has also been reported in animal toxicology and human clinical trials. In rats, a toxicology study with fucoidan derived from L japonica was described wherein doubling of clotting time after oral dosing of male and female rats with a high dose of 2500 mg/kg per day was observed. Notably, during 6 months of treatment at this high dose, there were no adverse changes in hematology, serum chemistry and body weight.35
The dual activity of AV513 as pro- and anticoagulant does call for careful monitoring during human testing. Interestingly, safe outcomes were reported during multiday and multiweek times at dose levels up to 3 g/day in human clinical studies with fucoidan derived from Undaria pinnatifida orally administered to herpes patients,36 oncology patients, and healthy volunteers.37 An apparently broad window of efficacy and safety for AV513 was affirmed in the current hemophilia A dog studies wherein 3 series of multiweek studies with 6 dogs were completed with no adverse events in behavior or clinical pathology endpoints. Thus, our results along with the published findings suggest that fucoidans appear well tolerated and that safe hemostasis can be achieved.
In the development of AV513, one would ideally correlate efficacy with plasma or serum drug levels. However, with a botanical drug candidate like fucoidan, it is very challenging to analytically measure plasma levels of AV513 because of the heterogeneous molecular weight, branched structure, and similarity in monosaccharide composition to mammalian polysaccharides. Although there are limitations to labeling fucoidan due to its chemical structure, methods have been developed to achieve low density fluorescent labeling.31 With 2.6% of fluorescent moieties on AV513, a labeling density that reduces detection sensitivity to 10 μg/mL, we determined the intravenous AV513 pharmacokinetics in rats. The heterogeneous species of Fla-AV513 exhibited an apparent elimination half-time of 1.25 hours with a relatively low clearance rate of 36 mL/kg per hr. These PK parameters are superior to the properties reported for low molecular weight fucoidan,14 which had a shorter half-life and was eliminated faster from circulation. Fucoidan is known to interact with L- and P-selectins10,38,–40 expressed on hematopoietic and vascular cells. It is possible that, aside from its interaction with coagulation protein target(s), AV513 may bind to vascular cells, given the potential for selectin interactions affecting its true half life.
AV513, administered either subcutaneously or orally, appears to provide therapeutically significant hemostatic improvement in low FVIII or totally FVIII-deficient subjects (mice and dogs). Clearly, the current results underscore a need for individualized dose optimization in bleeding disorder patients. The combined efficacy and safety of AV513 by 2 routes of administration for extended time periods in 2 groups of hemophilia A dogs supports the assessment of this drug candidate in humans. One objective of future studies will be to ascertain whether AV513 is best suited to use as an oral hemostatic adjunct to factor replacement (eg, to complement prophylactic regimens) or whether its hemostatic efficacy is sufficient to act as a stand-alone procoagulant agent in some patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful for technical and administrative assistance by Tongyao Liu and Lance Sultzbaugh, respectively. In addition, Ken Chahine and Susannah Patarroyo-White are acknowledged for helpful discussions and encouragement. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis and a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
Authorship
Contribution: K.W.J., S. Prasad, and D.L. designed the studies, analyzed the data, and wrote the paper. A.L., T.K., E.B., S. Prasad, S. Powell, and S.K. performed the studies.
Conflict-of-interest disclosure: K.W.J., S. Prasad, and S.K. are employees of Avigen and interested in developing therapy for bleeding disorders. At the time these studies were performed, D.L. had no conflicts of interest. Subsequent to completion of the studies, D.L. has assumed a limited financial interest in Avigen. The remaining authors declare no competing financial interests.
Correspondence: Srinivasa Prasad, Avigen, 1301 Harbor Bay Parkway, Alameda, CA 94502; e-mail: sprasad@avigen.com.