Abstract
We conducted a prospective multicenter study to compare the efficacy of repeated immunosuppressive therapy (IST) with stem-cell transplantation (SCT) from an alternative donor in children with acquired aplastic anemia (AA) who failed to respond to an initial course of IST. Patients with severe (n = 86) and very severe disease (n = 119) received initial IST consisting of antithymocyte globulin (ATG) and cyclosporine. Sixty patients failed to respond to IST after 6 months from the initial IST and were eligible for second-line treatment. Among them, 21 patients lacking suitable donors received a second course of IST. Three patients developed an anaphylactoid reaction to ATG and could not complete the second IST. A trilineage response was seen in only 2 of 18 (11%) evaluable patients after 6 months. Thirty-one patients received SCT from an alternative donor. At 5 years from the initiation of second-line therapy, the estimated failure-free survival (FFS), defined as survival with response, was 83.9% (± 16.1%, SD) in the SCT group compared with 9.5% (± 9.0%) in the IST group (P = .001). These results suggest that SCT from an alternative donor offers a better chance of FFS than a second IST in patients not responding to an initial IST.
Introduction
Acquired aplastic anemia (AA) is a heterogeneous disorder characterized by pancytopenia of peripheral blood and hypocellular marrow. Currently 2 effective treatments are available for this disorder: hematopoietic stem cell transplantation (SCT) and immunosuppressive therapy (IST). There are several reports comparing bone marrow transplantation (BMT) and IST as first-line treatment for AA.1-4 These studies indicate that allogeneic BMT from an HLA-matched sibling donor is the treatment of choice for young patients. IST consisting of antithymocyte globulin (ATG) and cyclosporine (CyA) with or without granulocyte-colony stimulating factor (G-CSF) has been successfully used for patients with AA who lack an HLA-matched sibling donor or who are not eligible for SCT. Several reports indicate that 2- to 5-year survival following IST is between 60% and 90%.5-7 We reported results of a multicenter trial of IST for children younger than 18 years with AA (AA-92 trial).8 In the AA-92 trial, 119 children with newly diagnosed AA were enrolled, and the response rate at 6 months was 71%, with the probability of survival at 4 years greater than 90%. However, approximately 30% of the patients did not respond to an initial course of IST. Moreover, a significant proportion of patients subsequently relapsed and required second-line therapy.9 The optimal treatment for such patients has not been established
A repeated course of IST has been used for patients who fail to respond to, or who have relapsed after an initial course of, IST. Tichelli et al reported the results of a Basel study that consisted of repeated courses of IST, using ATG from the same species (horse) for nonresponders.10 In their study, repeated IST was well tolerated and the response rate was 63%. An Italian group reported the results of repeated IST using ATG from different species (horse to rabbit), where the response rate was also high.11 Investigators at the National Institutes of Health (NIH) recently reported the results of retreatment with rabbit ATG and CyA in 22 patients refractory to horse ATG and CyA. Contrary to the reports from Europe, the overall response rate was only 27% and no patients achieved complete response.12
SCT from an alternative donor has also been used as salvage therapy for patients not responding to IST because recent progress in the management of patients who undergo SCT, and better selection of donors by DNA typing of HLA loci, has improved the outcome for these patients.13,14 However, no prospective study has been performed to date comparing repeated IST versus SCT from an alternative donor as second-line therapy. Therefore, we conducted a prospective multicenter trial to compare these 2 treatment options for pediatric patients with severe and very severe AA who had failed to respond to initial IST.
Methods
Patients
This multicenter study was designed by the Japan Childhood Aplastic Anemia Study Group and involved 79 hospitals in Japan. The eligibility criteria were as follows: age younger than 18 years, diagnosis less than 180 days before registration, no specific prior treatment for AA, and severe to very severe disease. The definition of disease severity was determined according to currently used criteria.15 The disease was considered severe if at least 2 of the following were noted: a neutrophil count less than 0.5 × 109/L, a platelet count less than 20 × 109/L, and a reticulocyte count less than 20 × 109/L with hypocellular bone marrow. AA was considered very severe if the criteria for severe disease were fulfilled and the neutrophil count was less than 0.2 × 109/L. Patients were excluded if they had congenital AA. Patients were screened for paroxysmal nocturnal hemoglobinuria (PNH) by flow cytometry using anti-CD55 and anti-CD59 antibodies. Bone marrow cytogenetic studies were performed in all patients. Allogeneic SCT was recommended for patients with severe or very severe disease who had an HLA-matched sibling: these patients were not included in AA-97 study.
Treatment protocol
Patients with very severe disease were treated with IST, which consisted of horse ATG (Lymphoglobulin; IMTIX-SANGSTAT, Lyon, France) 15 mg/kg per day on days 1 through 5; CyA 6 mg/kg per day from day 1 until at least day 180, with subsequent adjustment according to whole blood CyA concentration between 100 and 200 ng/mL; methylprednisolone (MePred) 2 mg/kg per day for 5 days, with subsequent halving of the dose every week until discontinuation on day 28 for prophylaxis of allergic reaction of ATG; and G-CSF (Filgrastim, Kirin, Tokyo, Japan) 400 μg/m2 per day from day 1, with responding patients (neutrophil count > 109/L) receiving the same dose 3 times a week for 60 days (ATG/CyA/MePred/G-CSF). Patients with severe disease were given the same treatment regimen, with the exception that G-CSF was not given unless severe infection was documented (ATG/CyA/MePred).
The hematologic response was evaluated at 6 months after the initiation of therapy. A complete response (CR) was defined for all patients as a neutrophil count more than 1.5 × 109/L, a platelet count more than 100 × 109/L, and a hemoglobin level more than 11.0 g/dL.8 A partial response (PR) was defined as a neutrophil count more than 0.5 × 109/L, a platelet count more than 20 × 109/L, a hemoglobin level more than 80 g/L (8.0 g/dL) and no requirement of blood transfusions. Patients with very severe or severe disease who failed to respond to initial IST underwent SCT if they had a serologically HLA-matched unrelated donor, HLA-one antigen mismatched family donor, or HLA-matched or HLA-one antigen mismatched unrelated cord blood donor at the time of evaluation. Those lacking a suitable donor received a second course of IST. A second course of IST consisted of the same regimen (horse ATG/CyA/MePred) used in the initial treatment of each patient. To reduce the risk of an anaphylactoid reaction to treatment with horse ATG, patients were initially given a 100-fold diluted dose of ATG as a test dose. An antihistamine was administered to all patients receiving a second course of IST to suppress allergic reactions.
The recommended conditioning regimen for SCT from an alternative donor consisted of cyclophosphamide (CY, 120 mg/kg), rabbit ATG (Thymoglobulin, IMTIX-SANGSTAT, 10 mg/kg), and total body irradiation (TBI, 10 Gy) or CY (3000 mg/m2), rabbit ATG (10 mg/kg), fludarabine (100 mg/m2), and local field irradiation (3 Gy).16,17 Prophylaxis against graft versus host disease (GVHD) consisted of a combination of CyA (3mg/kg per day) or tacrolimus (0.02mg/kg per day) plus short-term methotrexate. CyA dose were adjusted to maintain whole blood concentration of 100 to 200 ng/mL and tacrolimus dose 5 to 10 ng/mL, respectively.
Informed written consent was obtained from all patients or their parents in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of each participating hospital. The list of participating hospitals can be found in Document S1, (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Statistical analysis
The primary end point of this study was failure-free survival (FFS) after second-line therapy, which was defined as survival with response. Death, no response by 6 months, disease progression requiring clinical intervention, or relapse were considered treatment failures.18 Overall survival and FFS were analyzed using the Kaplan-Meier method. Differences between the 2 arms of the study were evaluated by the log-rank test. P less than .05 was considered statistically significant.
Results
Patient characteristics
From October 1997 to April 2004, 205 patients with newly diagnosed severe (n = 86) and very severe AA (n = 119) were enrolled in the AA-97 study (Table 1). An interim analysis was performed in April 2005. Four patients were excluded from further analysis for the following reasons: IST without ATG (2 patients) or stem cell transplantation within 4 months of diagnosis (2 patients). Two patients without any granulocytes were not treated with ATG because of severe infections; both of them died of fungal pneumonia within 2 months of diagnosis. Both patients who underwent SCT within 4 months of diagnosis died of graft rejection or cardiac toxicity to the preconditioning regimen. There were 2 further deaths within 6 months of patient registration: hemolysis of unknown cause and aspiration pneumonia. None of the patients was diagnosed with PNH at the time of registration. Severe and very severe AA were associated with hepatitis in 32 patients, with other viral infection in 3 patients, and with medication use in 2 patients. The median days (range) from diagnosis to treatment of severe and very severe AA were 13 (1-94) days and 19 (1-179) days, respectively (Table 1).
Pretreatment characteristics
| . | SAA . | VSAA . |
|---|---|---|
| Registered | 86 | 119 |
| Evaluable | 84 | 117 |
| Sex (M/F) | 48/36 | 65/52 |
| Median age, y (range) | 8 (0-17) | 9 (0-15) |
| Cause of AA | ||
| Idiopathic | 73 | 91 |
| Hepatitis | 8 | 24 |
| Viral infection | 1 | 2 |
| Drug | 2 | 0 |
| Median days from diagnosis to treatment (range) | 13 (1-94) | 19 (1-179) |
| . | SAA . | VSAA . |
|---|---|---|
| Registered | 86 | 119 |
| Evaluable | 84 | 117 |
| Sex (M/F) | 48/36 | 65/52 |
| Median age, y (range) | 8 (0-17) | 9 (0-15) |
| Cause of AA | ||
| Idiopathic | 73 | 91 |
| Hepatitis | 8 | 24 |
| Viral infection | 1 | 2 |
| Drug | 2 | 0 |
| Median days from diagnosis to treatment (range) | 13 (1-94) | 19 (1-179) |
SAA indicates severe aplastic anemia; VSAA, very severe aplastic anemia
Trilineage hematologic response
At 3 months after the initiation of therapy, 49 patients (58%) with severe AA and 46 patients (39%) with very severe AA had responded to the initial course of IST (Table 2). By 6 months, 55 patients (66%) with severe AA and 83 patients (72%) with very severe AA had evidence of a trilineage response and had become transfusion-independent. Three patients died between 3 and 6 months. Overall, of 198 evaluable patients receiving an initial course of IST, 37 patients (19%) had a complete response and 101 patients (51%) showed a partial response, for an overall response rate of 70% after 6 months. Sixty patients (30%), 28 (34%) with severe AA and 32 (28%) with very severe AA, did not attain CR or PR status at 6 months, and were therefore eligible for second-line therapy (Fig 1).
Response to treatment after initial treatment
| . | SAA . | VSAA . |
|---|---|---|
| 3 months | ||
| Evaluable | 84 | 117 |
| CR | 9* | 6† |
| PR | 40* | 40† |
| NR | 35 | 71 |
| Alive | 34 | 71 |
| Dead | 1 | 0 |
| 6 months | ||
| Evaluable | 83 | 115 |
| CR | 17‡ | 20§ |
| PR | 38‡ | 63§ |
| NR | 28 | 32 |
| Alive | 27 | 31 |
| Dead | 1 | 1 |
| . | SAA . | VSAA . |
|---|---|---|
| 3 months | ||
| Evaluable | 84 | 117 |
| CR | 9* | 6† |
| PR | 40* | 40† |
| NR | 35 | 71 |
| Alive | 34 | 71 |
| Dead | 1 | 0 |
| 6 months | ||
| Evaluable | 83 | 115 |
| CR | 17‡ | 20§ |
| PR | 38‡ | 63§ |
| NR | 28 | 32 |
| Alive | 27 | 31 |
| Dead | 1 | 1 |
Data are numbers (%) of responders.
NR indicates no response.
*These classes combined were 58% to the total number.
†These classes combined were 39% to the total number.
‡These classes combined were 66% to the total number.
§These classes combined were 72% to the total number
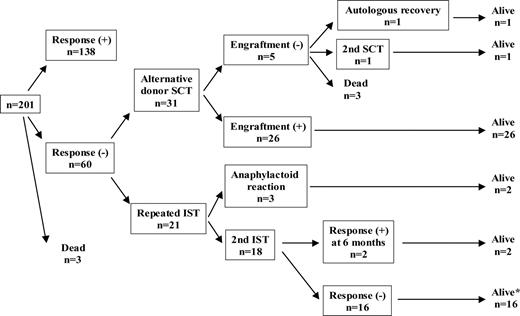
Overall outcome of 201 patients assigned to second-line therapy. *Among 16 patients who failed to respond to second IST, 8 patients received SCT and were alive. Four of the remaining 8 patients attained a late hematologic response and were alive. The other 4 patients were alive without response.
Overall outcome of 201 patients assigned to second-line therapy. *Among 16 patients who failed to respond to second IST, 8 patients received SCT and were alive. Four of the remaining 8 patients attained a late hematologic response and were alive. The other 4 patients were alive without response.
Repeated IST versus SCT as second-line therapy
Figure 1 shows the outcome of 201 patients with treatment assigned. Twenty-one patients lacking a suitable donor at the time of evaluation were assigned to receive a second course of IST. Three of these patients developed an anaphylactoid reaction to ATG and thus could not complete their second course of treatment. Anaphylactoid reactions were not observed during the first course of IST in these 3 patients. These patients were subsequently treated with corticosteroids, which rapidly resolved their symptoms. Among them, 1 patient died from complications of severe pancytopenia and 2 patients are alive with a late hematologic response.
Thirty-one patients received SCT from an alternative donor as follows: BMT from an HLA-matched unrelated donor (UBMT; n = 25), cord blood transplantation from an unrelated donor (UCBT; n = 2), and BMT from an HLA-mismatched family donor (n = 4). Twenty patients were conditioned with a CY, ATG, and TBI regimen and 4 received CY, Flu, ATG, and local field irradiation. Others received other types of conditioning regimen. Methotrexate and CyA were given for the prevention of GVHD in 5 patients. Tacrolimus was used instead of CyA in other patients. Five patients who had transformed to myelodysplastic syndrome (MDS) and 3 patients who were searching for an alternative donor still were excluded from the analysis.
In all, 52 patients were evaluated for response to second-line therapy. Characteristics of both groups are shown in Table 3. The median interval between the first course of IST and a second-line treatment was 7 months for the IST group and 8 months for the SCT group. At 6 months after the initiation of second IST, a trilineage response was seen in only 2 of 18 evaluable patients (11%). Among 16 nonresponders, 8 patients received UBMT as a third-line therapy and all of them are alive. They could not find a suitable donor at 6 months after initial therapy and received a second IST, but failed to respond. Marrow donors included HLA-one antigen mismatched unrelated donor (n = 3) and HLA serologically 6/6 matched unrelated donor (n = 5). Four patients attained late response and another 4 patients are alive with regular blood transfusions. Overall, 20 of 21 patients are alive with a median follow-up period of 66 months from the start of second IST (range: 9-80 months).
Characteristics of 52 patients who underwent second-line therapy
| . | SAA . | VSAA . |
|---|---|---|
| Patients | 21 | 31 |
| Sex, M/F | 14/7 | 14/17 |
| Median age, y (range) | 9 (2-17) | 8 (0-17) |
| Cause of AA | ||
| Idiopathic | 17 | 29 |
| Hepatitis | 4 | 2 |
| Severity of disease | ||
| SAA | 7 | 21 |
| VSAA | 14 | 10 |
| Median months from diagnosis to second-line therapy (range) | 7 (5-25) | 8 (5-20) |
| . | SAA . | VSAA . |
|---|---|---|
| Patients | 21 | 31 |
| Sex, M/F | 14/7 | 14/17 |
| Median age, y (range) | 9 (2-17) | 8 (0-17) |
| Cause of AA | ||
| Idiopathic | 17 | 29 |
| Hepatitis | 4 | 2 |
| Severity of disease | ||
| SAA | 7 | 21 |
| VSAA | 14 | 10 |
| Median months from diagnosis to second-line therapy (range) | 7 (5-25) | 8 (5-20) |
Data are numbers except where indicated.
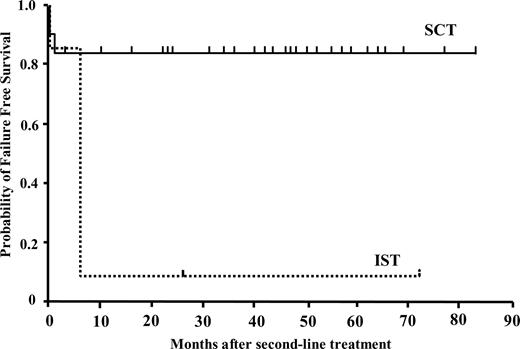
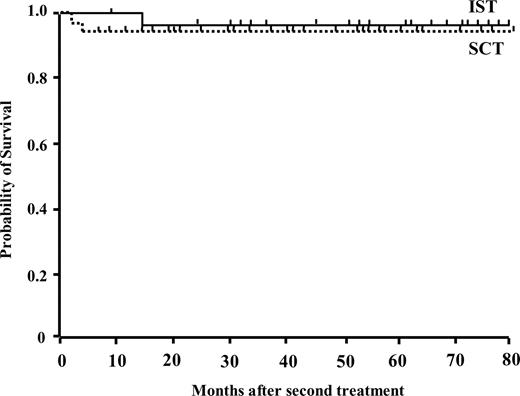
In the SCT group, 5 patients did not engraft. Bacterial or fungal infections resulted in the death of 2 patients at an early phase of SCT. One patient who received UCBT had recovery of autologous bone marrow function and is alive 68 months after the transplant. One patient transplanted from an HLA-mismatched sibling had a successful second transplant from an unrelated donor. Another patient who failed to engraft after UBMT was rescued by second transplant from an HLA-2 antigen mismatched mother. Blood count normalized in the remaining 26 patients and they are all alive. Four evaluable patients developed grade II to IV acute GVHD, and chronic GVHD was observed in 4 patients. Twenty-nine of 31 patients are alive with a median follow up period of 35 months from the alternative donor transplantation (range: 4-83 months). The probability of FFS was calculated after excluding deaths and patients failing to respond to a second-line treatment by 6 months and requiring further treatment, that is, including only patients who were alive with hematologic response. The estimated FFS at 5 years from the beginning of second-line therapy was 83.9% (± 16.1% SD) in the SCT group compared with 9.5% (± 9.0%) in the IST group (P = .001) (Fig 2). The overall survival rate was not different between the IST group (95.2 ± 6.7%) and the SCT group (93.5 ± 4.2%) after second-line treatment (Fig 3).
Actuarial probability of failure-free survival after second-line treatments with immunosuppressive therapy (n = 21) or stem-cell transplantation from an alternative donor (n = 31). FFS is defined as survival with response. Death, nonresponse by 6 months, disease progression requiring a second-line therapies, and relapse were considered as treatment failure.
Actuarial probability of failure-free survival after second-line treatments with immunosuppressive therapy (n = 21) or stem-cell transplantation from an alternative donor (n = 31). FFS is defined as survival with response. Death, nonresponse by 6 months, disease progression requiring a second-line therapies, and relapse were considered as treatment failure.
Actuarial probability of survival after second-line treatments with immunosuppressive therapy (n = 21) or stem cell transplantation from an alternative donor (n = 31).
Actuarial probability of survival after second-line treatments with immunosuppressive therapy (n = 21) or stem cell transplantation from an alternative donor (n = 31).
Cytogenetic analysis and clonal disease
At the time of diagnosis, a clonal cytogenetic abnormality (monosomy 7, trisomy 8) was detected in 2 patients, who had morphologically typical AA. The disappearance of monosomy 7 was observed in 1 patient,19 but trisomy 8 remained for 52 months after IST in another patient. New clonal cytogenetic abnormalities appeared in 10 patients after IST: monosomy 7 (5 patients), trisomy 8 (2 patients), trisomy 8 and del(7) (1 patient), monosomy X (1 patient), and t (3;3)(q21;q26) (1 patient). Eight patients underwent SCT from alternative donors and 6 of them are still alive.
Stem-cell transplantation
SCT was attempted in 52 patients in whom the initial IST failed (n = 31), the second IST failed (n = 8), who had relapse after initial response (n = 5), or who developed MDS and leukemia (n = 8). Alternative donors included unrelated bone marrow donors (n = 40), HLA-mismatched family donors (n = 6), and unrelated cord blood donors (n = 6). Five patients died: 3 received UCBT and 2 received UBMT. Causes of death were bacterial or fungal infections (n = 3), relapse of leukemia (n = 1), and venooclussive disease (n = 1).
Survival
We analyzed the actuarial survival of 201 enrolled patients according to the severity of their disease. The actuarial survival of all enrolled patients was 94.5% (± 1.7%) with a median follow-up period of 48 months (range: 12-90 months). The actuarial survival was 92.6% (± 2.8%) in the 117 patients with very severe AA and 96.8% (± 2.1%) in the 84 patients with severe AA. There were 6 deaths in the very severe AA group and 3 in the severe AA group. The causes of death were SCT-related toxicities (n = 5), MDS/acute myelogenous leukemia (AML) (n = 1), bacteremia (n = 1), hemolysis of unknown causes (n = 1), and aspiration pneumonia (n = 1).
Discussion
The introduction of intensive IST with ATG and CyA has dramatically improved the outcome of patients with severe and very severe AA.5-8 However, 30% to 40% of patients still fail to respond to IST and require second-line therapy. The treatment options for patients not responding to IST include further treatment with immunosuppressive agents or SCT from an alternative donor. At present, however, there is no consensus as to the best therapy for these patients. Recent studies have reported a high response rate and a favorable outcome after repeated ATG therapy in these patients, suggesting that SCT from an alternative donor should perhaps be considered third-line therapy.10,11 However, the majority of patients in these studies were adults. Because the outcome after alternative donor transplantation is better in children than in adults,20 the treatment choice may be different in children from in adults. Our prospective study showed that SCT from an alternative donor is superior to the repeated IST for FFS. At 6 months, the response rate to a second course of IST was only 11% (2/18), which increased to 33% (6/18) at 12 months, much lower than figures reported by others. The overall response was 63% in the Basel10 and 77% in the Italian11 studies. In addition, none of our patients achieved CR, whereas CR was achieved in 42% of patients in the Basel,10 and 30% in the Italian study.11 In the recent study from the National Institutes of Health, the overall response rate was 30%, although no one achieved a CR.12 The reasons for the discrepancy in the response rates among these studies are not known. However, there are a number of differences between our study (AA-97) and the others. First, our study group consisted of only pediatric patients, whereas other studies included both pediatric and adult patients. The median age of the patients was 9, 15, 18, and 31 years, in the AA-97, Basel, Italian, and National Institutes of Health studies, respectively. Until now, there have been no reports of repeated IST restricted to children with AA.
In the majority of patients with acquired AA, bone marrow failure is believed to result from immunologically mediated destruction of the hematopoietic progenitor cells.21 Whereas in some patients, a single course of ATG is not sufficient to achieve the degree of immunosuppression required to restore bone marrow function, necessitating further ATG therapy, the results of our study may indicate that pediatric patients are more susceptible than adult patients to the intensive IST currently used and that a single course of IST is adequate to discern their response to these immunosuppressive agents. Our results may also suggest that the efficacy of any immunosuppressive therapy for children with AA should be evaluated separately from adult patients.22
In the Italian study,11 the assessment of response to the first course of IST was carried out at 120 days and some patients received a second course of treatment as early as 2 months after initiation of immunosuppressive therapy. In our previous study (AA-92), we observed no further patient response to an initial course of IST after 6 months, thus making the time of assessment at 6 months.8 The timing of the evaluation of response to an initial course of IST is an important factor in determining the need for further treatment, making it difficult to compare the response rates to a second course of IST between our study and other studies.
In the AA-97 study, 31 severe and very severe patients who did not respond to immunosuppressive therapy received SCT from an alternative donor. Twenty-nine of these patients are alive with their bone marrow function restored. Importantly, all 26 engrafted patients are alive without failure. Of 2 patients who received UCBT, 1 died of fungal infection before engraftment, and the other reconstituted autologous bone marrow function. In a recent analysis of a large series of UCBT from the New York Blood Center, only 8 of 19 patients with severe AA engrafted after UCBT. The cohort of AA patients was among the group with the highest incidence of transplant-related mortality.23 Because of discouraging results in the early period, we thereafter recommend that UCBT not be used as a second-line therapy. In contrast, in our study, results after BMT from an unrelated donor were excellent. Twenty-four of the 25 patients are alive and well. The National Marrow Donor Program in the United States reported on the results of UBMT for IST-resistant AA patients.14 Fifty-one of 131 patients (39%) were alive at 11 to 94 months (median: 36 months) after transplantation. The major causes of death were graft failure and treatment-related events including GVHD and infections. Fifty-five patients were matched with donors using both serology and allele-level DRB1 typing; these patients had a survival rate of 56%. In a recent report from the Japan Marrow Donor Program, the overall survival rate for AA patients receiving HLA-matched unrelated BMT was 56%, with 81% survival in patients younger than 15 years and 32% survival in patients aged 16 and older.20 Therefore, younger patients clearly have a survival advantage after UBMT. Similarly, in our AA-92 study, 13 of 15 patients who failed IST and who were subsequently treated with UBMT are alive and well, with a median follow-up of 36 months.8 The duration of FFS of these pediatric patients with AA appeared to plateau at 2 years after SCT. Recently, 2 novel transplant regimens were reported: one from the United States and another from Europe.24,25 The first tested de-escalating doses of radiation from 6 Gy to 2 Gy. The best results were achieved with 2 Gy TBI. The European group designed a radiation-free preparative regimen consisted of fludarabine, cyclophosphamide, and ATG. The Japan Marrow Donor Program is now performing high-resolution HLA typing at the DNA level at loci A and B as well as DRB1. It is expected that more precise HLA matching between patient and donor will further improve the outcome for UBMT recipients.
On the other hand, IST appears to be associated with an increased risk of evolution of clonal diseases such as MDS and PNH.26,27 In our previous study, 11 of 50 children with AA that were treated with IST developed MDS/AML. None of the 48 patients who underwent SCT developed a clonal disorder.28 In the Basel study, clonal disease developed in 53% of patients who received multiple courses of IST.10 In the current study, there were 5 patients (8.3%) with MDS among 60 patients in whom initial IST was not effective, whereas no patient developed MDS/AML after SCT from an alternative donor treated as second-line therapy. This issue must be taken into consideration in the discussion of the appropriate second-line therapy for patients with AA.
Our study clearly demonstrates that SCT from an alternative donor provides a better chance of FFS than a second course of IST for children with AA who have failed to respond to an initial course of IST. Thus, we recommend SCT, and in particular BMT from an alternative donor, rather than a second course of IST as salvage therapy for these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.B., T.N., I.T., and S. K. designed the study. Y.K., H.Y., K.S., and S.K. analyzed results and wrote the manuscript. R.K., H.A., T.K., H.Y., M.T., H.M., A.O., A.M., Y. O., and S.O. enrolled the patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Kojima MD, PhD, Department of Pediatrics, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, 466-8550, Japan; e-mail: kojimas@med.nagoya-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal