Abstract
Safety and efficacy of the fully human anti-CD20 monoclonal antibody, ofatumumab, was analyzed in a multicenter dose-escalating study including 33 patients with relapsed or refractory chronic lymphocytic leukemia. Three cohorts of 3 (A), 3 (B), and 27 (C) patients received 4, once weekly, infusions of ofatumumab at the following doses: (A) one 100 mg and three 500 mg; (B) one 300 mg and three 1000 mg; (C) one 500 mg and three 2000 mg. Sixty-seven percent of the patients were Binet stage B, and the median number of previous treatments was 3. The maximum tolerated dose was not reached. The majority of related adverse events occurred at first infusion, and the number of adverse events decreased at each subsequent infusion. Seventeen (51%) of 33 patients experienced infections, 88% of them of grade 1-2. One event of interstitial pneumonia was fatal; all other cases resolved within one month. The response rate of cohort C was 50% (13/26), one patient having a nodular partial remission and 12 patients partial remission. In conclusion, ofatumumab was found to be well tolerated in patients with chronic lymphocytic leukemia (CLL) in doses up to 2000 mg. Preliminary data on safety and objective response are encouraging and support further studies on the role of ofatumumab in CLL patients. This trial was registered at www.clinicaltrials.gov as no. NCT00093314.
Introduction
The clinical course of chronic lymphocytic leukemia (CLL) is often indolent, but the disease remains incurable.1,2 Symptomatic patients have a survival between 1 and 7 years, even with the best therapeutic approaches.2,3 Initial therapeutic options for patients with stage B or C are chlorambucil, fludarabine alone (F regimen), fludarabine combined with cyclophosphamide (FC regimen), or fludarabine with rituximab (FC R regimen).3-5 Several phase 2 studies have shown an improvement in response rates and progression-free survival when chemotherapy is combined with rituximab.6 7 However, all patients eventually experienced disease progression, and there are then limited therapeutic options, especially for patients who are refractory to fludarabine.8
Two monoclonal antibodies (MAbs) have been widely used for progressing patients: rituximab and alemtuzumab. Rituximab alone is not widely used because of its low response rate in pretreated and untreated patients.9 Alemtuzumab yields good response rates but is also associated with high toxicity, particularly opportunistic infections.10,11 Although a number of combination regimens were evaluated for relapsed and refractory patients, at present no regimen has been shown to be convincingly superior.
There is growing evidence that MAb therapeutic activity in vivo is dependent upon complement activation and subsequent complement-dependent cytotoxicity (CDC).12,13 Effective CDC might be dependent on the distance between the plasma membrane and the constant parts of the sensitizing antibody.14 This would explain why MAb directed at membrane proximal targets such as CD20 and CD52 are more effective than those targeted at more extended yet equally expressed molecules such as CD45.15 Another property that can substantially affect the ability of a MAb to induce potent CDC is whether it is able to redistribute the target antigen into Triton X-100–insoluble areas of the plasma membrane.16 Rituximab and ofatumumab both target CD20 and show equivalent performance in the Tx100 insolubility assay.16
Ofatumumab is a new fully human anti-CD20 MAb with higher in vitro efficacy on CLL cells.16 Ofatumumab is able to lyse rituximab-resistant Raji cells, which express low levels of CD20 and high levels of complement-regulatory proteins CD55 and CD59. Ofatumumab can also lyse CD20 low-expressing CLL cells in the presence of human plasma or unfractionated blood. It is hypothesized that ofatumumab efficacy in these cases is due to a slower off-rate and more stable CD20 binding in comparison with rituximab.16 Ofatumumab targets a different epitope than rituximab of the CD20 antigen and this may explain some of the difference between rituximab and ofatumumab.17 Because of these properties, ofatumumab might be an effective treatment for relapsed or refractory patients who have already been treated with rituximab. We present here the safety and efficacy results of the first study of ofatumumab in patients with relapsed or refractory CLL.
Methods
Patients
All patients had relapsing or refractory CLL with circulating lymphocyte count more than 5 × 109/L (5000/μL) and were 18 years or older with a life expectancy greater than 3 months. Circulating lymphocytes in the screening blood sample had to be CD5+, CD20+, and CD23+. The main exclusion criteria were significant acute or chronic comorbidity; treatment with rituximab, alemtuzumab, or autologous stem-cell transplantation within the last 6 months; and previous treatment with allogeneic stem cell transplantation. Patients with known positivity for hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV) were excluded. Furthermore, patients with Richter syndrome, platelet count lower than 75 × 109/L (75 000/μL), neutrophil count lower than 1.5 × 109/L (1500/μL), creatinine level higher than 1.5 times the upper normal limit (UNL), bilirubin level higher than 1.5 UNL, and transaminases or alkaline phosphatases higher than 2.5 UNL were also excluded. The study was performed in accordance with Good Clinical Practices and local regulatory requirements. The study protocol was approved by the appropriate national authorities and institutional review boards/ethics committees of each participating institution (see affiliations list). Each patient gave written informed consent in accordance with the Declaration of Helsinki to participate.
Study design
This open-label phase 1/2 study was performed between September 2004 and April 2006. Twelve sites have included patients: Denmark (3 sites), France (2 sites), the Netherlands (1 site), Poland (5 sites), and United States (1 site). The primary objective was to evaluate the safety and efficacy of ofatumumab in patients with refractory or relapsing CLL. Patients received 4 weekly infusions of ofatumumab at 3 different doses. The 3 patients in cohort A received a first infusion of 100 mg ofatumumab and 3 subsequent infusions of 500 mg. The 3 patients of cohort B received a first infusion of 300 mg and 3 subsequent infusions of 1000 mg. And the 27 patients of cohort C received a first infusion of 500 mg and 3 subsequent infusions of 2000 mg. Before each infusion, patients received 1 g oral paracetamol (acetaminophen) 30 to 60 minutes prior to treatment and intravenous antihistamine 30 minutes prior to treatment. Before the first and second infusion, patients received intravenous glucocorticoid equivalent to 50 mg prednisolone 30 minutes prior to treatment. The intravenous glucocorticoid dose was repeated before the third and fourth infusion for patients who had an infusion-related adverse event grade 3 or more at the previous infusion. Inclusion in cohorts B and C was done when the 3 patients in the previous cohort had received the 4 infusions and had satisfactory safety evaluations after 1 week of follow-up. During the treatment phase, the patients were followed for safety assessments, and samples for efficacy measurements (flow cytometric analyses) and pharmacokinetics were obtained. No infection prophylaxis was sched-uled in the protocol.
In case of adverse events during infusion, the infusion rate was temporarily decreased or halted according to the investigator's judgment and the patients were treated with best clinical practice. If the patient showed signs of cytokine release syndrome with beginning signs of breathing difficulty, the infusion had to be stopped immediately and the patient treated according to best clinical practice. Whether to resume the infusion upon the patient's complete recovery was to be decided by the investigator. In case of very slow infusion rates due to infusion-related adverse events, intravenous glucocorticoids equivalent to 50 mg prednisolone could be given. Low infusion rates resulting in overnight stay at the hospital were not considered a serious adverse event (SAE).
Safety assessments
Adverse events (AEs) were reported throughout the study period and graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (Bethesda, MD). Related grade 3 AEs and serious AEs (SAEs) were followed until resolved or stable. Biochemistry and hematology parameters were assessed from blood samples on days 1, 3, 5, 22, and 25. Vital signs and electrocardiogram (ECG) were monitored regularly. Flow cytometry at screening and at week 4 was performed to analyze CD3+-, CD4+-, CD8+-, CD16+-, and CD56+-expressing cells to follow whether T-cell subpopulations or natural killer (NK) cells were affected by treatment with ofatumumab. The formation of human antihuman antibodies against ofatumumab was assessed at baseline and at end of study by an enzyme-linked immunosorbent assay.
Efficacy assessments
The primary efficacy variable was objective response over the period from screening to week 19 and was reported according to the NCI-Working Group 1988 and 1996 criteria as complete remission (CR), nodular partial remission (nPR), partial remission (PR), progressive disease (PD), stable disease (SD), and nonevaluable patients (NEs).18,19 Response was assessed on the following parameters: (a) lymph node size measured as the sum of the product of diameters (SPDs) of the largest palpable nodes in each of the following sites: cervical, axillary, supraclavicular, inguinal, and femoral; lymph nodes larger than 1 cm were included; (b) hepatomegaly and splenomegaly were measured in centimeters under the costal margin; (c) hemoglobin level, and platelet, lymphocyte, and neutrophil counts; (d) unilateral bone marrow aspirate and biopsy were performed at week 19 or 27 for patients with blood lymphocyte count less than 4.5 × 109/L (4500/μL); (e) computed tomography (CT) scans of neck, thorax, abdomen, and pelvis were performed before treatment and at week 19 or 27 depending on the date of the clinical response. CT scans were used to confirm the response in responding patients.
The secondary efficacy variables were overall tumor response up to week 27; CLL cells (CD5+CD20+ and CD5+CD19+) and normal B-cell (CD20+CD5−) levels in peripheral blood assessed by flow cytometry; time to progression; duration of response; and time to next anti-CLL therapy.
Statistical analyses
Under the assumption that 50% of the patients would achieve an objective response, a 2-sided 95% confidence interval using the large sample normal approximation for the proportion of patients achieving an objective response was 31% to 69%. The lower limit was therefore higher than 30%, which was considered to be the lowest response level that was clinically relevant. Based on this, 26 patients were planned for cohort C. However, 1 patient had already signed the informed consent and commenced screening when the target of 26 patients was reached, and therefore a total of 27 patients were included in the study. The efficacy analysis was performed on all patients exposed to the study drug in group C (full analysis population).
The primary efficacy end point was objective tumor response over the period from screening to week 19. Overall tumor response was assessed on data from physical examinations and evaluation of the peripheral blood and bone marrow as defined by NCI-WG Guidelines in CLL.18,19 According to these criteria, the response is required to last for a period of 2 months. Therefore clinical response first obtained at week 11 or 19 had to be confirmed at week 19 or 27, respectively. The number of patients achieving objective tumor response over the period from screening to week 19 was derived for cohort C and presented together with 95% confidence interval. The confidence interval was derived for the full analysis population (primary analysis) and for the per protocol population (secondary analysis). Time to progression was defined as the number of days from day 0 to first disease progression or death related to CLL. Response duration was defined as number of days from first documentation of response (defined as CR, nPR, or PR) to first disease progression. Time to next treatment was defined as days from day 0 to the date when any other anti-CLL therapy is started.
Results
Forty-two patients were screened, and 9 failed screening; thus, 33 patients took part in the study. Maximum tolerated dose was not reached. Three patients were allocated to each of cohorts A and B and 27 patients to cohort C. All patients completed treatment except one patient in cohort C who was withdrawn after the first infusion due to drug-related SAE.
The patient population is described in Table 1. Median age was 61 years, ranging from 27 to 82 years. Median time from diagnosis of CLL to study was 6.3 years (range, 1.2-14 years), and median time since latest CLL treatment was 0.8 years (range, 0.1-5.3 years). Median number of prior treatments was 3 (range, 1-9). Most patients had Rai stage I or II (39% and 45%, respectively) or Binet stage A or B (21% and 67%, respectively) at time of study entry.
Demographics and baseline characteristics
| Parameters . | Median . |
|---|---|
| Demography | |
| Age, y (min-max) | 61 (27-82) |
| Male, no. (%) | 19 (58%) |
| White, no. (%) | 33 (100%) |
| Disease characteristics (min-max) | |
| Duration of CLL, y | 6.3 (1.2-14) |
| Time from latest treatment, y | 0.8 (0.1-5.3) |
| No. of previous treatments | 3 (1-9) |
| Modified Rai stage at screening, no. (%) | |
| 0 | 1 (3) |
| I | 13 (39) |
| II | 15 (45) |
| III | 1 (3) |
| IV | 3 (9) |
| Modified Binet stage at screening, no. (%) | |
| A | 7 (21) |
| B | 22 (67) |
| C | 4 (12) |
| Constitutional symptoms, no. (%) | |
| Extreme fatigue | 2 (6) |
| Night sweats | 5 (15) |
| Fever | 0 |
| Weight loss | 0 |
| Hematologic symptoms, no. (%) | |
| Anemia, hemoglobin level less than 10 g/L | 7 (21) |
| Neutropenia, neutrophil count less than 1.5×109/L | 0 (0) |
| Thrombocytopenia, platelet count less than 120×109/L | 9 (27) |
| Lymphocytes/109/L (min-max) | 51.3 (8.2-227.2) |
| LDH level above normal value, no. (%) | 7 (21) |
| Lymph nodes, no. (%) | |
| None | 5 (15) |
| More than 5 cm in diameter | 4 (12) |
| SPD more than 50 cm2; | 4 (12) |
| Splenomegaly, no. (%) | |
| No | 22 (67) |
| More than 5 cm below diaphragm | 2 (6) |
| Parameters . | Median . |
|---|---|
| Demography | |
| Age, y (min-max) | 61 (27-82) |
| Male, no. (%) | 19 (58%) |
| White, no. (%) | 33 (100%) |
| Disease characteristics (min-max) | |
| Duration of CLL, y | 6.3 (1.2-14) |
| Time from latest treatment, y | 0.8 (0.1-5.3) |
| No. of previous treatments | 3 (1-9) |
| Modified Rai stage at screening, no. (%) | |
| 0 | 1 (3) |
| I | 13 (39) |
| II | 15 (45) |
| III | 1 (3) |
| IV | 3 (9) |
| Modified Binet stage at screening, no. (%) | |
| A | 7 (21) |
| B | 22 (67) |
| C | 4 (12) |
| Constitutional symptoms, no. (%) | |
| Extreme fatigue | 2 (6) |
| Night sweats | 5 (15) |
| Fever | 0 |
| Weight loss | 0 |
| Hematologic symptoms, no. (%) | |
| Anemia, hemoglobin level less than 10 g/L | 7 (21) |
| Neutropenia, neutrophil count less than 1.5×109/L | 0 (0) |
| Thrombocytopenia, platelet count less than 120×109/L | 9 (27) |
| Lymphocytes/109/L (min-max) | 51.3 (8.2-227.2) |
| LDH level above normal value, no. (%) | 7 (21) |
| Lymph nodes, no. (%) | |
| None | 5 (15) |
| More than 5 cm in diameter | 4 (12) |
| SPD more than 50 cm2; | 4 (12) |
| Splenomegaly, no. (%) | |
| No | 22 (67) |
| More than 5 cm below diaphragm | 2 (6) |
Safety results
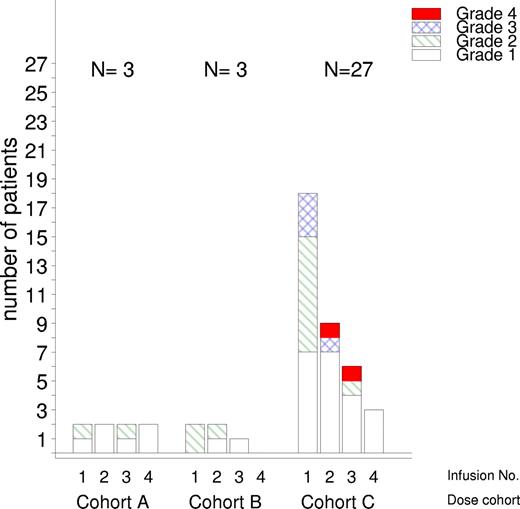
Of the 33 patients enrolled in the study, 32 received all 4 infusions. One patient withdrew because of a SAE the day after the first infusion (cytolytic hepatitis, grade 3). This patient, who also had temporarily increased liver enzymes before enrollment, completely recovered after 3 days. Twenty-seven patients reported 246 AEs of which 92% were mild (grade 1-2) and only 19 AEs were grade 3 or 4 (Table 2). Ten of the AEs were serious (Table 3). Of all AEs, 61% (150/246) were assessed as related to the treatment. The majority of AEs, 138 (56%), were reported on an infusion day, and of these 84 (61%) were reported on the day of the first infusion. The infusion-related events usually occurred within the first few hours after starting the infusion with the most common symptoms being transient rigors, pyrexia, fatigue, rash, and increased sweating. Infusion-related AEs tended to decrease in number and in intensity for subsequent infusion days (Figure 1).
Summary of toxicity grades 3 and 4 according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0
| MedDRA System Organ Class preferred term . | No. of patients (%) . | No. of events . |
|---|---|---|
| Total no. of subjects with grade 3 or 4 adverse events | 10 (30) | 18 |
| Blood and lymphatic system disorders | 4 (12) | 7 |
| Thrombocytopenia | 3 (9) | 4 |
| Neutropenia | 2 (6) | 2 |
| Anemia NOS | 1 (3) | 1 |
| Infections and infestations | 3 (9) | 3 |
| Herpes zoster | 1 (3) | 1 |
| Nasopharyngitis | 1 (3) | 1 |
| Pneumonia NOS | 1 (3) | 1 |
| General disorders and administration site conditions | 2 (6) | 3 |
| Pyrexia | 2 (6) | 2 |
| Pain NOS | 1 (3) | 1 |
| Nervous system disorders | 2 (6) | 2 |
| Carotid artery stenosis | 1 (3) | 1 |
| Dizziness | 1 (3) | 1 |
| Cardiac disorders: angina pectoris | 1 (3) | 1 |
| 1 (3) | 1 | |
| Hepatobiliary disorders: cytolytic hepatitis | 1 (3) | 1 |
| 1 (3) | 1 | |
| Respiratory, thoracic, and mediastinal disorders: hypoxia | 1 (3) | 1 |
| 1 (3) | 1 |
| MedDRA System Organ Class preferred term . | No. of patients (%) . | No. of events . |
|---|---|---|
| Total no. of subjects with grade 3 or 4 adverse events | 10 (30) | 18 |
| Blood and lymphatic system disorders | 4 (12) | 7 |
| Thrombocytopenia | 3 (9) | 4 |
| Neutropenia | 2 (6) | 2 |
| Anemia NOS | 1 (3) | 1 |
| Infections and infestations | 3 (9) | 3 |
| Herpes zoster | 1 (3) | 1 |
| Nasopharyngitis | 1 (3) | 1 |
| Pneumonia NOS | 1 (3) | 1 |
| General disorders and administration site conditions | 2 (6) | 3 |
| Pyrexia | 2 (6) | 2 |
| Pain NOS | 1 (3) | 1 |
| Nervous system disorders | 2 (6) | 2 |
| Carotid artery stenosis | 1 (3) | 1 |
| Dizziness | 1 (3) | 1 |
| Cardiac disorders: angina pectoris | 1 (3) | 1 |
| 1 (3) | 1 | |
| Hepatobiliary disorders: cytolytic hepatitis | 1 (3) | 1 |
| 1 (3) | 1 | |
| Respiratory, thoracic, and mediastinal disorders: hypoxia | 1 (3) | 1 |
| 1 (3) | 1 |
Serious adverse events (SAEs) observed in the 3 cohorts
| . | No. of patients, n=33 . | No. of SAEs . |
|---|---|---|
| Totals of subjects and events | 9 | 10 |
| Herpes zoster | 1 | 1 |
| Pneumonia | 1 | 1 |
| Sinusitis | 1 | 1 |
| Neutropenia | 2 | 2 |
| Hemolytic anemia | 1 | 1 |
| Angina pectoris | 1 | 1 |
| Cytolytic hepatitis | 1 | 1 |
| Carotid artery stenosis | 1 | 1 |
| Interstitial lung disease | 1 | 1 |
| . | No. of patients, n=33 . | No. of SAEs . |
|---|---|---|
| Totals of subjects and events | 9 | 10 |
| Herpes zoster | 1 | 1 |
| Pneumonia | 1 | 1 |
| Sinusitis | 1 | 1 |
| Neutropenia | 2 | 2 |
| Hemolytic anemia | 1 | 1 |
| Angina pectoris | 1 | 1 |
| Cytolytic hepatitis | 1 | 1 |
| Carotid artery stenosis | 1 | 1 |
| Interstitial lung disease | 1 | 1 |
Seventeen patients (51%) reported infections. The most frequently observed infection was nasopharyngitis (8/25 infectious AEs) with a median onset 139 days after first infusion (range, 10-354 days). All but one patient recovered within a month. The majority of the infections were of grade 1 or 2, whereas 3 events were grade 3: herpes zoster (SAE), nasopharyngitis, pneumonia (SAE), and 1 was fatal (infectious interstitial lung disease). In addition, another patient had a grade 2 event of sinusitis that was reported as an SAE.
Hematologic toxicity was reported in 15% of patients (8 events in 5 patients). Four of those AEs were considered related to the treatment: 2 cases of neutropenia and 2 thrombocytopenias. The 2 neutropenias were grade 4 and reported as SAEs. The 2 patients with thrombocytopenia had entered the study with medical histories of thrombocytopenia that aggravated to grade 3 during the study. Three patients had recovered by the end of the reporting period. One CTC grade 2 SAE of hemolytic anemia was reported in a 50-year-old man who was hospitalized for transfusions. This patient had a history of fludarabine-induced hemolytic anemia, and the event was considered not related to study drug. For hemoglobin, a modest downward shift within the normal range was observed during treatment.
In total, 9 patients reported 10 SAEs (Table 2) during the study, of which 5 were considered treatment related: herpes zoster (1 patient), neutropenia (2 patients), interstitial lung disease (1 patient), and cytolytic hepatitis (1 patient). The event of infectious interstitial lung disease was fatal. The patient, an 82-year-old man, had previously had 4 lines of treatment for CLL and had profound chronic hypogammaglobulinemia. He had 3 episodes of interstitial pneumonia the previous year. Although the event was most likely caused by the underlying disease, a contribution of the study drug could not be completely excluded. The event of cytolytic hepatitis occurred in a 74-year-old male patient who showed an increase in hepatic enzymes during routine examination the day of first infusion. The patient had elevated liver parameters before enrollment; he fully recovered from the event. In addition, 2 events with fatal outcome were reported after the end of the study. One patient who was withdrawn from treatment due to disease progression and had started chemotherapy died of pneumonia 4 months after the end of ofatumumab treatment. One patient died of disease progression 11 months after final treatment. Both events were considered unrelated to ofatumumab treatment.
Mean serum immunoglobulin A (IgA) and IgG remained stable, within or slightly lower than the normal range. Mean serum IgM tended to decrease to lower than normal during the study. Overall, more than half of the patients did not show any change in terms of T cells or NK cells. Human antihuman antibodies were not detected in any patient.
Efficacy results
The primary efficacy variable, objective response over the period from screening to week 19, is presented in Table 4. The remission rate in cohort C was 50% (95% confidence interval [CI]: 30%-70%). There were no complete remissions, but in cohort A 1 patient reached a PR and in cohort C 1 patient had a nPR and 12 patients had PR. One cohort C patient presenting with PR showed all features of nPR, except that CT scanning identified residual lymphadenopathy. In cohort C, 62% (16/26) obtained response as evaluated by physical examination and peripheral blood 4 weeks after commencing treatment. Three patients did not respond until week 7 or 11. Not all of these responses were sustained, and at week 19 the number of patients with sustained response was limited to 9. Of the patients previously treated with rituximab (7), alemtuzumab (6), and/or fludarabine (20), 7 responded to ofatumumab treatment. Two patients maintained their response to week 27, while the remaining evaluable patients had progressive disease by this time with a median progression-free survival of 106 days but a median time to next treatment of 1 year.
Primary end point: objective response from screening to week 19
| . | Cohort A, n = 3 . | Cohort B, n = 3 . | Cohort C, n = 27 . | Total, n = 33 . |
|---|---|---|---|---|
| Not evaluable | 0 | 0 | 1 | 1 |
| Objective response (%) | 1 (33) | 0 | 13 (50) | 14 (44) |
| 95% CI, % | 1-91 | — | 30-70 | 29-67 |
| . | Cohort A, n = 3 . | Cohort B, n = 3 . | Cohort C, n = 27 . | Total, n = 33 . |
|---|---|---|---|---|
| Not evaluable | 0 | 0 | 1 | 1 |
| Objective response (%) | 1 (33) | 0 | 13 (50) | 14 (44) |
| 95% CI, % | 1-91 | — | 30-70 | 29-67 |
— indicates not applicable.
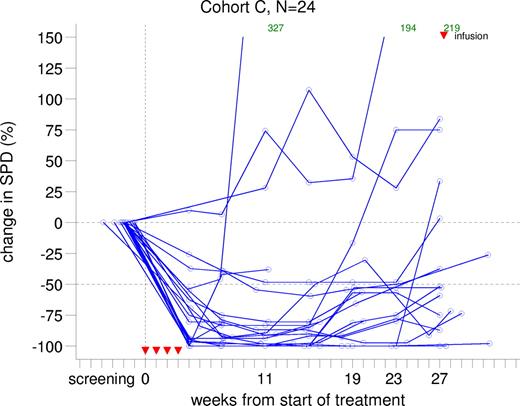
Most patients in cohort C showed more than 50% decrease in lymph node size from week 4. This shrinkage was sustained until week 15 after which SPD gradually progressed. A subset of patients demonstrated a sustained, reduced lymph node size until week 27, the end of the reporting period. Lymph node size over time for cohort C patients is presented in Figure 2.
Change from baseline in sum of products of greatest lymph node diameters in cohort C.
Change from baseline in sum of products of greatest lymph node diameters in cohort C.
Hematology parameters may reflect efficacy if they improve on treatment. Of the 7 anemic patients (hemoglobin level < 120 g/L [12 g/dL]) at baseline, 6 had an improvement (1 in cohort B and 5 in cohort C). Of the 9 patients with thrombocytopenia at baseline, 8 improved on therapy (2 in cohort A, 1 in cohort B, and 5 in cohort C). Lymphocyte, neutrophil, and platelet counts over time for cohort C are presented in Table 5.
Hemoglobin level, neutrophil count, and platelet count at screening, during treatment, and after treatment
| . | Screening . | Time from screening . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 7 . | Wk 11 . | Wk 15 . | Wk 19 . | Wk 23 . | Wk 27 . | Mo 9 . | Mo 12 . | ||
| Hemoglobin level, g/L | |||||||||||||
| N | 33 | 33 | 32 | 32 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 131 | 122.5 | 123 | 123 | 125 | 129 | 131 | 135 | 138 | 141 | 137 | 145 | 140 |
| Median | 135 | 123 | 122 | 121 | 124 | 130 | 133 | 135 | 138 | 139 | 132 | 147 | 140 |
| Minimum | 96 | 79 | 100 | 91 | 94 | 109 | 100 | 107 | 108 | 113 | 106 | 121 | 122 |
| Maximum | 154 | 151 | 146 | 146 | 159 | 150 | 162 | 168 | 174 | 169 | 165 | 175 | 156 |
| Neutrophil count,109/L | |||||||||||||
| N | 33 | 33 | 32 | 32 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 16.12 | 4.63 | 3.89 | 4.04 | 3.43 | 3.43 | 3.62 | 6.27 | 4.47 | 3.97 | 4.11 | 4.51 | 4.34 |
| Median | 6.20 | 3.50 | 3.15 | 2.80 | 2.95 | 3.15 | 2.90 | 4.00 | 3.90 | 3.65 | 3.50 | 4.20 | 3.95 |
| Minimum | 1.60 | 0.00 | 0.60 | 0.10 | 0.10 | 0.20 | 0.80 | 1.60 | 1.10 | 1.70 | 1.40 | 1.60 | 2.90 |
| Maximum | 147.1 | 19.30 | 15.20 | 33.50 | 16.50 | 7.90 | 16.00 | 32.60 | 21.40 | 8.20 | 12.50 | 8.40 | 6.00 |
| Platelet count, 109/L | |||||||||||||
| N | 33 | 33 | 32 | 31 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 152 | 158 | 168 | 168 | 179 | 182 | 177 | 172 | 179 | 181 | 170 | 185 | 180 |
| Median | 145 | 163 | 170 | 155 | 171 | 172 | 169 | 161 | 169 | 175 | 180 | 190 | 173 |
| Minimum | 69 | 48 | 41 | 75 | 84 | 68 | 49 | 50 | 47 | 104 | 45 | 142 | 132 |
| Maximum | 279 | 312 | 329 | 291 | 311 | 348 | 352 | 368 | 374 | 307 | 271 | 219 | 227 |
| . | Screening . | Time from screening . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 1 . | Wk 2 . | Wk 3 . | Wk 4 . | Wk 7 . | Wk 11 . | Wk 15 . | Wk 19 . | Wk 23 . | Wk 27 . | Mo 9 . | Mo 12 . | ||
| Hemoglobin level, g/L | |||||||||||||
| N | 33 | 33 | 32 | 32 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 131 | 122.5 | 123 | 123 | 125 | 129 | 131 | 135 | 138 | 141 | 137 | 145 | 140 |
| Median | 135 | 123 | 122 | 121 | 124 | 130 | 133 | 135 | 138 | 139 | 132 | 147 | 140 |
| Minimum | 96 | 79 | 100 | 91 | 94 | 109 | 100 | 107 | 108 | 113 | 106 | 121 | 122 |
| Maximum | 154 | 151 | 146 | 146 | 159 | 150 | 162 | 168 | 174 | 169 | 165 | 175 | 156 |
| Neutrophil count,109/L | |||||||||||||
| N | 33 | 33 | 32 | 32 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 16.12 | 4.63 | 3.89 | 4.04 | 3.43 | 3.43 | 3.62 | 6.27 | 4.47 | 3.97 | 4.11 | 4.51 | 4.34 |
| Median | 6.20 | 3.50 | 3.15 | 2.80 | 2.95 | 3.15 | 2.90 | 4.00 | 3.90 | 3.65 | 3.50 | 4.20 | 3.95 |
| Minimum | 1.60 | 0.00 | 0.60 | 0.10 | 0.10 | 0.20 | 0.80 | 1.60 | 1.10 | 1.70 | 1.40 | 1.60 | 2.90 |
| Maximum | 147.1 | 19.30 | 15.20 | 33.50 | 16.50 | 7.90 | 16.00 | 32.60 | 21.40 | 8.20 | 12.50 | 8.40 | 6.00 |
| Platelet count, 109/L | |||||||||||||
| N | 33 | 33 | 32 | 31 | 32 | 30 | 30 | 27 | 23 | 20 | 18 | 7 | 8 |
| Mean | 152 | 158 | 168 | 168 | 179 | 182 | 177 | 172 | 179 | 181 | 170 | 185 | 180 |
| Median | 145 | 163 | 170 | 155 | 171 | 172 | 169 | 161 | 169 | 175 | 180 | 190 | 173 |
| Minimum | 69 | 48 | 41 | 75 | 84 | 68 | 49 | 50 | 47 | 104 | 45 | 142 | 132 |
| Maximum | 279 | 312 | 329 | 291 | 311 | 348 | 352 | 368 | 374 | 307 | 271 | 219 | 227 |
Posttreatment bone marrow examination was performed on 11 patients in cohort C. In the 8 patients who responded to treatment, the median percentage of marrow lymphocytes was 78% (range, 30%-90%) before treatment and 50% (range, 5%-75%) after treatment. After treatment, 3 patients had less than 30% lymphocytes (5%, 10%, and 25%) but all had a nodular growth pattern.
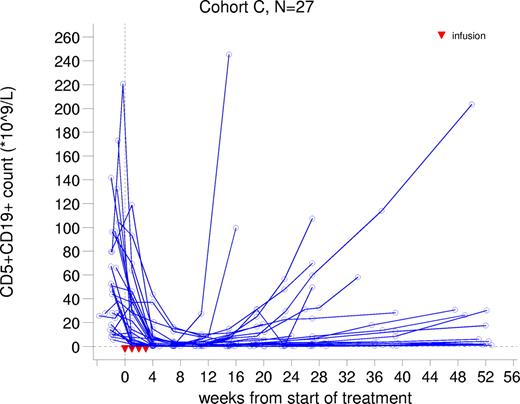
In cohort C, a median 55% reduction in malignant CD5+CD19+ B cells in peripheral blood was observed after the first infusion. After the fourth infusion, the median percentage reduction from baseline was 97% ranging from 15% to 100% (Figure 3). Observations were similar for CD5+CD20+ B cells. Normal B cells were also depleted. For the majority patients, this depletion was sustained until approximately week 24, after which the number of B cells gradually rose.
Discussion
This study presents the results of ofatumumab, a fully human anti-CD20 monoclonal antibody, in CLL patients and shows a response (PR or nPR) in half of the patients treated in the phase 2 part. This is superior to the results obtained with rituximab in the same setting even if it is very difficult to compare patients' status and probability of response in different phase 1/2 studies. Huhn et al obtained a response in 7 (25%) of the 28 treated patients.20 Itala et al obtained a response in 8 (35%) of the 24 treated patients.21 Response rates increased to 58% with CR in untreated patients with CLL.22 Response rates increased to 45% with rituximab alone in relapsing patients when rituximab was given 3 times a week.9 Similarly, O'Brien et al found a correlation between dose and response in CLL patients treated with rituximab alone: 22% for patients treated at 500 to 825 mg/m2, 43% for those treated at 1000 to 1500 mg/m2, and 75% for those treated at the highest dose of 2250 mg/m2.23 Median duration of response was short in all these studies, except in the O'Brien et al study, ranging from 10 to 20 weeks in patients treated with standard dose of rituximab but longer (8 months) in patients receiving higher dose of rituximab. In our series, median PFS was 15 weeks.
In vitro, compared with rituximab at the same concentration, ofatumumab has a greater activity on CLL cells for CDC, and this was related to a lower off-rate of the Ig from the cell surface.16 Ofatumumab also targets a different epitope of the CD20 molecule and this is also correlated to the CDC activity.17 So, there are several biologic reasons that may explain the greater activity in patients on ofatumumab compared with rituximab. However, the efficacy of rituximab was determined on its use in thousands of patients and this relatively small phase 1/2 study is not sufficient to definitively conclude that ofatumumab is superior. Only 7 patients had previously received rituximab, alone or in combination with chemotherapy, and 1 of them reached a PR after ofatumumab.
These results showed a clear relationship between dose and efficacy with only one responder among cohorts A and B and few improvements in lymphocytes counts or SPD in these 2 cohorts with lower dose. In another phase 1/2 study with ofatumumab in patients with follicular lymphoma, the dose effect tested between 300 mg and 1000 mg flat dose was not evident as described for rituximab in follicular lymphoma patients.24 Can we conclude that the higher efficacy of ofatumumab in CLL patients compared with rituximab is related to the higher dose of the antibody used? Only a comparison study with rituximab will allow conclusions to be drawn, but the in vitro data are clearly in favor of a superior efficacy of ofatumumab in relapsing CLL patients.
Of the 33 patients enrolled in the trial, 32 received all 4 infusions. One patient was withdrawn from treatment after the first infusion due to a grade 3 cytolytic hepatitis. Almost all (92%) reported AEs were of CTC grade 1 or 2, and most were observed during or just after the first infusion. Thus, these AEs were not completely prevented by the use of premedication regimen of paracetamol, antihistaminic, and glucocorticoids. The most common infusion-related symptoms were transient rigors, pyrexia, fatigue, rash, and increased sweating, which probably resulted from the cytokine-release syndrome to be expected with anti-CD20 treatment in CLL patients. Infusion-related AEs reduced in number and intensity at each subsequent infusion. This toxicity is similar to the one described with rituximab in CLL patients.25
Infection due to immunosuppression is a concern when depleting a patient's normal B cells, and 51% of our patients had at least one infection. The majority of the infections were of grade 1 or grade 2 intensity, and nasopharyngitis was the most frequently observed infectious event. One fatal event of infectious interstitial lung disease was seen in a patient with 4 previous lines of treatment and profound chronic hypogammaglobulinemia. The infection rate compares favorably with those seen with other treatments such as fludarabine or alemtuzumab in CLL patients.11,26,27 Cohort C mean serum IgA and IgG remained stable despite the long-lasting reduction in the number of peripheral CD20+ B cells. Mean serum IgM level tended to decrease lower than normal during the study, which is expected with long-lasting depletion of normal B cells.28 T-cell counts (CD3, CD4+CD8−, or CD4−CD8+) and NK cells remained within normal ranges for all patients.
There were 8 events (3% of total) of hematologic toxicity. A grade 2 hemolytic anemia occurred in a patient with a known fludarabine-induced hemolytic anemia. Worsening of thrombocytopenia was observed in 4 events in 3 patients who had a medical history of thrombocytopenia. One of these patients also had neutropenia (SAE) and anemia. One additional SAE of neutropenia was recorded. These cytopenias are common in patients with CLL, and a relationship with ofatumumab is not certain. However, such cytopenias have been described with rituximab in lymphoma patients; their mechanisms are not completely understood.29 Flow cytometry results showed that ofatumumab did result in the rapid and prolonged depletion of circulating normal B lymphocytes in all patients. Serial evaluation demonstrated that recovery to normal levels was not observed until 5 to 6 months after completion of therapy, and in a subset of patients the depletion was sustained until the end of the study period, month 12. These results are similar to what was described with rituximab.25
In the present study, ofatumumab, a fully human anti-CD20 monoclonal antibody, was found to be well tolerated in patients with CLL in doses up to 2000 mg. Preliminary data on objective response are encouraging and support further studies on the role of ofatumumab in CLL patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.C., M.F., J.P. conceived and designed the study; B.C., S.L., L.M.,P., O.G., H.F., M.H.J.O., J.W., J.K., J.H., A.H., J.W., and T.R. provided study materials or patients; M.F. and J.P. collected and assembled data; B.C., M.F., and J.P. analyzed and interpreted data; B.C. wrote the paper; B.C., S.L., L.M.P., O.G., H.F., M.H.J.O., J.W., J.K., J.H., A.H., J.W., M.F., J.P., and T.R. approved the final paper.
Conflict-of-interest disclosure: M.F. and J.P. are employees of Genmab. B.C. was a Consultant with Genmab, Roche, and Genentech before this study. The remaining authors declare no competing financial interests.
Correspondence: Bertrand Coiffier, Département d'Hématologie, Centre Hospitalier Lyon Sud, Pierre-Benite Cedex, France; e-mail: bertrand.coiffier@chu-lyon.fr.