Abstract
The immunoregulatory cytokine interleukin6 (IL6) acts in a pro- and anti-inflammatory fashion. Synthesized by myeloid cells, fibroblasts and endothelial cells, IL6 on target cells, binds to the IL6 receptor (IL6R) and signals via complex formation with the ubiquitously expressed gp130 receptor. Paradoxically, most cells that respond to IL6 during inflammatory states do not express the IL6R and are themselves not directly responsive to the cytokine. A naturally occurring soluble form of the IL6R renders all cells responsive to IL6. This alternative signaling process is called IL6 transsignaling. Here we developed a transgenic strategy based on the overexpression of the soluble form of gp130, which specifically blocks all IL6 responses mediated by the soluble IL6R but does not affect IL6 responses via the membrane bound IL6R. In these mice, inflammatory processes are blocked as in IL6−/− mice, strongly arguing for a major role of the soluble IL6R during inflammation in vivo.
Introduction
IL6 has major functions in inflammatory reactions of the body.1,2 Mice with a targeted inactivation of the IL6 gene are completely protected in animal models of rheumatoid arthritis3,4 and multiple sclerosis.5 Furthermore, regenerative reactions such as wound healing and liver regeneration are severely compromised in IL6−/− mice.6
A soluble form of the IL6R can bind IL6 with the same affinity as the membrane-bound form and the complex of IL6 and the soluble IL6R (sIL6R) can induce signaling in a process called IL6 transsignaling.7,8 Because the IL6R is only sparely expressed, IL6 transsignaling dramatically increases the number of potential IL6 target cells.9 This is relevant for inflammatory processes in vivo, because endothelial cells and smooth muscle cells, which play key roles in inflammation, lack IL6R expression. The interest in the role of IL6 in inflammatory processes has recently been intensified by the finding that the differentiation of TH17 cells, which is a prerequisite for inflammatory damage during autoimmune disease, shows a dependence on IL6 in combination with TGFβ.10
Recent studies with animal models of inflammatory bowel disease,11,12 peritonitis,13 rheumatoid arthritis,14,15 and inflammatory colon cancer16 suggest that IL6 transsignaling serves as the major proinflammatory paradigm of IL6 signaling under pathophysiologic conditions.
To further analyze the relevance of IL6-transsignaling in vivo we generated a mouse model in which IL6-transsignaling is specifically abrogated. There have been reports describing the generation of knock-in mice with noncleavable L-selectin and noncleavable TNF-α, showing that shedding of membrane proteins was important for antigen-stimulated lymphocyte migration and for secondary lymphoid organ architecture.17,18 Such a strategy is impractical in the case of the sIL6R because its generation is complex and can be generated by ectodomain shedding as well as by translation from an alternatively spliced mRNA. Furthermore, shedding of the IL6R was also shown to occur at multiple cleavage sites and performed by multiple and distinct sheddase activities.19-22
We therefore decided to generate transgenic mice overexpressing the sgp130Fc-protein that had been demonstrated to completely block IL6 transsignaling without affecting activities via the membrane bound IL6R. Blocking of IL6 transsignaling via sgp130Fc is independent of the mode of sIL6R generation. The need for a large molar excess of the sgp130Fc-protein necessitated the development of a codon optimization strategy that should be relevant for the construction of all animal models, which are based on the overexpression of heterologous proteins.
The data presented here clearly show that sgp130Fc transgenic mice display an IL6-transsignaling knockout-like phenotype and establish them as the only possible in vivo model of this IL6-transsignaling paradigm. Here, we demonstrate in the air-pouch model of inflammatory activation that the transition from the neutrophil-dominated phase to the mononuclear cell–dominated phase relies on IL6 transsignaling rather than classic IL6 signaling, highlighting the important role of the sIL6R-mediated processes in innate immunity.
Methods
Cells and proteins
HepG2 cells were obtained from the American Type Culture Collection (Manassas, VA). All cells were grown in DMEM high glucose culture medium (PAA Laboratories, Pasching, Austria) supplemented with 10% fetal calf serum, penicillin (60 mg/L), and streptomycin (100 mg/L) at 37°C with 5% CO2 in a humidified atmosphere. Hyper-IL6 is a fusion protein of IL6 and sIL6R connected by a flexible polypeptide linker.23 Soluble sgp130Fc is a fusion protein of the extracellular portion of gp130 and the Fc-portion of IgG1.24 Both recombinant proteins were expressed and purified as previously described. The anti-mIL6R D7715A7 monoclonal antibody (mAB) was from BIOZOL (Eching, Germany). All restriction enzymes were obtained from MBI Fermentas (St Leon-Rot, Germany).
Transfection
HepG2 cells were transfected using DEAE Dextran as previously described.25 The transfection efficiency as visualized after 24 hours by GFP expression (Axiovert 200 Microscope, Zeiss) was approximately 80%.
Cloning
We used the plasmid pTZ-PEPCK-βglob.intron to subclone the cDNA coding for the human fusion gene sgp130Fc into the XbaI site (pTZ-PEPCK-sgp130Fc-βglob.intron) and into the XhoI site (pTZ-PEPCK-βglob.intron-sgp130Fc). The plasmid pCR-Script-opt_sgp130Fc containing the optimized cDNA coding for the human fusion gene sgp130Fc (opt_sgp130Fc) was synthesized as described by GeneArt (Regensburg, Germany). We used the plasmid pTZ-PEPCK-βglob.intron to subclone the cDNA coding for opt_sgp130Fc into the XbaI site (pTZ-PEPCK-opt_sgp130Fc-βglob.intron) and into the XhoI site (pTZ-PEPCK-βglob.intron-opt_sgp130Fc).
Generation of sgp130Fc and opt_sgp130Fc transgenic mice
The BamHI-BamHI fragment of pTZ-PEPCK-sgp130Fc-βglob.intron containing the entire construct (Figure S4, available on the Blood website; see the Supplemental Materials link at the top of the online article) was gel purified and microinjected into the pronuclei of fertilized eggs of B6D2 mice. Mice were screened for the presence of sgp130Fc by Southern blot of NcoI-digested tail DNA. The EarI-EarI fragment of pTZ-PEPCK-βglob.intron-opt_sgp130Fc containing the entire construct (Figure 1B) was gel purified and microinjected into the pronuclei of fertilized eggs of B6D2 mice. Mice were screened for the presence of opt_sgp130Fc by Southern blot of XhoI-digested tail DNA.
Generation of transgenic mice with the optimized opt_sgp130Fc cDNA. (A) cDNA-optimization and intron localization positively influence sgp130Fc protein accumulation in transiently transfected HepG2-cells. The constructs differ in (1) position of the intron (upstream/downstream of the transgene) and (2) optimization status (wild-type [WT]/optimized cDNA). 1. pTZ-PEPCK-sgp130Fc-βglob.intron, 2. pTZ-PEPCK-βglob.intron-sgp130Fc, 3. pTZ-PEPCK-opt_sgp130Fc-βglob.intron, 4. pTZ-PEPCK-βglob.intron-opt_sgp130Fc. sgp130Fc was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. (B) opt_sgp130Fc construct injected into fertilized mouse eggs (pTZ-PEPCK-βglob.intron-opt_sgp130Fc). (C) sgp130Fc from serum of founder animals was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. * indicates an unspecific band. A vertical line has been inserted to indicate where a gel lane was cut. This gel came from one experiment; irrelevant lanes have been cut out. (D) Expression of sgp130Fc protein in opt_sgp130Fc transgenic mice. Serum was prepared from 8- to 10-week-old WT mice, heterozygous (+/−) and homozygous (+/+) transgenic mice of the lines opt2 and opt3 and sgp130Fc protein levels were determined by ELISA. Data represent mean values plus or minus SD (n) mice per group.
Generation of transgenic mice with the optimized opt_sgp130Fc cDNA. (A) cDNA-optimization and intron localization positively influence sgp130Fc protein accumulation in transiently transfected HepG2-cells. The constructs differ in (1) position of the intron (upstream/downstream of the transgene) and (2) optimization status (wild-type [WT]/optimized cDNA). 1. pTZ-PEPCK-sgp130Fc-βglob.intron, 2. pTZ-PEPCK-βglob.intron-sgp130Fc, 3. pTZ-PEPCK-opt_sgp130Fc-βglob.intron, 4. pTZ-PEPCK-βglob.intron-opt_sgp130Fc. sgp130Fc was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. (B) opt_sgp130Fc construct injected into fertilized mouse eggs (pTZ-PEPCK-βglob.intron-opt_sgp130Fc). (C) sgp130Fc from serum of founder animals was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. * indicates an unspecific band. A vertical line has been inserted to indicate where a gel lane was cut. This gel came from one experiment; irrelevant lanes have been cut out. (D) Expression of sgp130Fc protein in opt_sgp130Fc transgenic mice. Serum was prepared from 8- to 10-week-old WT mice, heterozygous (+/−) and homozygous (+/+) transgenic mice of the lines opt2 and opt3 and sgp130Fc protein levels were determined by ELISA. Data represent mean values plus or minus SD (n) mice per group.
Animal treatment
All experiments were carried out in accordance with the animal care guidelines of the University of Kiel (acceptance no.: V 742-72 241.121-3 (20-2/04) and (76-7/00)). Mice were maintained in a 12-hour light-dark cycle under standard conditions and were provided with food and water ad libitum. Procedures involving animals and their care were conducted in conformance with national and international laws and policies. Blood was drawn from the mice by tail bleeding or by cardiac puncture under general anesthesia.
RNA expression
Mice were killed by cervical dislocation and RNA was isolated from different organs and subjected to Northern blot analysis.26 Five micrograms of heat-denatured RNA per sample was fractionated on a 1% agarose gel with 7% formaldehyde. The separated RNA was transferred to Hybond-N + (Amersham Biosciences, Freiburg, Germany) according to the manufacturer's instructions and hybridized with 32P-cDNA fragments labeled by random priming. The following probes were used: a PCR-fragment of murine SAA2 cDNA and an XhoI restriction fragment of sgp130_opt cDNA.
Enzyme-linked immunosorbent assays
Enzyme-linked immunosorbent assays (ELISAs) for sgp130 (DuoSet human gp130 ELISA Kit), IL6 (DuoSet mouse IL6 ELISA Kit), KC (DuoSet mouse KC ELISA Kit) (all from R&D Systems, Wiesbaden, Germany) and MCP1 (Ready-SET-Go! mouse MCP1 ELISA Kit, eBiosciences, San Diego, CA) were performed according to the manufacturers' instructions. Microtitre plates (Greiner Microlon, Solingen, Germany) were coated with antimurine IL6R pAB AF1830 (R&D Systems, 0.8 μg/mL) in phosphate-buffered saline (PBS). After blocking, 100-μL aliquots of cell lysates or culture supernatants were added. IL6R bound to the plate was detected by biotinylated goat anti–mouse IL6R pAb BAF1830 (R&D Systems; working concentration 0.2 μg/mL) followed by streptavidin-horseradish peroxidase (R&D Systems). The enzymatic reaction was performed with soluble peroxidase substrate (BM blue POD; Roche, Mannheim, Germany) at 37°C and the absorbance was read at 450 nm on an SLT Rainbow plate reader (Tecan, Maennedorf, Switzerland).
Immunoprecipitation
Forty microliters of murine serum from transgenic and wild-type (WT) animals were incubated with or without 0.5 μg Hyper-IL6 and 900 μL PBS/0.01% Tween overnight at 4°C. Immune complexes were precipitated with 30 μL protein A-Sepharose (50% slurry, CL-4B, Amersham Biosciences) for 2 hours at 4°C and separated by 10% SDS-PAGE, and subjected to Western blot analysis using a mAB against hsgp130 (BP-4, Milenia Biotec, Bad Nauheim, Germany) or hslL6R (clone 14-18).27
Air-pouch model
The air-pouch model of local inflammation was performed with transgenic and WT mice according to Edwards et al.28 In brief, mice were anesthetized with ether and subcutaneous dorsal pouches were created by injection of 6 mL of sterile air. After 3 days, the pouches were reinjected with 4 mL of air. On day 6, 1 mL of 1% carrageenan (Sigma-Aldrich, Deisenhofen, Germany) in sterile PBS was injected into the pouches. The corresponding WT mice received sterile PBS. At different time points after treatment, the animals were killed and the pouches were washed with 3 mL of PBS. The lavage fluid was immediately cooled on ice and centrifuged at 5000 rpm for 10 minutes at 4°C. The cells were analyzed by FACS and the supernatant was analyzed by ELISA. The neutralizing IL6R mAb (100 μg/mouse) and sgp130Fc (100 μg/mouse) was administered intraperitoneally 6 hours before carrageenan injection. Neutrophil depletion was induced with a rat anti–mouse mAb against the neutrophil maturation antigen, GR1 (Ly6G mAb; BD Biosciences-Pharmingen, San Diego, CA). The Ly6G mAb (100 μg) was administered intraperitoneally 18 hours before carrageenan injection.
Flow cytometric analysis
Aliquots of the air pouch lavage fluid containing 2 × 105 cells were used for FACS analysis, whereby the mABs Ly6GC (BD Biosciences, Heidelberg, Germany), F4/80 (Caltag-Invitrogen, Karlsruhe, Germany), CD3, and B220 (both from BD Biosciences) were used to count infiltrating neutrophils, mononuclear cells (macrophages), T cells, and B cells. In brief, cells were washed once with 500 μL FACS buffer (1× PBS, 1% BSA, 1 g/L N3Na). To block Fc-receptors on neutrophils and mononuclear cells, the cell suspension was incubated with Mouse BD Fc Block CD 16/32 mAb (BD Biosciences). The cells were subsequently treated with the fluorescence-coupled mAbs against Ly6GC or F4/80 for 1 hour to specifically detect neutrophils and mononuclear cells, respectively. B cells and T cells were stained with fluorescence-coupled mAbs against B220 (CD45R) and CD3, respectively. Cells were washed once with 500 μL FACS antibody buffer and resuspended in 1 mL FACS buffer and analyzed by FACS (FACS-Canto; Becton Dickinson, Heidelberg, Germany). In general, data were acquired from 10 000 gated events per sample.
Results
Optimizing the sgp130Fc-cDNA to achieve high-level protein accumulation in transgenic mice
Codon scanning of the sgp130Fc open reading frame highlighted a significant number of codons that were classified as being rarely used for translation. We therefore chose to codon optimize the sgp130Fc cDNA to ensure maximal expression of the transgene. Collectively, 22% of the 2511 nucleotides of the sgp130Fc cDNA were modified, and attention was given to avoid a high or low GC content. In addition, all internal inhibitory motifs, AT-rich instability motifs, repeat sequences, splice donor and acceptor sites, and poly(A) sites were eliminated (Figures S1Figure S2. cDNA analysis of sgp130Fc and opt_sgp130Fc (PDF, 3.88 MB)–3). A sequence alignment of the cDNA coding for sgp130Fc and the codon-optimized variant (opt_sgp130Fc) is depicted in Figure S3. We also considered the position of the β-globin intron present in the transgenic construct under the control of the PEPCK-promotor.29 As shown in Figure 1A, the codon-optimized and nonoptimized sgp130Fc cDNA was cloned either upstream or downstream of the β-globin intron. Individual constructs were subsequently transfected into HepG2 cells and sgp130Fc expression gauged by Western blot analysis of sgp130Fc precipitated from the culture supernatants. This was confirmed by ELISA analysis of the culture supernatants (data not shown). These experiments highlighted that optimal sgp130Fc expression necessitated insertion of the codon-optimized cDNA downstream of the β-globin intron. This modified construct was subsequently used to generate opt_sgp130Fc transgenic mice (Figure 1B). Fourteen transgenic mice were obtained, which were screened for sgp130Fc expression. Four opt_sgp130Fc founder animals expressed high μg/mL serum levels of the sgp130Fc protein (Figure 1C). Two founder animals were subsequently bred to homozygosity and shown to express more than 50-fold higher sgp130Fc levels than those detected in the nonoptimized sgp130Fc transgenic mice (Figure 1D; compare with Figure S4C). We obtained several sgp130Fc- and opt_sgp130Fc expressing founder animals. The sgp130Fc protein serum levels for all WT sgp130Fc transgenics were in the nanogram range, whereas for all opt_sgp130Fc transgenics they were in the microgram range. This observation makes it unlikely that the different expression levels between optimized and nonoptimized sgp130Fc are the consequence of chromosomal integration site events, rather than being a direct cause of the expression optimization applied for opt_sgp130Fc.
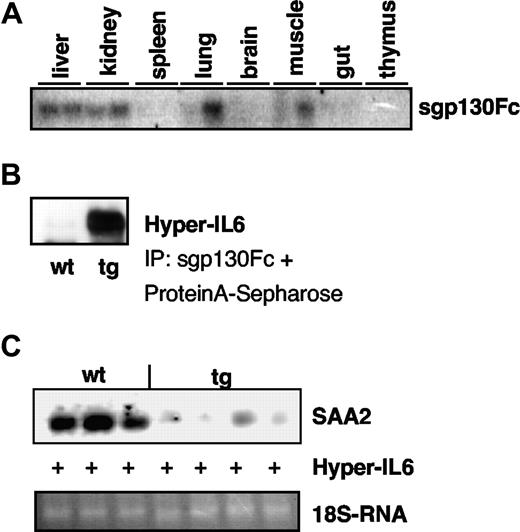
Two animals were analyzed for opt_sgp130Fc-mRNA-expression. The opt_sgp130Fc-mRNA was expressed in liver, kidney, and to some extent in lung and muscle, whereas little or no mRNA expression could be detected in spleen, brain, gut, and thymus (Figure 2A). The sgp130Fc was biologically active because it bound to and precipitated Hyper-IL6 protein, a fusion protein of IL6 and sIL6R connected by a flexible linker (Figure 2B).
Characterization of opt_sgp130Fc transgenic mice. (A) Northern blot analysis showing opt_sgp130Fc mRNA expression in different organs of 2 homozygous opt_sgp130Fc transgenic mice. Mice were killed and total RNA from the indicated organs and tissues was prepared. Eight micrograms total RNA were separated and then hybridized with a 32P-labeled XhoI restriction fragment comprising the opt_sgp130Fc cDNA. (B) sgp130Fc from opt_sgp130Fc transgenic mice could bind to Hyper-IL6. Hyper-IL6 coprecipitates with sgp130Fc that is present in the serum of opt_sgp130Fc transgenic mice. Hyper-IL6 was detected by Western blotting against sIL6R. One homozygous animal of the line opt3 and one WT animal are depicted. (C) Induction of acute-phase response by Hyper-IL6 in opt_sgp130Fc transgenic and WT mice. Northern blot analysis showing the liver expression of serum amyloid A (SAA2) 4 hours after intraperitoneal injection of 500 ng Hyper-IL6 per mice. Five micrograms total liver RNA were seperated and probed with a 32P-labeled PCR fragment of the mouse SAA2 gene.
Characterization of opt_sgp130Fc transgenic mice. (A) Northern blot analysis showing opt_sgp130Fc mRNA expression in different organs of 2 homozygous opt_sgp130Fc transgenic mice. Mice were killed and total RNA from the indicated organs and tissues was prepared. Eight micrograms total RNA were separated and then hybridized with a 32P-labeled XhoI restriction fragment comprising the opt_sgp130Fc cDNA. (B) sgp130Fc from opt_sgp130Fc transgenic mice could bind to Hyper-IL6. Hyper-IL6 coprecipitates with sgp130Fc that is present in the serum of opt_sgp130Fc transgenic mice. Hyper-IL6 was detected by Western blotting against sIL6R. One homozygous animal of the line opt3 and one WT animal are depicted. (C) Induction of acute-phase response by Hyper-IL6 in opt_sgp130Fc transgenic and WT mice. Northern blot analysis showing the liver expression of serum amyloid A (SAA2) 4 hours after intraperitoneal injection of 500 ng Hyper-IL6 per mice. Five micrograms total liver RNA were seperated and probed with a 32P-labeled PCR fragment of the mouse SAA2 gene.
Nontransgenic WT mice and homozygous sgp130Fc transgenic mice were injected with 500 ng Hyper-IL6, a fusion protein of IL6 and sIL6R, which effectively mimics IL6 transsignaling but not classic signaling.23 When the induction of the acute phase gene SAA2 was analyzed, WT mice showed a massive induction of SAA2 mRNA, whereas only a marginal induction was seen in opt_sgp130Fc mice (Figure 2C). No significant inhibition of Hyper-IL6–induced SAA2 mRNA-expression could be seen in the nonoptimized sgp130Fc transgenic mice (Figure S4D). These results confirmed that selective optimization of the sgp130Fc transgene and its expression construct sufficiently elevated circulating sgp130Fc levels to enable efficient abrogation of IL6 transsignaling in vivo. Sgp130Fc transgenic mice were fertile, developed normally, and showed no overt phenomenologic abnormalities. Analysis of blood cells revealed no significant changes in the number of neutrophils, B cells, or T cells (data not shown).
IL6 transsignaling governs inflammation in the air-pouch model
Subcutaneous injection of sterile air under the skin of mice results in the formation of an air pouch with a lining tissue morphologically and functionally similar to the synovium.30 Administration of the irritant carrageenan into the cavity induces a local inflammatory response that can be easily accessed to monitor changes in leukocyte recruitment and the cytokine network.31-33
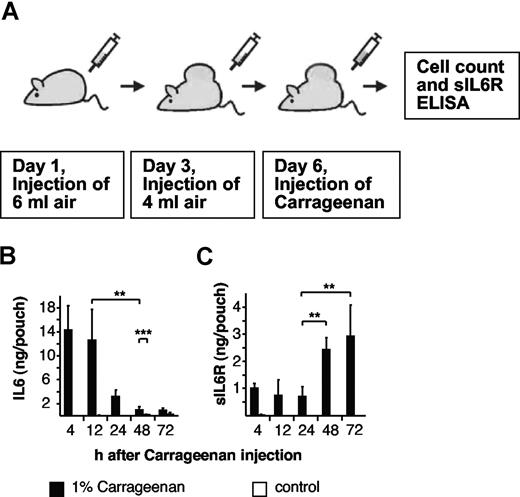
One percent carrageenan solution or PBS was administered into 6-day-old air pouches of 8- to 10-week-old male WT mice to induce an inflammatory response (Figure 3A). As shown in Figure 3B, IL6 levels rapidly peaked at 4 hours after the administration of carrageenan and thereafter declined within 48 hours and 72 hours. Conversely, increases in sIL6R levels occurred later in the ensuing immune response and were elevated 48 to 72 hours after induction of inflammation (Figure 3C), indicating that the accumulation of these proteins is governed by different mechanisms or that the cellular source of these mediators are derived from distinct cell types.
Cytokine levels during the course of the air-pouch model of acute inflammation in mice. (A) Scheme of the air-pouch model of local inflammation as outlined in “Air-pouch model.” (B) Levels of endogenous IL6 in inflamed and noninflamed air pouches at different time points were measured by enzyme-linked immunosorbent assay (ELISA). (C) Levels of endogenous sIL6R in inflamed and noninflamed air pouches at different time points were measured by ELISA. In noninflamed air pouches, IL6 and sIL6R were hardly detectable. Four to 7 mice per time point. Data are represented as mean values plus or minus standard deviation (**P < .01; ***P < .05).
Cytokine levels during the course of the air-pouch model of acute inflammation in mice. (A) Scheme of the air-pouch model of local inflammation as outlined in “Air-pouch model.” (B) Levels of endogenous IL6 in inflamed and noninflamed air pouches at different time points were measured by enzyme-linked immunosorbent assay (ELISA). (C) Levels of endogenous sIL6R in inflamed and noninflamed air pouches at different time points were measured by ELISA. In noninflamed air pouches, IL6 and sIL6R were hardly detectable. Four to 7 mice per time point. Data are represented as mean values plus or minus standard deviation (**P < .01; ***P < .05).
Indeed, IL6 is known to be secreted by activated monocytes, whereas the sIL6R has recently been shown to be produced by dying neutrophils via activation of the membrane bound shedding protease ADAM17.27 No IL6 and sIL6R induction was observed in PBS-treated air pouches.
In a study using the air-pouch model, Mantovani et al studied the influence of IL6 during an acute inflammation.31 This group postulated that the sIL6R is involved in cellular transmigration during acute inflammation, although they could not measure sIL6R levels due to the lack of available assays. Their conclusion was based on in vivo experiments comparing immune cell transmigration in WT and IL6−/− mice, which showed that IL6−/− mice have a reduced capacity to attract immune cells (polymorphonuclear leukocytes, mononuclear cells, and lymphocytes). In vitro experiments revealed that the action of IL6 was not mediated by the membrane bound IL6R. Endothelial cells responsible for secreting a pattern of chemokines directing transmigration of specific subsets of immune cells only express gp130 but not the IL6R. By combining in vivo and in vitro data, the authors hypothesized that the IL6-induced migration of immune cells is mediated by the sIL6R via transsignaling, although an experimental proof was missing because an in vivo model of IL6 transsignaling was not available. This study was extended by Hurst et al using an experimental peritonitis model of acute inflammation.13 Here the invasion of mononuclear cells could be augmented by injection of an IL6/sIL6R fusion protein into the peritoneum of IL6−/− mice, which was caused by massive gp130-stimulated expression of the monocyte-attracting chemokine MCP-1. The authors concluded from these results that transmigration of monocytes was dependent on IL6 transsignaling, even though direct experimental evidence was still missing because the effect of inhibition of IL6 transsignaling by sgp130 on monocyte infiltration has not been evaluated. We have recently shown that the sIL6R is mostly derived from activated neutrophils, which are the first infiltrating cells found in the air pouch upon carrageenan injection. Animals that have been depleted of neutrophils showed no increase in sIL6R levels in air pouches, and these changes were associated with a marked reduction in the number of infiltrating mononuclear cells.27 We suggest that sIL6R liberated from the infiltrating neutrophil population is required to drive the recruitment of inflammatory mononuclear cells to the site of inflammation.27
Because we were especially interested in the influence of IL6 transsignaling on monocyte recruitment during the late phase of acute inflammation, we next evaluated the inflammatory regulation of leukocytes in the opt_sgp130Fc transgenic mice.
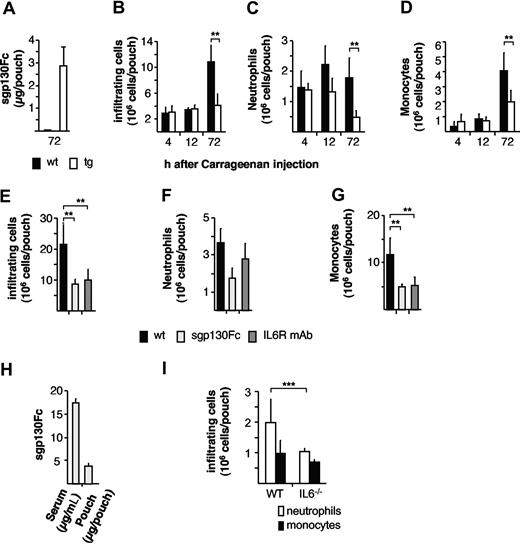
In vitro experiments revealed that at least a 10-fold molar excess of sgp130Fc to IL6/sIL6R is sufficient to completely block IL6-transsignaling.24 Air pouches from transgenic animals contained sgp130Fc protein in the range of 3 μg, which is a 100-fold molar excess of sgp130Fc to IL6/sIL6R and therefore should be sufficient to block IL6 transsignaling–mediated processes in vivo during acute inflammation (Figure 4A). When total cells infiltrating the air pouch were analyzed in opt_sgp130Fc transgenic mice, cellular infiltration was strongly reduced (Figure 4B). Consistent with previous findings in IL6−/− mice (Table S1),34,35 the initial influx of neutrophils (4 hours) is unaffected in opt_sgp130Fc transgenic mice. However, as inflammation proceeds, neutrophil recruitment and that of the subsequent monocytic cell infiltration is temporally compromised in opt_sgp130Fc transgenic mice (Figure 4C,D). Confirmation that these events were directed by IL6 transsignaling was substantiated by the administration of WT mice with either recombinant sgp130Fc protein or a blocking anti-IL6R antibody (Figure 4E-G). In this respect, intraperitoneal delivery of sgp130Fc resulted in systemic and local sgp130Fc concentrations comparable to that achieved in transgenic opt_sgp130Fc animals (Figure 4H). In line with the data from Mantovani et al, we could show that IL6−/− mice exhibit a statistically significant reduction of neutrophil presence at the site of inflammation after 12 hours, confirming the role of IL6 on neutrophil presence during inflammation (Figure 4I). Collectively these studies endorse a role for IL6 transsignaling in limiting the overall trafficking of inflammatory neutrophils and monocytic cells.
Reduced cell infiltration in opt_sgp130Fc transgenic mice during acute inflammation. (A) Levels of transgenic sgp130Fc-protein in inflamed air pouches of opt_sgp130Fc transgenic mice. 72 hours after carrageenan stimulation, the levels of sgp130Fc-protein were assessed by ELISA. Data are represented as mean values plus or minus SD of 8 animals. (B) Quantitative flow cytometry analysis showing inflammatory cells (x106 per pouch) of homozygous opt_sgp130Fc transgenic and WT animals (wt) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan. (C) Neutrophils (x106/pouch) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan as measured by flow cytometry. (D) Monocytes (x106/pouch) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan as measured by flow cytometry. Four to 7 mice per group. Data are represented as mean values plus or minus SD (**P < .01; *P < .05). (E) Quantitative flow cytometry analysis showing inflammatory cells (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan. Comparison of WT animals treated with phosphate-buffered saline (PBS), 100 μg sgp130Fc, or 100 μg anti-IL6R mAb 6 hours before carrageenan injection. (F) Neutrophils (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan as measured by flow cytometry. Comparison of WT animals administered with PBS, sgp130Fc, or anti-IL6R mAb 6 hours before carrageenan injection. (G) Monocytes (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan as measured by flow cytometry. Comparison of WT animals administered with PBS, sgp130Fc, or anti-IL6R mAb 6 hours before carrageenan injection. (H) Levels of recombinant sgp130Fc-protein in serum (μg/mL) and in inflamed air pouches (μg/pouch) of WT mice 78 hours after administration of sgp130Fc. Sgp130Fc-protein levels were assessed by ELISA. Data are represented as mean values plus or minus SD of 5 animals (**P < .01). (I) Neutrophils and monocytes (x106/pouch) infiltrating the air pouch 12 hours after injection of 1% carrageenan as measured by flow cytometry of IL6−/− and WT animals. Data are represented as mean values plus or minus SD of 3 animals (***P < .015).
Reduced cell infiltration in opt_sgp130Fc transgenic mice during acute inflammation. (A) Levels of transgenic sgp130Fc-protein in inflamed air pouches of opt_sgp130Fc transgenic mice. 72 hours after carrageenan stimulation, the levels of sgp130Fc-protein were assessed by ELISA. Data are represented as mean values plus or minus SD of 8 animals. (B) Quantitative flow cytometry analysis showing inflammatory cells (x106 per pouch) of homozygous opt_sgp130Fc transgenic and WT animals (wt) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan. (C) Neutrophils (x106/pouch) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan as measured by flow cytometry. (D) Monocytes (x106/pouch) infiltrating the air pouch 4 hours, 12 hours, and 72 hours after injection of 1% carrageenan as measured by flow cytometry. Four to 7 mice per group. Data are represented as mean values plus or minus SD (**P < .01; *P < .05). (E) Quantitative flow cytometry analysis showing inflammatory cells (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan. Comparison of WT animals treated with phosphate-buffered saline (PBS), 100 μg sgp130Fc, or 100 μg anti-IL6R mAb 6 hours before carrageenan injection. (F) Neutrophils (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan as measured by flow cytometry. Comparison of WT animals administered with PBS, sgp130Fc, or anti-IL6R mAb 6 hours before carrageenan injection. (G) Monocytes (x106/pouch) infiltrating the air pouch 72 hours after injection of 1% carrageenan as measured by flow cytometry. Comparison of WT animals administered with PBS, sgp130Fc, or anti-IL6R mAb 6 hours before carrageenan injection. (H) Levels of recombinant sgp130Fc-protein in serum (μg/mL) and in inflamed air pouches (μg/pouch) of WT mice 78 hours after administration of sgp130Fc. Sgp130Fc-protein levels were assessed by ELISA. Data are represented as mean values plus or minus SD of 5 animals (**P < .01). (I) Neutrophils and monocytes (x106/pouch) infiltrating the air pouch 12 hours after injection of 1% carrageenan as measured by flow cytometry of IL6−/− and WT animals. Data are represented as mean values plus or minus SD of 3 animals (***P < .015).
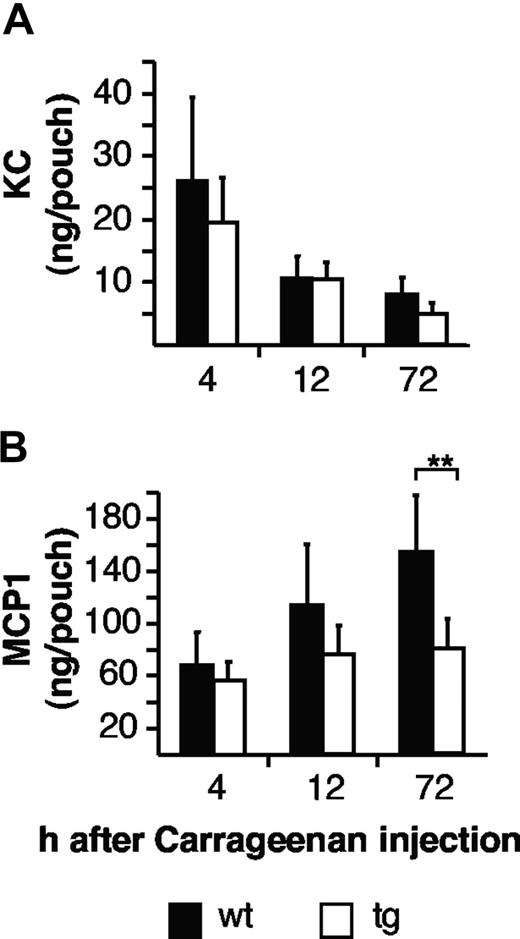
It is well established that infiltrating neutrophils and macrophages are attracted by a family of factors called chemokines. The CXC chemokine KC, which is the functional homologue of the human chemokine GROα/CXCL1, has been shown to be responsible for neutrophil attraction, whereas the CC chemokine MCP-1 is involved in the recruitment of macrophages. Importantly, endothelial cells, which secrete inflammatory chemokines, only express the gp130 signal transducing chain but not the IL6-specific IL6R.31,36 In an experimental peritonitis model, the induction of MCP-1 on mesothelial cells can be induced by injection of Hyper-IL6 into the peritoneum of IL6−/− mice.13 We therefore asked whether the infiltration of mononuclear cells in vivo relies on IL6 transsignaling by specifically blocking IL6 transsignaling. First, we measured the levels of these chemokines in the air-pouch model. As shown in Figure 5A, the chemokine KC was elevated already at 4 hours compatible with its role of neutrophil recruitment. The accumulation of KC was not different in WT and transgenic mice at all phases of inflammation (Figure 5A). In contrast, the induction of MCP-1 in WT mice was most pronounced at the late phase (72 hours after carrageenan injection), whereas in sgp130Fc mice, the induction of MCP-1 but not of KC was completely abolished (Figure 5B), indicating that in the air-pouch model this response is under the control of the complex of IL6 and sIL6R.
Reduced mononuclear cells attracting chemokines in opt_sgp130Fc transgenic mice. (A) Levels of endogenous KC in inflamed air pouches of opt_sgp130Fc transgenic and WT mice 4 hours, 12 hours, and 72 hours after carrageenan challenge. KC concentrations were determined by ELISA. (B) MCP1 in inflamed air pouches of opt_sgp130Fc transgenic and WT mice 4 hours, 12 hours, and 72 hours after carrageenan challenge. KC and MCP1 concentrations were determined by ELISA. Four to 7 mice per group. Data are represented as mean values plus or minus SD (**P < .01).
Reduced mononuclear cells attracting chemokines in opt_sgp130Fc transgenic mice. (A) Levels of endogenous KC in inflamed air pouches of opt_sgp130Fc transgenic and WT mice 4 hours, 12 hours, and 72 hours after carrageenan challenge. KC concentrations were determined by ELISA. (B) MCP1 in inflamed air pouches of opt_sgp130Fc transgenic and WT mice 4 hours, 12 hours, and 72 hours after carrageenan challenge. KC and MCP1 concentrations were determined by ELISA. Four to 7 mice per group. Data are represented as mean values plus or minus SD (**P < .01).
Discussion
There are three major findings in this report. First, we developed a strategy to obtain robust and systemic expression of a transgenic protein in mice that is high enough to block an endogenous pathway. Furthermore, we showed that the sgp130Fc protein when expressed in mice leads to inhibition of IL6 transsignaling in vivo. Finally, we demonstrated, using our transgenic mice, that in the air-pouch model of inflammation, recruitment of inflammatory mononuclear cells strictly depends on the IL6 transsignaling pathway.
The aim of this study was to produce sufficient amounts of sgp130Fc protein in transgenic mice to suppress systemic as well as local IL6-transsignaling responses. For the expression of the transgene, we used the liver-specific PEPCK promoter, which we had used before to express the sIL6R in transgenic mice and that had led to microgram expression levels in the plasma of the animals.29,37 In mammals, gluconeogenesis does not occur until birth and is paralleled by the expression of the PEPCK gene.38 Therefore, expression of the sgp130Fc transgene driven by the PEPCK promoter is only expressed after birth of the animals and did not influence embryonic development.
We realized, however, that the plasma levels of transgenic mice expressing sgp130Fc from the natural sgp130Fc cDNA were only in the range of 60 to 500 ng/mL. These levels were not high enough to neutralize the endogenous acute–phase reaction induced by the fusion protein of IL6 and sIL6R, Hyper-IL6. Therefore, we attempted to further increase the protein expression level in transgenic mice by use of an optimized sgp130Fc-cDNA.
Optimization of gene sequences including the change of codon usage, the removal of cryptic splice sites, and mRNA-destabilizing motifs has been shown to often drastically increase protein expression levels.39 Cell-culture experiments clearly indicated that the expression of an optimized sgp130Fc-cDNA resulted in increased protein accumulation in the supernatant. It is further known that intron-containing transgenes are better expressed than intronless transgenes.40 Using an optimized sgp130Fc cDNA and varying the position of the open reading frame in relation to the β-globin intron, we could show in cell-culture experiments that placing the optimized sgp130Fc-cDNA downstream of the intron resulted in the highest levels of secreted sgp130Fc protein. Using this expression construct topology, we generated opt_sgp130Fc transgenic mice that exhibited a more than 50-fold increase in transgenic protein accumulation as compared with the nonoptimized sgp130Fc transgenic mice.
Here for the first time we describe a strategy that might be generally transferable to other applications that rely on the massive overexpression of an inhibitory or stimulatory protein in transgenic mice. Many therapeutic antiinflammatory strategies are based on proteins (ie, monoclonal antibodies or soluble receptors), which require a molar excess to ensure appropriate inhibition of biologic responses. The test of such strategies in vivo in transgenic mouse models is of particular importance for the evaluation of consequences of the life-long exposure to an inhibitory protein. Furthermore, our approach could be important for human gene therapies, which rely on the efficient cell-based delivery of therapeutic proteins. We have shown in the case of the sgp130Fc protein that conventional transgenic strategies failed to deliver amounts adequate for the competitive inhibition of IL6 transsignaling.
The opt_sgp130Fc transgene is transcribed mainly in the liver and kidney and is secreted into the circulation, where it is found in microgram amounts. The endogenously produced sgp130Fc protein was able to bind to and precipitate the fusion protein of IL6 and sIL6R, Hyper-IL6. Indeed, overexpression of the sgp130Fc transgene selectively blocked the biologic activity of the IL-6/sIL-6R complex in vivo. Significantly, sgp130 has previously been noted to exhibit low affinity for certain other IL6-related cytokines such as leukemia inhibitory factor (LIF).15,24,41 However, these additional interactions appear to have limited biologic impact because sgp130Fc transgenic mice bred normally, unlike LIF-deficient female mice, which are infertile.42 Importantly, we could also demonstrate that the sgp130Fc protein was not only found in the circulation but also in the air pouch compartment where the concentrations of the protein were high enough to achieve neutralization of IL6 transsignaling. This is important because we could earlier demonstrate that the activity in closed compartments such as the peritoneum13 or the joint14,15 is of pathophysiologic relevance and that the addition of recombinant sgp130Fc protein acted to modulate the outcome of the ensuing inflammatory response.
Previous reports have shown that IL6−/− mice display altered leukocyte recruitment.13,14,31,34,35,43 Within the context of the air-pouch model of carrageenan-induced inflammation, it has been reported that although the migratory capacity of leukocytes derived from WT and IL6−/− mice was comparable, chemokine-mediated recruitment was markedly impaired in IL6−/− mice.31 We have extended this view by demonstrating that IL6 in association with its soluble receptor is important for neutrophil and monocytic cell infiltration during inflammation.
Measurements of IL6 and sIL6R protein levels showed that the expression of both proteins is differentially regulated during the course of an inflammatory episode. During early phase of inflammation (until 12 or 24 hours), IL6-protein levels within the air pouch increased rapidly. Thereafter, the level of IL6 declined to approximately 1 ng per pouch during the late phase (48-72 hours), which still was approximately 100- to 1000-fold above normal serum levels, which are below 5 pg/mL.44 Interestingly, the level of sIL6R was rising during the late phase of inflammation. Taking into account that the molecular weight of the sIL6R is approximately twice that of IL6, the IL6 levels in the late phase of inflammation are still high enough to bind to soluble IL6R molecules and initiate IL6 transsignaling. Such temporal differences in the profile of IL6 and sIL6R secretion is not uncommon clinically, and is postulated to act as a control mechanism for determining the stage of the inflammatory response that is reliant on IL6 transsignaling.13
Soluble cytokine receptors such as sIL6R function to maintain the circulating half-life of their inflammatory ligands.29 Consequently, we propose that local increases in sIL6R after neutrophil infiltration act to sequester IL6 generated early in the inflammatory response by stromal tissue cells. Generation of sIL6R is therefore the rate-limiting step for activation events governed by IL6 transsignaling within the air pouch. In this respect, differences in neutrophil and mononuclear cell infiltration are only evident in opt_sgp130Fc-transgenic mice once sIL6R levels are suitably elevated. Consequently, early changes in neutrophil trafficking (4 hours), presumably orchestrated by other inflammatory mediators such as IL1β and TNFα, remain unabated in opt_sgp130Fc-transgenic mice. Such conclusions are substantiated in WT mice, where selective blockade of IL6 transsignaling with sgp130Fc gives rise to a similar profile of leukocyte infiltration.
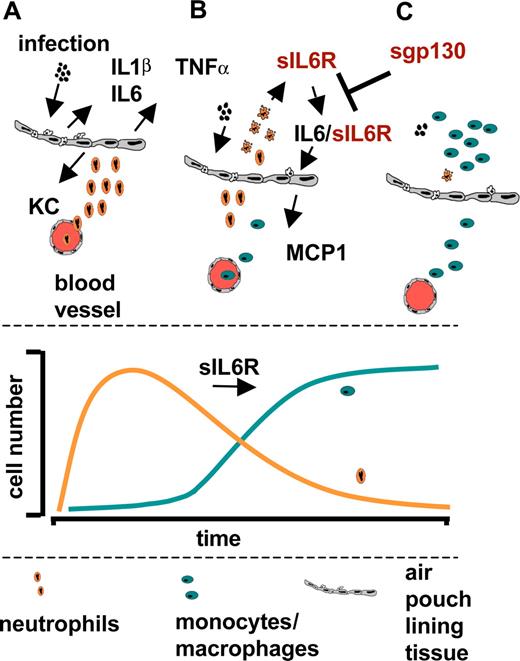
IL6 itself is unable to directly elicit mononuclear cell infiltration because the structural cells lining the air pouch lack expression of the cognate IL6R.31,36 The studies outlined herein highlight that regulation of IL6 transsignaling relies primarily on the initial influx of neutrophils, which has been shown in vitro to shed IL6R in response to inflammatory chemokines, lipid mediators, complement components, C-reactive protein, and neutrophil apoptosis.13,27,36,45-47 The subsequent appraisal of leukocyte recruitment and inflammatory chemokine expression in opt_sgp130Fc transgenic mice showed that IL6 transsignaling was responsible for triggering the chemokine-mediated recruitment of mononuclear phagocytes. Indeed, the over-expression of sgp130Fc led to impaired expression of the inflammatory chemokine MCP-1/CCL2. The consequence of this control process is outlined in Figure 6. An acute inflammation stimulates tissue-resident mononuclear cells to secrete the proinflammatory cytokines TNFα, IL1β and IL6, which in turn induce CXC-chemokines like KC secretion from endothelial cells (Figure 6A). Importantly, endothelial cells do not express membrane-bound IL6R and are therefore not responsive toward IL6 alone. Infiltrating neutrophils shed their IL6R upon induction of apoptosis, and the IL6/sIL6R complex stimulates endothelial cells to produce the CC-chemokine MCP1 (Figure 6B). MCP1 subsequently attracts mononuclear cells to the site of inflammation. Finally, the replacement of neutrophils by mononuclear cells is an important intermediate step in the resolution of inflammation, which is therefore controlled by the presence of sIL6R (Figure 6C).
Role of IL6 transsignaling during acute inflammation. (A) Acute inflammation stimulates tissue-resident mononuclear cells to secrete the proinflammatory cytokines TNFα, IL6, and IL1β, which in turn induce CXC-chemokines like KC secretion from endothelial cells. (B) Endothelial cells do not express membrane-bound IL6R and are therefore not responsive toward IL6 itself. Infiltrating neutrophils shed their IL6R upon apoptosis and the IL6/sIL6R complex stimulates endothelial cells to produce the CC-chemokine MCP1. MCP1 subsequently attracts mononuclear cells to the site of inflammation. (C) The replacement of neutrophils by mononuclear cells is an important intermediate step in the resolution of inflammation, which is controlled by the presence of sIL6R.
Role of IL6 transsignaling during acute inflammation. (A) Acute inflammation stimulates tissue-resident mononuclear cells to secrete the proinflammatory cytokines TNFα, IL6, and IL1β, which in turn induce CXC-chemokines like KC secretion from endothelial cells. (B) Endothelial cells do not express membrane-bound IL6R and are therefore not responsive toward IL6 itself. Infiltrating neutrophils shed their IL6R upon apoptosis and the IL6/sIL6R complex stimulates endothelial cells to produce the CC-chemokine MCP1. MCP1 subsequently attracts mononuclear cells to the site of inflammation. (C) The replacement of neutrophils by mononuclear cells is an important intermediate step in the resolution of inflammation, which is controlled by the presence of sIL6R.
Besides orchestration of the infiltration of inflammatory cells to the site of inflammation, IL6 has been shown to play a role in the establishment and maintenance of inflammatory bowel disease and colon cancer.11,16 In the latter case, it is not entirely clear which cells are stimulated by the complex of IL6 and sIL6R in vivo. However, we have recently shown that the induction of Foxp3 expressing regulatory T cells by TGFβ is much more effectively blocked by IL6 and sIL6R than by IL6 alone, indicating a role for IL6 transsignaling in establishing the balance between regulatory T cells and TH17 cells.48 These results strongly indicate a much more global role for IL6 transsignaling in the regulation of the immune system in which IL6 acting via the membrane-bound IL6R governing the hepatic acute-phase reaction49 and regulating B-cell stimulation50 and IL6 acting via the soluble IL6R is primarily activated under pathophysiologic conditions.11-16 In this respect it is interesting that thermal stress in the range of fever temperatures increased the expression of intercellular adhesion molecule 1 (ICAM1) exclusively in high endothelial venules, which was accompanied by increased lymphocyte trafficking across high endothelial venules, and that the thermal induction of ICAM1 was governed by the IL6-transsignaling pathway.51
The sgp130Fc transgenic mice will be challenged in more prolonged inflammatory events such as T-cell effector/memory functions and chronic disease progression. Such studies will allow for the first time in vivo definition of the balance between classical IL6 signaling and IL6 transsignaling in inflammatory states, in infectious diseases, and in tumorigenesis. Moreover, because the sgp130Fc protein is a candidate protein for the treatment of chronic inflammatory diseases, the sgp130Fc transgenic mice are an ideal animal model for the evaluation of the physiologic consequences of a life-long blockade of IL6 transsignaling. The precise comparison of the phenotype of these transgenic mice with that of IL6−/− mice will allow us to estimate the possible advantages of a specific blockade of IL6 transsignaling over a global inhibition of the entire IL6 pathway with neutralizing IL6R antibodies, which are already being tested in the clinic.44 Our mouse model will therefore be invaluable in defining the involvement of IL6 transsignaling in conditions such as infection, inflammation, regeneration, and cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stefanie Schnell for excellent technical assistance, and Tamas Laskay and Christian Idel (Institute of Medical Microbiology and Hygiene, University of Lübeck, Lübeck, Germany) for technical help and fruitful discussions.
This work was supported by grants SFB415, Project B5, and Project C6 from the Deutsche Forschungsgemeinschaft, Bonn, Germany (J.S. and S.R.-J.).
Authorship
Contribution: B.R., A.C., and U.M. performed the experiments; G.H.W., D.S., A.S.W., and S.A.J. contributed techniques and materials and analyzed data; S.R.-J. and J.S. designed the study and wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: G.H.W. and D.S. are employees of the CONARIS Research Institute, Kiel, Germany. S.R.-J. is the inventor on the patent describing the function of sgp130Fc and is a shareholder of the CONARIS Research Institute Kiel, Germany. All other authors declare no competing financial interests.
Correspondence: Stefan Rose-John, Department of Biochemistry, Christian-Albrechts-University, Kiel, Olshausenstraβe 40, D-24098 Kiel, Germany; e-mail: rosejohn@biochem.uni-kiel.de.

![Figure 1. Generation of transgenic mice with the optimized opt_sgp130Fc cDNA. (A) cDNA-optimization and intron localization positively influence sgp130Fc protein accumulation in transiently transfected HepG2-cells. The constructs differ in (1) position of the intron (upstream/downstream of the transgene) and (2) optimization status (wild-type [WT]/optimized cDNA). 1. pTZ-PEPCK-sgp130Fc-βglob.intron, 2. pTZ-PEPCK-βglob.intron-sgp130Fc, 3. pTZ-PEPCK-opt_sgp130Fc-βglob.intron, 4. pTZ-PEPCK-βglob.intron-opt_sgp130Fc. sgp130Fc was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. (B) opt_sgp130Fc construct injected into fertilized mouse eggs (pTZ-PEPCK-βglob.intron-opt_sgp130Fc). (C) sgp130Fc from serum of founder animals was precipitated from cell supernatant with Protein A sepharose and detected by immunoblotting. * indicates an unspecific band. A vertical line has been inserted to indicate where a gel lane was cut. This gel came from one experiment; irrelevant lanes have been cut out. (D) Expression of sgp130Fc protein in opt_sgp130Fc transgenic mice. Serum was prepared from 8- to 10-week-old WT mice, heterozygous (+/−) and homozygous (+/+) transgenic mice of the lines opt2 and opt3 and sgp130Fc protein levels were determined by ELISA. Data represent mean values plus or minus SD (n) mice per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/3/10.1182_blood-2007-07-102137/6/m_zh80050813650001.jpeg?Expires=1769832426&Signature=LbjC9oBc66bT758X0FwV9t~WBw46FcoGFDnJvyx6Pgg~B35iyxixayb4iKPL8IeS-44j5ytPwEhDbRqDgUjbfs2j6b9YNN-gjvbQkI8Nj-gq6wmnAXnmJoVLjpvVEZfBP52filuxxNFA0NThG9h4xbzENiVzvmpO-4QX8fIhUvaa1kzU7ety7SyWsW4gRWONm0wTr9NB6X7o0abGoEsp4BUM6jU4ILrcO1KIKRkfzeP-UKlGPkd14NnUsQywYnoOK7EpAUH8gFEH1-LBrgOxatEtHBefJ5wPV08neXX5wl1uzVMSGrFXBRzP4ms0cZP3tWS-xKhkSY465KQ1K4sysw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal