Abstract
CD4+ helper T (Th) cells play a crucial role in the delicate balance between host defense and autoimmune disease. Two important populations of helper T cells are the proinflammatory, interleukin-17 (IL-17)–producing (Th17) cells and the anti-inflammatory forkhead box P3–positive (FoxP3+) T regulatory (Treg) cells. Here we show that all-trans retinoic acid (ATRA) and other agonists of the retinoic acid receptor alpha (RARα) inhibit the formation of Th17 cells and promote FoxP3 expression. Conversely, inhibition of retinoic acid signaling constrains transforming growth factor beta (TGF-β1) induction of FoxP3. The effect of ATRA is mediated independently of IL-2, signal transducer and activator of transcription 5 (Stat5) and Stat3, representing a novel mechanism for the induction of FoxP3 in CD4 T cells. As previous studies have shown that vitamin A derivatives are protective in animal models of autoimmune disease, the current data suggest a previously unrecognized role for RARα in the regulation of CD4+ T-cell differentiation and provide a mechanism for the anti-inflammatory effects of retinoic acid.
Introduction
Immune responses are orchestrated by subsets of CD4+ helper and effector T cells. Classically, differentiated T cells have been classified as belonging either to T-helper 1 (Th1) or Th2 lineages.1 Th1 cells secrete interferon-gamma (IFN-γ) in response to interleukin-12 (IL-12) and require the transcription factors T-box21 (T-bet) and signal transducer and activator of transcription 4 (Stat4)2 and Stat1, whereas Th2 cells secrete IL-4, IL-5, and IL-13 and require the transcription factors GATA-binding protein 3 (GATA-3) and Stat6.3 While this simple paradigm was instrumental in understanding immune responses to model pathogens, it was less clear how such a model explained the pathogenesis of autoimmune disease.
More recently, additional fates for CD4+ T cells have emerged that provide more insights into the mechanisms of tolerance and immune-mediated disease. One of these new subsets of T cells, expressing the transcription factor FoxP3, is termed regulatory T cells (Tregs) and serves to inhibit immune responses. Deficiency of FoxP3 in mice and humans is associated with fatal autoimmune disease.4,5 FoxP3+ Tregs normally develop in the thymus, but FoxP3 expression and Treg function also develop when naive CD4+ T cells are stimulated in vitro with transforming growth factor-β1 (TGF-β1) and IL-2.6 Development and differentiation of Tregs and expression of FoxP3 are dependent upon another transcription factor, Stat5.7,8
Another new subset of T cells that may be related to Tregs is a subset that produces the proinflammatory cytokine IL-17 (Th17 cells). Overproduction of IL-17 has been noted in a variety of models of autoimmune disease, including experimental autoimmune encephalomyelitis and collagen-induced arthritis.9-13 Importantly, increased levels of IL-17 are also seen in human diseases, such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease.14-16 Th17 cells are generated by the cytokines TGF-β1 and IL-6, the latter of which acts via Stat3 to induce the transcription factor retinoic acid orphan receptor gamma (RORγt).17 Overexpression of RORγt induces IL-17 production, whereas deficiency of this transcription factor virtually abrogates Th17 differentiation.
RORγt belongs to a superfamily of retinoid nuclear receptors that include the retinoic acid receptors (RARs), retinoid X receptors (RXRs), and other RORs.18 A connection between retinoids and CD4+ T-cell differentiation has long been recognized. Memory T cells from vitamin A–deficient mice have been found to overproduce IFN-γ and underproduce interleukin-4 (IL-4).19 In contrast, administration of the active metabolite of vitamin A, retinoic acid (RA), has been reported to suppress memory cell IFN-γ production and increase IL-4 secretion.20 Later, it emerged that vitamin A can be metabolized into retinoic acid isomers with different ligand-receptor affinities: 9-cis retinoic acid, with a higher affinity for the RXR family, and all-trans retinoic acid (ATRA), with a higher affinity for the RAR family.21 Acting through RXRα, 9-cis retinoic acid skews naive T cells toward the Th2 phenotype.22 In turn, ATRA actually prevents naive T cells from differentiating into either the Th1 or Th2 phenotypes.20
Consistent with the inhibitory effects of ATRA observed in vitro, ATRA analogs have shown benefit in the treatment of a variety of autoimmune diseases.23-25 For instance, ATRA has long been documented to inhibit EAE in animal models.24,26 However, a mechanism underlying these findings has not been defined. In light of the recently recognized importance of Th17 and Treg cells in the pathophysiology of EAE, we explored a possible role for ATRA in Th17/Treg polarization.
Methods
Mice
Wild-type C57BL/6 mice were purchased from Taconic (Hudson, NY). C57BL/6 mice bearing loxP-flanked (fl/fl) alleles of Stat5 and Stat3 and expressing Cre under the control of CD4 have been described previously,7,27 as have FoxP3 enhanced green fluorescent protein (eGFP) reporter mice.28 Cre-negative littermates or wild-type C57BL/6 mice were used as controls. Il2−/− mice on a Bl10 Rag2−/− TcR transgenic (5C.C7) background were obtained from Taconic, along with Bl6 Rag2−/− TcR transgenic (OTII) animals. All animals were handled and housed in facilities in accordance with National Institutes of Health Animal Care and Use Committee guidelines.
Cell isolation
Peripheral T cells were obtained from spleens and lymph nodes of 8- to 12-week-old mice by mechanical disruption through a 70-μm cell strainer. Bulk CD4+ T cells were obtained using positive selection with anti-CD4 microbeads using an autoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+CD62L+ cells were obtained by first enriching for CD4+ T cells via negative selection using a Mouse CD4+ T Cell Isolation Kit (Miltenyi Biotec), followed by positive selection using indirect labeling with anti-CD62L allophycocyanin (APC)-conjugated antibodies (eBioscience, San Diego, CA) and anti-APC microbeads (Miltenyi Biotec). CD4+ single-positive thymocytes were obtained by negative selection with anti-CD8 microbeads (Miltenyi Biotec). CD11c+ cells were obtained from mouse splenocytes by positive selection with anti-CD11c microbeads (Miltenyi Biotec). Flow cytometry–sorted naive CD4+ T cells were obtained by surface staining cells with anti-CD4, anti-CD62L, anti-CD44, and anti-CD25 antibodies (eBioscience), followed by flow cytometric cell sorting using a Mo-Flo cell sorter (Dako, Carpinteria, CA). Cell purity was routinely greater than 99.5%.
Cell culture
All cell cultures were performed in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum, 2 mM glutamine, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, 0.25 μg/mL amphotericin B, and 2 μM β-mercaptoethanol. Cells were activated at a concentration of 106 cells/mL media in 96-well cell culture plates (Nalge Nunc, Rochester NY) either with plate-bound anti-CD3 (5 μg/mL) and anti-CD28 (5 μg/mL) as indicated (BD PharMingen, San Diego, CA) or 2.5 × 105 CD11c+ cells/mL pulsed with 1 mM OVA peptide (ISQAVHAAHAEINEAGR; Peptides International, Louisville, KY). Th-neutral conditions (Th0) contained no exogenous cytokines or anticytokines. Th1 cultures were supplemented with 10 ng/mL IL-12 (R&D Systems, Minneapolis, MN) with 10 μg/mL anti–IL-4 (BD PharMingen); Th2 conditions contained 10 ng/mL IL-4 (R&D Systems) with 10 μg/mL anti–IFN-γ (BD PharMingen); and Th17 conditions contained 10 ng/mL IL-6 (Invitrogen, Carpinteria, CA), 5 ng/mL TGF-β1 (R&D Systems), 10 μg/mL anti–IL-4, and 10 μg/mL anti–IFN-γ. Where indicated, IL-2 was added at 100 IU/mL and TGF-β1 alone at 5 ng/mL. ATRA, TTNBP (4-[E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]) benzoic acid, and A7980 were obtained from Sigma (St Louis, MO). LE540 and AM580 were obtained from Wako (Osaka, Japan). All retinoids were dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 0.01 M and stored at −80°C in lightproof containers. Stocks were thrown away after 4 freeze-thaw cycles. Cultures containing retinoids were protected from light throughout the time of culture; unless stated otherwise, ATRA was used at a final concentration of 1 μM.
In vitro suppression assays
Various numbers (2500 to 40 000) of natural (CD4+ CD25+) Tregs or induced (CD4+ FoxP3-GFP+) Tregs were added to 50 000 naive (CD4+ CD25−) T cells and 50 000 irradiated Thy-1− cells in a total volume of 200 μL media with soluble anti-CD3 at 1 μg/mL. After 4 days, 0.2 μCi of 3H-thymidine (Amersham Biosciences, Piscataway, NJ) was added, and incorporation was measured after a further 6 hours of incubation using a scintillation counter (PerkinElmer, Waltham MA).
Measurement of cytokines
Anti–IFN-γ-APC and fluorescein isothiocyanate (FITC), anti–IL-17-PE, and anti–IL-4-PE and APC antibodies for intracellular cytokine staining were obtained from BD Biosciences. Anti–IL-17 Alexa Fluor 647 and anti–FoxP3-FITC and APC antibodies were obtained from eBioscience. In brief, cells were stimulated for 4 to 5 hours with phorbol myristate acetate, ionomycin, and Brefeldin A. Cells were stained using the Cytofix/Cytoperm Kit (BD Biosciences) with the indicated antibodies. Events were analyzed on a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA). Events were collected and analyzed with FlowJo software version 8.2 (Tree Star, Ashland, OR). Cytokine concentrations of IFN-γ, IL-4, and IL-17 in cell culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) using the appropriate Quantikine kits (R&D Systems).
Quantitative real-time PCR analysis
Total RNA was isolated from cell cultures using the RNeasy kit (Qiagen, Hilden, Germany). RNA was quantitated using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA concentrations were equalized for each experiment before making cDNA using random hexamers and reverse transcriptase (Applied Biosystems, Foster City, CA). Quantitative PCR was performed with an ABI 7500 Fast Real-Time PCR System using Taqman site-specific primers and probes (Applied Biosystems). Results were normalized to β-actin levels. For comparisons, Th-neutral culture conditions were assigned an arbitrary value of 1.
Statistics
Unless otherwise stated, statistical analysis was performed using the 2-sample, two-tailed Student t test. P values less than .05 were considered significant.
Results
Retinoids inhibit IL-17 and promote FoxP3 expression
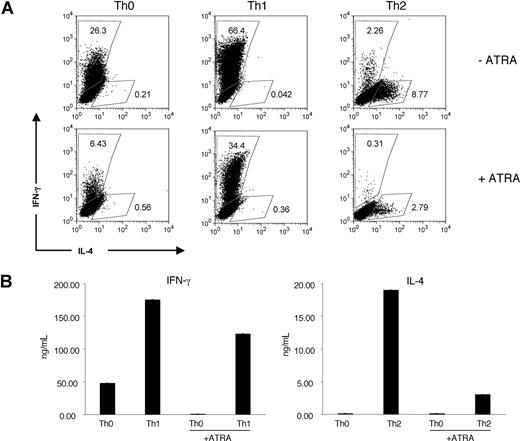
We began our studies by revisiting the effect of ATRA on the polarization of the traditional CD4 T-cell subsets. CD4+CD62L+ T cells from wild-type C57Bl6 mice were stimulated for 2 days in vitro under neutral, Th1-favoring (IL-12, anti–IL-4), or Th2-favoring (IL-4, anti–IFN-γ) conditions with or without 1 μM ATRA and then expanded for 3 days with polarizing cytokines and IL-2, again with or without ATRA (Figure 1A,B). As expected, the different polarizing conditions led to selective secretion of the signature cytokines. Moreover, consistent with previous reports,29,30 ATRA reduced IFN-γ and IL-4 production by both Th1 and Th2 cells, respectively.
ATRA inhibits Th1 and Th2 polarization. Polyclonal CD4+CD62L+ cells isolated by MACS from C57Bl6J mice were polarized for 5 days under neutral, Th1 (IL-12 and anti–IL-4), or Th2 (IL-4 and anti–IFN-γ) favoring culture conditions with or without 1 μM ATRA. (A) Cytokine production at day 5 by intracellular staining. Numbers on plots are percentages of total cells. (B) Cells were washed thoroughly after 5 days in culture, then resuspended at 0.5 × 106 cells/mL and restimulated overnight with plate-bound anti-CD3 and anti-CD28 in media without additional cytokines. Supernatants were collected and measured by ELISA; error bars represent standard deviations (n = 3). These experiments are representative of 3 independent experiments.
ATRA inhibits Th1 and Th2 polarization. Polyclonal CD4+CD62L+ cells isolated by MACS from C57Bl6J mice were polarized for 5 days under neutral, Th1 (IL-12 and anti–IL-4), or Th2 (IL-4 and anti–IFN-γ) favoring culture conditions with or without 1 μM ATRA. (A) Cytokine production at day 5 by intracellular staining. Numbers on plots are percentages of total cells. (B) Cells were washed thoroughly after 5 days in culture, then resuspended at 0.5 × 106 cells/mL and restimulated overnight with plate-bound anti-CD3 and anti-CD28 in media without additional cytokines. Supernatants were collected and measured by ELISA; error bars represent standard deviations (n = 3). These experiments are representative of 3 independent experiments.
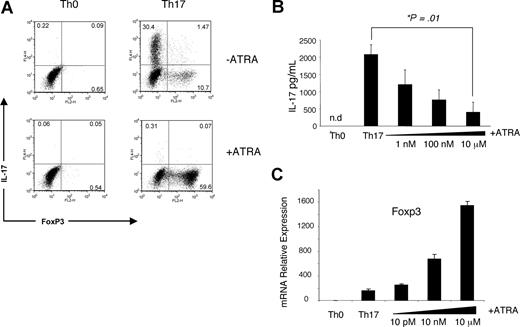
We next turned our investigation to the effect of retinoids on Th17 differentiation. Naive CD4+ T cells from Rag2−/− TCR-transgenic (OTII) mice were stimulated using CD11c+ cells pulsed with cognate peptide for 3 days in vitro under established Th17-favoring conditions (IL-6, TGF-β1, anti–IL-4, and anti–IFN-γ) with or without 1 μM ATRA. On the third day, IL-17 expression was determined by intracellular staining. In the absence of ATRA, the conditions generated substantial proportions of IL-17-producing cells, which were reduced by inclusion of this compound (Figure 2A). We confirmed that this inhibition was dose dependent in polyclonally stimulated naive CD4+ T cells stimulated under Th17 conditions by ELISA (Figure 2B). At least in vitro, the differentiation of Th17 cells is related to another subset, inducible Treg cells, as TGF-β1 appears to contribute to the generation of both.28 It was notable, therefore, that in addition to inhibiting Th17-cell generation, ATRA also increased the percentage of FoxP3+ cells (Figure 2A) and FoxP3 mRNA (Figure 2C) under otherwise Th17-favoring conditions, the latter in a dose-dependent manner. It should be noted that in contrast with Figure 2A, the differentiation of T cells in Figure 2B,C was induced without antigen-presenting cells, indicating that ATRA must be acting directly upon T cells.
ATRA inhibits Th17 polarization and promotes FoxP3 expression. (A) CD4+ T cells isolated by MACS beads from OTII TCR transgenic mice were incubated with isolated CD11c+ cells pulsed with 1 μg OVA peptide and cultured for 3 days under neutral or Th17-favoring (IL-6, TGF-β1, anti–IFN-γ, and anti–IL-4) conditions with or without 1 μM ATRA. The figure depicts intracellular staining for IL-17 production and FoxP3 expression, and it is representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B,C) Polyclonal CD4+CD62L+CD25−CD44− cells isolated by flow cytometry were stimulated for 3 days with anti-CD3 and anti-CD28 under neutral or Th17-favoring conditions with or without ATRA. (B) Average IL-17 concentration in supernatants from 3 independent experiments as measured by ELISA. The P value was determined by a 2-tailed paired t test; a single asterisk denotes significance (P < .05). (C) FoxP3 expression normalized to β-actin levels and relative to Th-neutral conditions without ATRA; error bars represent standard deviation (n = 3); the data are representative of 2 independent experiments.
ATRA inhibits Th17 polarization and promotes FoxP3 expression. (A) CD4+ T cells isolated by MACS beads from OTII TCR transgenic mice were incubated with isolated CD11c+ cells pulsed with 1 μg OVA peptide and cultured for 3 days under neutral or Th17-favoring (IL-6, TGF-β1, anti–IFN-γ, and anti–IL-4) conditions with or without 1 μM ATRA. The figure depicts intracellular staining for IL-17 production and FoxP3 expression, and it is representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B,C) Polyclonal CD4+CD62L+CD25−CD44− cells isolated by flow cytometry were stimulated for 3 days with anti-CD3 and anti-CD28 under neutral or Th17-favoring conditions with or without ATRA. (B) Average IL-17 concentration in supernatants from 3 independent experiments as measured by ELISA. The P value was determined by a 2-tailed paired t test; a single asterisk denotes significance (P < .05). (C) FoxP3 expression normalized to β-actin levels and relative to Th-neutral conditions without ATRA; error bars represent standard deviation (n = 3); the data are representative of 2 independent experiments.
Critical role of RARα in CD4+ T-cell differentiation
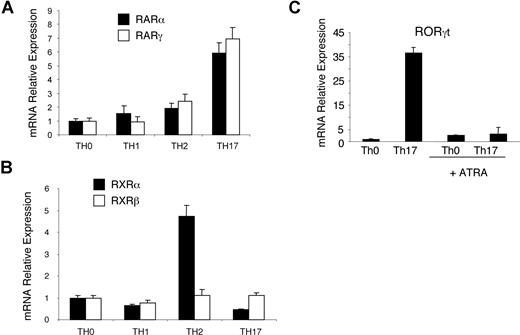
The retinoic acid receptor family is divided into RAR, RXR, and ROR subfamilies, and RAR and RXR family members are known to bind ATRA. We began our investigation by examining which retinoic acid family receptors are expressed by each T-cell subset. Naive CD4+ T cells from wild-type C57Bl6 mice were stimulated in vitro for 3 days with plate-bound anti-CD3 and anti-CD28 antibodies under polarizing conditions for Th-neutral, Th1, Th2, or Th17 cells (Figure 3A,B). Receptor expression was analyzed by quantitative RT-PCR. Consistent with the reports of other groups, we found that Th2 cells preferentially express high levels of RXRα.31 However, we also found that Th17 cells were distinct in expressing high levels of RARα and RARγ. In addition, Th17 cells also express high levels of the orphan receptor RORγt, which was downregulated by ATRA (Figure 3C).
Th17 cells express high levels of RARα, RARγ, and RORγt mRNA. (A,B) CD4+CD62L+ cells isolated by MACS were polyclonally stimulated for 3 days under Th-neutral, Th1, Th2, or Th17 conditions. Relative expression of RAR and RXR receptors was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels under Th-neutral conditions, and error bars represent standard deviation (n = 3). Detected RAR and RXR receptors are shown (RAR-β and RXR-γ were not present). (C) Naive CD4+ T cells were polyclonally stimulated for 3 days under Th-neutral or Th17 conditions in the presence or absence of 1 μM ATRA. Relative expression of RORγt mRNA was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels and relative to Th-neutral conditions; error bars represent standard deviation (n = 3). The inhibition of RORγt mRNA was confirmed in 3 independent experiments.
Th17 cells express high levels of RARα, RARγ, and RORγt mRNA. (A,B) CD4+CD62L+ cells isolated by MACS were polyclonally stimulated for 3 days under Th-neutral, Th1, Th2, or Th17 conditions. Relative expression of RAR and RXR receptors was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels under Th-neutral conditions, and error bars represent standard deviation (n = 3). Detected RAR and RXR receptors are shown (RAR-β and RXR-γ were not present). (C) Naive CD4+ T cells were polyclonally stimulated for 3 days under Th-neutral or Th17 conditions in the presence or absence of 1 μM ATRA. Relative expression of RORγt mRNA was analyzed by quantitative RT-PCR. Expression is normalized to β-actin levels and relative to Th-neutral conditions; error bars represent standard deviation (n = 3). The inhibition of RORγt mRNA was confirmed in 3 independent experiments.
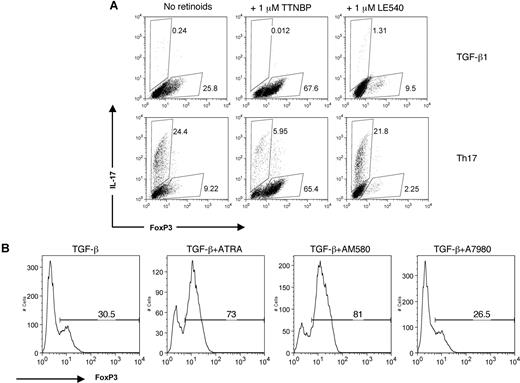
To test the potential functional significance of RAR in Th17 cells, we compared the effects of (4-[E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl] benzoic acid (TTNBP), a pan-RAR agonist, and LE540, a pan-RAR antagonist, on FoxP3 and IL-17 expression (Figure 4A). Like ATRA, TTNBP increased the proportion of FoxP3+ cells, with a reciprocal decrease in the proportion of IL-17–producing cells. In contrast, the RAR antagonist, LE540, had the opposite effect, actually reducing the ability of TGF-β1 to induce FoxP3, without noticeable effects on IL-17 production. Next, we assessed the relative contributions of RARα and RARγ to FoxP3 expression in naive cells by using the synthetic retinoids AM580 and A7980, selective RARα and RARγ agonists, respectively (Figure 3B). Whereas AM580 was as effective as ATRA at increasing the percentage of FoxP3+ cells, A7980 had little demonstrable effect, thus strongly implying a role for RARα as the key player in FoxP3 expression. These data are also notable in that they imply a direct role for retinoids in controlling FoxP3, as the studies were performed using isolated T cells.
RARα is necessary for TGF-β1 induction of FoxP3. (A) Naive CD4+ cells were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone for 3 days with TTNBP (pan-RAR agonist) or LE540 (pan-RAR antagonist). IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Numbers on plots are percentages of total cells. (B) Naive CD4+ T cells were polyclonally stimulated for 3 days with TGF-β1 and 1 μM ATRA, 1 μM A7980 (selective RARγ agonist), or 1 μM AM580 (selective RARα agonist). FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on graphs are percentages of cells that are FoxP3.+
RARα is necessary for TGF-β1 induction of FoxP3. (A) Naive CD4+ cells were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone for 3 days with TTNBP (pan-RAR agonist) or LE540 (pan-RAR antagonist). IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Numbers on plots are percentages of total cells. (B) Naive CD4+ T cells were polyclonally stimulated for 3 days with TGF-β1 and 1 μM ATRA, 1 μM A7980 (selective RARγ agonist), or 1 μM AM580 (selective RARα agonist). FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on graphs are percentages of cells that are FoxP3.+
The effect of retinoids is independent of Stat3, Stat5, and IL-2
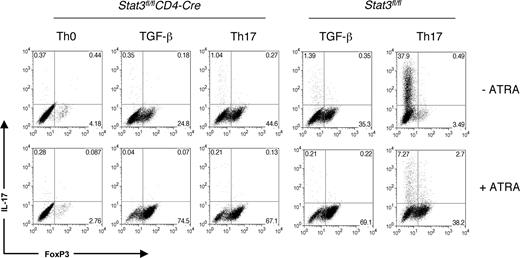
Several lines of evidence point to key roles for Stat5 and Stat3 as essential regulators of FoxP3 and IL-17.27,32-34 Specifically, in the presence of TGF-β1, IL-2 acting through Stat5 is known to increase FoxP3 and inhibit IL-17. In contrast, IL-6 acting through Stat3 increases IL-17 and inhibits FoxP3. The ability of ATRA to both reduce IL-17 and enhance FoxP3 expression in polyclonally stimulated T cells in the presence of IL-6 and TGF-β1 was reminiscent of the effects of altering Stat3 and Stat5 signaling. We therefore examined the relationship between the effect of ATRA and the requirement for these transcription factors. Using T cells from Stat3fl/flCD4-Cre mice, we first investigated whether ATRA might be inhibiting Th17 cells and promoting FoxP3 indirectly by blocking IL-6 signaling through Stat3 (Figure 5). In the absence of Stat3, the combination of TGF-β1 and IL-6 failed to induce IL-17–producing T cells, as noted previously. In this setting, these cytokines produced a higher proportion of FoxP3+ T cells. Nonetheless, the presence of ATRA was able to further increase the expression of FoxP3 in the absence of Stat3, suggesting that ATRA enhances FoxP3 in a Stat3-independent manner.
ATRA enhances FoxP3 expression independent of Stat3. Peripheral CD4+ cells from Stat3fl/flCD4-Cre mice or WT controls were polyclonally stimulated for 3 days under Th-neutral conditions, Th17 conditions, or with TGF-β1 alone in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
ATRA enhances FoxP3 expression independent of Stat3. Peripheral CD4+ cells from Stat3fl/flCD4-Cre mice or WT controls were polyclonally stimulated for 3 days under Th-neutral conditions, Th17 conditions, or with TGF-β1 alone in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression was measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
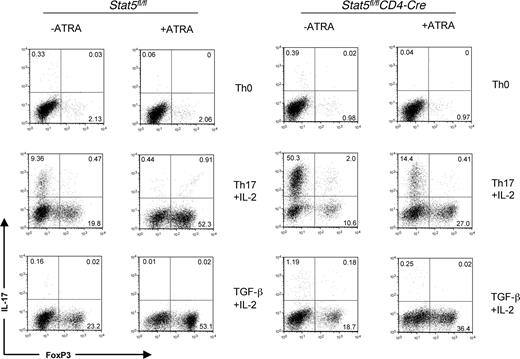
Next, using thymocytes as a source of naive cells from tissue-specific Stat5fl/flCD4-Cre knockouts, we evaluated the idea that ATRA might affect Stat5 signaling. As we have shown previously, the addition of IL-2 inhibited IL-17 in a Stat5-dependent manner. However, in the absence of Stat5, ATRA still was able to inhibit IL-17 and combine with TGF-β1 to enhance expression of FoxP3, even in the absence of Stat5 (Figure 6). Thus, we conclude from these data that the negative regulatory effect of ATRA on IL-17 expression and its positive effect of FoxP3 are independent of the Stats typically involved in controlling expression of these key target genes.
ATRA enhances FoxP3 expression independent of Stat5. CD8-depleted thymocytes from Stat5fl/flCD4-Cre mice were polyclonally stimulated for 3 days with media alone, IL-6, IL-2, and TGF-β1, or IL-2 and TGFβ-1 in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression were measured on CD4+ gated fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
ATRA enhances FoxP3 expression independent of Stat5. CD8-depleted thymocytes from Stat5fl/flCD4-Cre mice were polyclonally stimulated for 3 days with media alone, IL-6, IL-2, and TGF-β1, or IL-2 and TGFβ-1 in the presence or absence of 1 μM ATRA. IL-17 and FoxP3 expression were measured on CD4+ gated fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells.
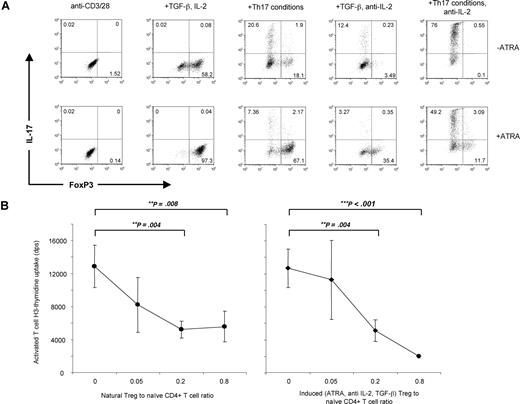
Previous work has shown that the presence of IL-2 is critical to induce FoxP3 expression in naive T cells.7 The ability of ATRA to induce FoxP3 expression in Stat5-deficient CD4+ T cells led us to investigate whether ATRA could replace the action of IL-2 as a cofactor for FoxP3 induction by TGF-β1. Using Rag2−/− TCR-transgenic (OTII) naive T cells, we examined the effect of the addition of ATRA with or without addition of exogenous IL-2 or its blocking antibody. As shown in Figure 7A, TGF-β1 in the presence of anti–IL-2 was a modest inducer of FoxP3 expression, whereas the addition of human IL-2 greatly increased the effect of TGF-β1. In contrast, the combination of ATRA and TGF-β1 in the presence of anti–IL-2 generated substantial proportions of FoxP3-positive cells (35%). Addition of exogenous IL-2 increased the proportions even further (>90%). In the presence of TGF-β1 and IL-6 and absence of IL-2, substantially greater numbers of IL-17–producing cells are generated, as previously reported.27,35 The addition of ATRA inhibited IL-17 expression in the absence of IL-2 while enhancing FoxP3 expression (Figure 7A, lower right panel). We confirmed these results using IL-2–deficient Rag2−/− TCR-transgenic (5C.C7) naive T cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We next determined whether ATRA-induced FoxP3 cells in the presence of anti–IL-2 were as suppressive as natural (CD4+CD25+) Tregs (Figure 7B). Using a transgenic FoxP3-GFP mouse, GFP-positive cells induced by 3 days of polyclonal stimulation in the presence of TGF-β1, ATRA, and anti–IL-2 were isolated by flow cytometry and show a similar ability to inhibit polyclonally stimulated CD4+ CD25+ cells in comparison to natural Tregs.
ATRA enhances FoxP3 expression independent of IL-2. (A) Peripheral CD4 cells from Rag2−/− transgenic (OTII) mice were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone, in the presence of either IL-2 or anti–IL-2. All stimulations were performed with (lower panels) or without (upper panels) 1μM ATRA. IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B) CD4+CD25− MACS-purified FoxP3-GFP cells were polyclonally stimulated for 3 days in the presence of TGF-β1, ATRA, and anti–IL-2. FoxP3-positive cells were isolated by flow cytometry and used to inhibit CD4+CD25− cells sorted by flow cytometry and stimulated with soluble anti-CD3 in the presence of irradiated Thy1.1− cells. Proliferation was measured by 3H-thymidine uptake and was compared with CD4+CD25+ (natural Treg) cells purified by flow cytometry. Error bars denote the standard error of the mean (n = 3), and significance was determined by an unpaired t test, and data are representative of 2 independent experiments.
ATRA enhances FoxP3 expression independent of IL-2. (A) Peripheral CD4 cells from Rag2−/− transgenic (OTII) mice were polyclonally stimulated for 3 days under Th17 conditions or TGF-β1 alone, in the presence of either IL-2 or anti–IL-2. All stimulations were performed with (lower panels) or without (upper panels) 1μM ATRA. IL-17 and FoxP3 expression were measured on fixed cells by intracellular staining. Data are representative of 2 independent experiments. Numbers on plots are percentages of total cells. (B) CD4+CD25− MACS-purified FoxP3-GFP cells were polyclonally stimulated for 3 days in the presence of TGF-β1, ATRA, and anti–IL-2. FoxP3-positive cells were isolated by flow cytometry and used to inhibit CD4+CD25− cells sorted by flow cytometry and stimulated with soluble anti-CD3 in the presence of irradiated Thy1.1− cells. Proliferation was measured by 3H-thymidine uptake and was compared with CD4+CD25+ (natural Treg) cells purified by flow cytometry. Error bars denote the standard error of the mean (n = 3), and significance was determined by an unpaired t test, and data are representative of 2 independent experiments.
Discussion
In this study, we examined the role of the vitamin A metabolite ATRA in Th17 generation and FoxP3 induction. We found that as previously reported, ATRA negatively regulates expression of the effector cytokines IFN-γ and IL-4. In addition, we found that ATRA also directly inhibits T-cell production of the key inflammatory cytokine IL-17. However, given the putative relatedness of Th17 and Treg cells, it was even more striking that, concomitant with reduced IL-17 expression, we noted induction of FoxP3 expression by ATRA. Furthermore, the finding that inhibition of RAR function in isolated T cells inhibited Foxp3 expression and enhanced IL-17 production argues for a role of endogenous retinoids in regulating the balance between Treg and Th17 differentiation. Because of the increasingly prominent roles of these subsets in many autoimmune and autoinflammatory disorders, there is great interest in the relationship between Th17 and Treg cells, both of which can be induced in vitro in the presence of TGF-β1. The present data point to a role of retinoids acting via RARα as the likely candidate for mediating this effect.
Our findings argue that in addition to the well-known regulators of Th17/Treg differentiation, retinoids appear to be another physiologic regulator of CD4+ T-cell lineage commitment. The strongest data for this argument are the dramatic effects of inhibiting RARα. In addition, these data provide potential insights into the regulation of CD4+ T-cell differentiation in the gut. Mucosal dendritic cells (DCs) are well known to be immunosuppressive.36 These subsets also influence the tropism of T cells by influencing expression of homing receptors.37 Recently, dendritic cells in the gut-associated lymphatic tissue have been shown to synthesize ATRA from dietary beta-carotene.38 The ability of mucosal DCs to produce retinoids may explain why the relative distribution of Th17 cells in the gut is limited, although IL-6 expression is widespread.17,39 It is possible that DCs use retinoic acid as a T-cell modulator, increasing secretion to suppress inflammation and decreasing production when an inflammatory response is warranted. Thus, retinoid receptors and local production of retinoids may represent novel physiologic regulators of the Th17/Treg axis.
The mechanisms by which retinoids regulate IL-17 and FoxP3 expression are not yet clear. Based on expression and the effect of a selective antagonist, our data would argue that the major mediator of the effect of ATRA is RARα. However, deficiency of RARα results in early postnatal lethality40 ; therefore, establishing the precise role of RARα will await tissue-specific deletion strategies. It is notable that ATRA treatment is also associated with downregulation of RORγt, the RAR-related transcription factor that drives Th17 differentiation. It will be important to determine the mechanisms by which RARα influences RORγt expression in CD4+ T-cell differentiation. Although Stat3 and Stat5 are key regulators of Th17/Treg differentiation,6,7,27,32-34,41 our data indicate that ATRA appears to exert its effect independently of these factors. As a transcription factor itself, RARα might directly bind the promoters of the Foxp3, Il17, and/or RORγ genes; in the latter case, the expectation would be that it serves as a repressor. Nonetheless, our data suggest a model in which TGF-β1, Stat5, and RARα each provide unique and additive signals to increase FoxP3 expression. Conversely, Stat5 and RARα both contribute to IL-17 suppression, antagonizing the positive effects of TGF-β1 and Stat3.
Beyond potential roles as physiologic regulators of CD4+ T-cell differentiation, retinoids and retinoid receptors are attractive pharmacologic agents. The ability to affect Th17/Treg polarization has attracted great clinical interest.42-44 Altering this ratio in favor of increased numbers of Treg cells would be useful for a wide range of autoimmune and autoinflammatory diseases. Conversely, suppressing Treg formation has potential benefits for developing tumor immunotherapies. In fact, ATRA analogs have shown efficacy in in vivo models of autoimmune disease.23,25,26 This suggests that retinoid-based pharmaceutical strategies may offer a promising alternative to our current approach of managing immunologic diseases. It will be important to elucidate precisely how retinoids regulate IL-17 and FoxP3 to optimize therapeutic regimens and to generate agents with increased selectivity in these actions.
In summary, ATRA and other ligands for RARα inhibit Th17 polarization and promote FoxP3 expression. This occurs independently of IL-2, Stat5, and Stat3, which have been implicated previously in Th17 and Treg homeostasis. Not only may this be an important physiologic regulator of T-cell lineage commitment but, in addition, ATRA analogs are likely to be useful as pharmacologic agents. Understanding the precise role for retinoic acid in normal Treg development and function remains an interesting subject for future investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
K.M.E. is a Howard Hughes Medical Institute–National Institutes of Health Research Scholar.
Authorship
Contribution: K.M.E. and A.L. designed and performed experiments. T.D., G.S., and Y.K. designed experiments and helped analyze data. E.S. and J.J.O. supervised planning of experiments and assisted in data interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arian Laurence, Building 10, Room 9N262, 10 Center Drive, MSC-1820, Bethesda, MD 20892-1820; e-mail: laurencea@mail.nih.gov.
References
Author notes
K.M.E. and A.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal