Abstract
The prevalence, clinical characteristics, and prognostic significance of immune thrombocytopenia (IT) in patients with chronic lymphocytic leukemia (CLL) have not been clearly determined. To clarify this, we retrospectively analyzed 1278 consecutive newly diagnosed patients with CLL. Criteria for IT diagnosis included the following: rapid (< 2 weeks) and severe fall (half of the initial level and below 100 × 109/L) in platelet count; normal or augmented megakaryocytes in bone marrow; no or limited (not palpable) splenomegaly; no cytotoxic treatment in the preceding month. Sixty-four patients (5%) were diagnosed with IT. The median time to IT from CLL diagnosis was 13 months (range, 0-81 months), and median platelet count at IT diagnosis was 14 × 109/L (range, 1-71 × 109/L). Fifty-six of the 64 patients (87%) received treatment for IT. The probability of responding to treatment for IT was significantly higher for patients receiving chemotherapy with or without steroids than for patients treated with intravenous immunoglobulins with or without steroids (P = .01). The development of IT was significantly associated with unmutated IgVh, a positive direct antiglobulin test, and the occurrence of autoimmune hemolytic anemia. Patients with CLL and IT had poorer survival than other patients with CLL (5-year overall survival 64% vs 82%, P < .001), and this effect was independent from common clinical prognostic variables.
Introduction
Autoimmune hemolytic anemia (AHA), immune thrombocytopenia (IT), and pure red cell aplasia represent the autoimmune hematologic conditions more frequently associated with chronic lymphocytic leukemia (CLL).1 However, the association of IT with CLL has been described in the literature only with small series of patients or isolated case reports, leaving its true prevalence unknown. It has been estimated that IT can complicate the course of CLL in approximately 2% of patients.2
Both Rai3 and Binet4 staging classifications recognize a platelet count less than 100 × 109/L at CLL diagnosis as a very unfavorable prognostic factor, regardless of the etiology of the thrombocytopenia. Assuming a low platelet count in CLL patients is usually due to involvement of the bone marrow by the primary disease, no recognized criteria have been postulated so far for IT diagnosis. The lack of sufficient sensitivity and specificity in available platelet auto-antibody tests,5,6 which would parallel the direct Coomb test for red blood cells, certainly limits the interpretation of some acute thrombocytopenias. However, the occurrence of a rapid and deep “unexplained” fall in the platelet count, together with some adjuvant conditions, such as platelet rise after therapy with prednisone or high-dose intravenous immunoglobulins (IVIg), an augmented number of megakaryocytes in the bone marrow, or the absence of hypersplenism, have been used for IT diagnosis in past reports.7-11
Previous studies also reported that IT during CLL can be sometimes successfully treated with steroids,7 cyclosporin A,11 or splenectomy,12 with a variable duration of response. Furthermore, long-term responses have been described after treatment with cytotoxic agents,13 or monoclonal antibodies.14-16 Based on survival results from a few small series, the occurrence of IT may not impair the prognosis of CLL patients.9,12,17
The role of normal or neoplastic B cells, and of their interactions with CD4+ T lymphocytes in the development of auto-immunity, is still a matter of debate.18 IgVh DNA sequence analysis of CLL B cells has revealed a significantly higher risk for unmutated cells to develop IT during the course of the disease.19
To identify the clinical characteristics of patients developing IT in the course of CLL, the most effective therapeutic approach, and the impact of IT on the natural history and survival of CLL patients, we retrospectively analyzed the clinical records of 1278 consecutive patients with CLL.
Methods
Approval was obtained from the San Bortolo Hospital institutional review board for this study. Informed consent was not required while reporting anonymous data.
Patients with CLL
Our study population consisted of 1278 consecutive patients with newly diagnosed B-CLL who presented at the 3 participating institutions (Ospedale San Bortolo, Vicenza, Italy; Ospedale Regina Apostolorum, Albano Laziale, Italy; University of Verona, Verona, Italy) between January 1, 1996, and December 31, 2004. CLL diagnosis was based on the diagnostic criteria of the National Cancer Institute.20
The median age at diagnosis was 67 years, and 758 (59%) were males. The Rai stage was 0 in 458 patients (36%), 1 in 394 (31%), 2 in 236 (18%), 3 in 87 (7%), and 4 in 103 (8%). After a median follow-up of 60 months from CLL diagnosis, 755 patients (59%) required cytotoxic treatment with a median time to CLL treatment of 15 months (range 0-120). Treatment was based on staging and risk assessment in each different Institution, according to local practice guidelines, and mainly consisted of monochemotherapy (Table 1). Induction therapy consisted of chlorambucil alone in 70%, fludarabine alone or in combination in 27%, and of cyclophosphamide, vincristine, prednosone (COP) or cyclophosphamide, doxorubicine, vincristine, prednisone (CHOP) regimens in the remaining 3%.
Clinical and pathological characteristics of 1278 patients with CLL according to IT occurrence
| . | CLL (1214) . | % . | CLL and IT (64) . | % . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 68 (32-91) | — | 66 (48-80) | — | .08* |
| 60 or younger | 313 | 26 | 18 | 28 | — |
| Older than 60 | 901 | 74 | 46 | 72 | — |
| Sex | |||||
| M | 716 | 59 | 42 | 65 | .27 |
| F | 498 | 41 | 22 | 35 | — |
| RAI stage | |||||
| 0 | 439 | 36 | 19 | 30 | .14 |
| 1 | 371 | 31 | 23 | 36 | — |
| 2 | 220 | 18 | 16 | 25 | — |
| 3 | 82 | 7 | 5 | 8 | — |
| 4 | 102 | 8 | 1 | 1 | — |
| Baseline | |||||
| WBC, ×109/L | 31 (2.2-560) | 43.1 (6.2-450) | .019* | ||
| Hb, g/L | 131 (38-177) | 130 (53-162) | .32* | ||
| Plt, ×109/L | 191 (7-768) | 152 (3-528) | <.001* | ||
| Splenomegaly | |||||
| Yes | 397 | 33 | 21 | 33 | .58 |
| No | 817 | 67 | 43 | 67 | — |
| Positive DAT | 61 | 5 | 14 | 22 | <.001 |
| Elevated LDH | 339 | 28 | 15 | 26 | .31 |
| AHA | 37 | 3 | 10 | 16 | <.001 |
| IgVh status | |||||
| Mutated† | 93 | 54 | 5 | 18 | <.001 |
| Unmutated | 78 | 46 | 22 | 82 | — |
| Months to CLL treatment | 14.6 (0-120) | — | 15.4 (0-108) | — | .16* |
| Therapy for CLL | |||||
| No | 510 | 42 | 13 | 18 | .001 |
| Yes | 704 | 58 | 51 | 82 | — |
| First-line therapy | |||||
| Chl‡ | 506 | 72 | 44 | 86 | .09 |
| Fludarabine-based | 198 | 28 | 7 | 14 | — |
| Any fludarabine§ | 276 | 23 | 18 | 28 | .17 |
| . | CLL (1214) . | % . | CLL and IT (64) . | % . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 68 (32-91) | — | 66 (48-80) | — | .08* |
| 60 or younger | 313 | 26 | 18 | 28 | — |
| Older than 60 | 901 | 74 | 46 | 72 | — |
| Sex | |||||
| M | 716 | 59 | 42 | 65 | .27 |
| F | 498 | 41 | 22 | 35 | — |
| RAI stage | |||||
| 0 | 439 | 36 | 19 | 30 | .14 |
| 1 | 371 | 31 | 23 | 36 | — |
| 2 | 220 | 18 | 16 | 25 | — |
| 3 | 82 | 7 | 5 | 8 | — |
| 4 | 102 | 8 | 1 | 1 | — |
| Baseline | |||||
| WBC, ×109/L | 31 (2.2-560) | 43.1 (6.2-450) | .019* | ||
| Hb, g/L | 131 (38-177) | 130 (53-162) | .32* | ||
| Plt, ×109/L | 191 (7-768) | 152 (3-528) | <.001* | ||
| Splenomegaly | |||||
| Yes | 397 | 33 | 21 | 33 | .58 |
| No | 817 | 67 | 43 | 67 | — |
| Positive DAT | 61 | 5 | 14 | 22 | <.001 |
| Elevated LDH | 339 | 28 | 15 | 26 | .31 |
| AHA | 37 | 3 | 10 | 16 | <.001 |
| IgVh status | |||||
| Mutated† | 93 | 54 | 5 | 18 | <.001 |
| Unmutated | 78 | 46 | 22 | 82 | — |
| Months to CLL treatment | 14.6 (0-120) | — | 15.4 (0-108) | — | .16* |
| Therapy for CLL | |||||
| No | 510 | 42 | 13 | 18 | .001 |
| Yes | 704 | 58 | 51 | 82 | — |
| First-line therapy | |||||
| Chl‡ | 506 | 72 | 44 | 86 | .09 |
| Fludarabine-based | 198 | 28 | 7 | 14 | — |
| Any fludarabine§ | 276 | 23 | 18 | 28 | .17 |
DAT indicates direct antiglobulin test; AHA, autoimmune hemolytic anemia; Chl, chlorambucil; and —, not applicable.
Mann-Withney test was adopted to calculate P values of continuous variables.
Values not available in all patients (see text).
Includes 19 patients treated with COP or CHOP.
Refers to treatments delivered before IT diagnosis when applicable.
Diagnostic criteria for IT and AHA
To be considered for inclusion, IT patients had to fulfill all the following criteria: rapid (< 2 weeks) and severe fall (at least half of the initial level and below 100 × 109/L) of the platelet count; a normal or augmented number of megakaryocytes in the bone marrow; no or limited (not palpable) splenomegaly; no cytotoxic treatment in the last month. Any other common cause of thrombocytopenia, such as pseudo-thrombocytopenia, disseminate intravascular coagulation, thrombotic thrombocytopenic purpura, HIV and HCV infections, acute infections, as well as heparin treatment, were ruled out by clinical and laboratory analysis, together with peripheral blood smear examination. Drug-induced thrombocytopenia was excluded based on the lack of any temporal relationship to new drugs.
For thrombocytopenias occurring in patients with advanced Rai stage (3 or 4) and extensive bone marrow involvement, the interpretation of the number of megakaryocytes was sometimes problematic. In these cases we considered essential requisites for the diagnosis of IT the following criteria: lack of response to platelet transfusion (in patients without known refractoriness to platelet concentrates), or a rapid (< 1 week) response to high-dose intravenous Ig (IVIg).
For patients whose IT was diagnosed at the time of CLL presentation we used the same diagnostic criteria, except for the rapid fall of the platelet count, which could not be established in patients presenting with no data on their previous platelet count.
The diagnosis of AHA required the presence of all the following criteria: Hb level less than or equal to 110 g/L in absence of any chemotherapy in the preceding month, positive direct antiglobulin test, one or more laboratory signs of hemolysis (increased bilirubin, elevated lactic dehydrogenase, reticulocytosis).
Treatment for IT and response definitions
Indications for treating patients with IT consisted of a platelet count less than 20 to 30 × 109/L or the presence of signs or symptoms of bleeding and a platelet count less than 50 × 109/L.
Therapy specifically administered for the treatment of IT included chemotherapy (chlorambucil, cyclophosphamyde, COP, and fractionated cyclophosphamide, vincristine, prednisone [CVP]) with or without steroids, steroids alone, IVIg with or without steroids, rituximab, and splenectomy.
The definition of response for IT was based on the following criteria: patients with platelet count of 150 × 109/L or higher were considered to be complete responders (CR); patients with platelet count above 50 × 109/L and less than 150 × 109/L were considered to be partial responders (PR); patients with platelet count above 20 × 109/L but less than or equal to 50 × 109/L were classified as minimal responders (MR); patients whose platelet count never rose above 20 × 109/L were not responders (NR). Time limits in the achievement of any response in the platelet count from the start of therapy were considered: one week for IVIg (400 mg/kg body weight [bw] per day for 5 days or 1 g/kg bw perday for 2 days), 3 weeks for steroids (1 mg/kg bw per day for 14 or more days), and 4 weeks for patients treated with chemotherapy. Response had to last for at least 2 weeks after the end of the specific treatment to be considered.
Patients still requiring treatment for IT after 6 months from diagnosis, but still responsive, were considered to have chronic responsive IT. Patients who never responded to any treatment, including or not splenectomy, or who were NR at the time of the last follow-up were considered refractory.
Supportive care
Tranexamic acid and irradiated platelet units from apheresis were infused only in case of grade 3 or 4 bleeding according to World Health Organization (WHO) score.21
All patients treated with steroids received a concomitant antimycotic prophylaxis with oral fluconazole or itraconazole depending on the treating physician. Wide-spectrum antibacterial prophylaxis with ciprofloxacine, antiviral prophylaxis with oral acyclovir, as well as oral trimethoprim-cotrimoxazole against Pneumocystis carinii were administered in all centers following local practice.
Biologic characterization of CLL patients
One-hundred ninety-eight consecutive patients with CLL who presented to the Vicenza Hematology Department between January 1, 2000, and December 31, 2003, were studied and characterized for their IgVh status and Vh gene repertoire, and for CD38 and ZAP-70 expression. To assess mutational status, DNA was obtained from peripheral blood or bone marrow specimens at diagnosis or, for the purposes of this study, in all patients with available specimens. Sequences were aligned to IMGT22 (Lefranc M.P., Montpellier, France) and V-BASE directories23 (M.R.C., Cambridge, United Kingdom) and analyzed using DNAPLOT and IMGT/VQUEST software. Sequences differing more than 2% from the corresponding germ-line gene were considered to be mutated. ZAP-70 and CD38 expression were assessed by immunophenotype on peripheral blood samples or bone marrow aspirates (positivity cut-off of 20% and 30% tumor cells, respectively).
Eighty-nine patients from the Vicenza Hematology Department with available specimens were studied by cytogenetic and fluorescent in situ hybridization (FISH) analysis. For the purposes of this analysis, the 61 patients (68%) with no cytogenetic alterations or with 13q14− were defined as favorable, while the remaining 28 patients with different results (11q23−, tri12q13, 17p13−, and complex karyotypes) were considered unfavorable.
Statistical analysis
Our statistical end points were as follows: to determine IT prevalence in CLL patients; to compare patients with CLL who developed IT and patients who did not for clinical, laboratory, and biologic characteristics; to find any variable at CLL presentation that would predict or be associated with IT occurrence; to verify the impact of IT development on survival of patients with CLL; to define the best initial therapeutic approach and general treatment for IT; to define IT clinical behavior and variables predicting long-term response; and to analyze the outcome of CLL patients developing IT.
Overall survival (OS) was defined as the time interval between CLL diagnosis and last follow-up or death. Patients without progressive disease who died for CLL-unrelated causes before receiving any treatment for CLL and before IT occurrence were censored at the time of death to eliminate the confounding effect of deaths from other causes unrelated to CLL or IT. Actuarial OS was estimated by the Kaplan-Meier method,24 and differences were analyzed by the log rank test.25 Proportional Cox Hazard Model was used for multivariate analysis.26
The comparison of presenting clinical and laboratory features between the groups of patients with and without IT was carried out with the χ2 test or with the nonparametric Mann-Whitney and Kruskal-Wallis tests, as appropriate. Serum levels of lactic acid dehydrogenase (LDH) were coded as a ratio above the normal value for each participating center. All variables found to have a P value less than or equal to .05 were considered to be statistically significant. All statistical calculations were performed using StatView (Abacus Concepts, Berkeley, CA).
Results
Prevalence and clinical characteristics of CLL patients developing IT
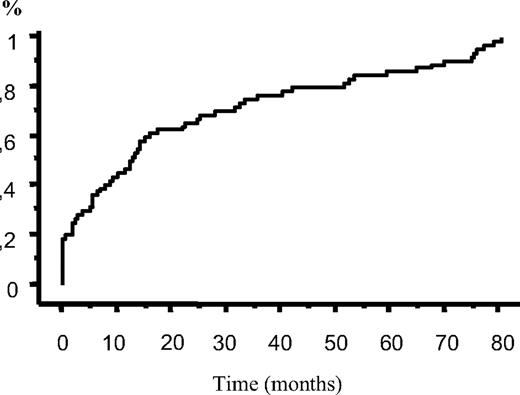
Of our 1278 patients with CLL, 64 (5%) developed IT during the course of the disease. Clinical and laboratory characteristics of these patients are listed in Table 1. Median platelet count at IT diagnosis was 14 × 109/L (range, 1-71 × 109/L). IT occurred concomitantly with CLL diagnosis in 14 patients (22%). None of these patients had a previous history of isolated thrombocytopenia, and the duration of IT before the concurrence of CLL was not established. Median time to IT from CLL diagnosis for the remaining 50 patients was 17 months (range 0-81 months; Figure 1).
Time to IT from CLL diagnosis (64 patients). IT occurred concomitantly with CLL diagnosis in 14 patients (22%), while median time to IT from CLL diagnosis for the remaining 50 patients was 17 months.
Time to IT from CLL diagnosis (64 patients). IT occurred concomitantly with CLL diagnosis in 14 patients (22%), while median time to IT from CLL diagnosis for the remaining 50 patients was 17 months.
Occurrence of IT was not temporally related to tumor progression or recurrence, with the majority of patients (67%) developing IT while CLL was stable and out of therapy. In only 11% of patients IT accompanied tumor progression or recurrence.
Sixteen patients (25%) were chemotherapy naive at the time of IT diagnosis. Of the 48 previously treated patients, 18 (28%) had received only one line of chemotherapy, 19 (30%) 2 lines, and the remaining 11 (17%) had received 3 or more lines of chemotherapy. Patients treated with or without fludarabine alone or in combination had a similar risk of developing IT (P = .17).
Twenty-six patients (41%) presented with WHO grade 1 or 2 bleeding signs at IT diagnosis. Five patients (8%) experienced grade 4 bleeding episodes during the observation period, all requiring hospitalization and transfusions. All of these patients had refractory IT, and 4 of them died because of this complication.
Clinical and biologic variables related to IT occurrence
Among common clinical and laboratory variables at CLL presentation, only a higher white blood cell count and a positive direct antiglobulin test were significantly associated with IT development, as shown in Table 1. Clinically relevant (needing specific treatment) AHA occurred in 47 (4%) patients, and was significantly more frequent in the group of patients with CLL and IT than in other patients with CLL (P < .001, Table 1). Of the 10 patients who developed AHA among the 64 patients with IT, AHA preceded the development of acute thrombocytopenia in 3, while it occurred concomitantly with IT in 7 (Evans syndrome).
Of the investigated biologic variables, unmutated IgVh status was significantly associated with patients developing IT (P < .001), confirming our previous findings.19 When characterizing the Vh gene repertoire, the V1 family resulted significantly more represented among CLL patients with IT (12/27, P = .01), while the V4 family was significantly less represented among patients with IT (1/27, P = .02) than in other CLL patients. The remaining families were equally represented between patients who developed IT and patients who did not. The most represented Vh genes in our IT patients were Vh1-69 (24%), Vh3-30 (14%), and Vh3-33 (10%).
ZAP-70 positivity, which was significantly associated in our series with un-mutated IgVh status (P = .001), also significantly correlated with increased risk of IT occurrence (P = .02).
CD38 positivity, in addition to unfavorable cytogenetics by FISH analysis, did not correlate with development of IT (P = .08, and P = .23, respectively).
First therapeutic approach and general treatment for IT
Fifty-six of the 64 patients (87%) received at least one treatment for IT. Of them, 21 patients (37%) achieved CR after the first therapeutic approach, 12 (21%) achieved PR, 5 (9%) MR, and 18 (32%) were NR. The first therapeutic approach and response to each treatment are shown in Table 2.
IT response to first therapeutic approach in 56 treated patients
| . | All patients (n = 56) . | Response to therapy . | |||
|---|---|---|---|---|---|
| CR (n = 21) . | PR (n = 12) . | MR (n = 5) . | NR (n = 18) . | ||
| Chemotherapy plus steroids* | 18 | 9 | 5 | 3 | 1 |
| Chemotherapy | 4 | 2 | 0 | 0 | 2 |
| Splenectomy | 1 | 1 | 0 | 0 | 0 |
| Steroids | 12 | 3 | 4 | 0 | 5 |
| IVIg | 8 | 1 | 2 | 0 | 5 |
| IVIg plus steroids | 13 | 5 | 1 | 2 | 5 |
| . | All patients (n = 56) . | Response to therapy . | |||
|---|---|---|---|---|---|
| CR (n = 21) . | PR (n = 12) . | MR (n = 5) . | NR (n = 18) . | ||
| Chemotherapy plus steroids* | 18 | 9 | 5 | 3 | 1 |
| Chemotherapy | 4 | 2 | 0 | 0 | 2 |
| Splenectomy | 1 | 1 | 0 | 0 | 0 |
| Steroids | 12 | 3 | 4 | 0 | 5 |
| IVIg | 8 | 1 | 2 | 0 | 5 |
| IVIg plus steroids | 13 | 5 | 1 | 2 | 5 |
Data are numbers of patients. Chemotherapy includes Chlorembucil alone, COP, and CVP.
Includes one patient treated with rituximab who obtained CR.
The probability of achieving at least a MR after first therapeutic approach for IT was significantly higher for the 22 patients treated with chemotherapy with or without steroids than for the 21 patients treated with IVIg with or without steroids (P = .01). Also steroids alone did not seem to be a sufficiently adequate therapeutic approach compared with chemotherapy with or without steroids (P = .05).
When we looked at the response obtained by treatment with IVIg alone or in combination with steroids for the 37 CLL patients who were treated at least once with IVIg during the observation period, 19 (51%) achieved at least a MR (8 CR, 5 PR, 6 MR). The addition of steroids did not influence platelet response (P = .79). On the other hand, chemotherapy with or without steroids (including chlorambucil, cyclophosphamyde, COP, and CVP) induced at least a MR in 27 of 41 patients (66%). Splenectomy was performed in 10 patients: 7 achieved long-term responses (6 CR, 1 PR), while the remaining 3 patients were NR and are still refractory to any treatment. Rituximab was administered to 4 patients with 2 of them responding and still in CR after 13 and 38 months.
IT clinical behavior and variables predicting long-term response
After a median follow-up from IT onset of 35 months, 14 of the 64 patients (22%) are still refractory to any treatment (mean platelet level at last follow-up 9 × 109/L, range 1-19 × 109/L), 10 patients (16%) have chronic responsive IT, and 40 patients (62%) maintain a durable response (8 never required treatment for their IT). Of these, 26 are in CR (2 spontaneous remissions were observed), and 14 are in PR.
When we looked at risk factors associated with IT refractoriness, we could not identify any clinical or biologic characteristic of CLL at diagnosis which significantly correlated with such adverse behavior. Neither the type of treatment for CLL (ie, chlorambucil alone vs fludarabine alone or in combination), the presence of splenomegaly, the CLL status at IT diagnosis, or the time to IT diagnosis had any influence on IT long-term outcome.
On the other hand, platelet response (at least MR) to the first therapeutic approach could predict the development of chronic responsive or refractory IT (P = .01), but no association was found between different induction therapies (IVIg, steroids, splenectomy, or chemotherapy) and IT long-term response or frequency of major bleeding episodes.
CLL outcome: univariate and multivariate analysis
Five- and 10-year overall survival (OS) for all patients was 73% and 50%, respectively. No difference in terms of OS was found between patients diagnosed in the 3 participating Institutions (P = .35).
Among clinical and laboratory variables that have been widely recognized as adverse prognostic factors in CLL patients at diagnosis,27 univariate analysis revealed age over 60 years (P < .001), absolute lymphocyte count (ALC) more than 30 × 109/L (P < .001), advanced Rai stage (P < .001), unmutated IgVh status (P < .001), ZAP-70 (P = .003), and CD38 positivity (P < .001), and unfavorable FISH analysis (P = .01) as adverse factors in terms of OS.
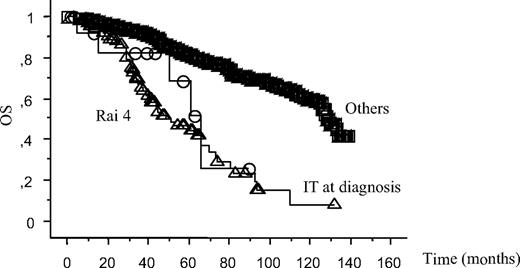
Univariate analysis also indicated that, similarly to low platelet count due to tumor infiltration (Rai 4), the 14 patients with IT at the time of CLL diagnosis had a significantly worst survival than other CLL patients (P = .03; Figure 2; Table 3). Thus, noteworthy, low platelet count at CLL diagnosis was associated with impaired survival, regardless the pathogenesis of the thrombocytopenia, with no difference between Rai 4 patients (5-year OS 45%), and IT patients (51%, P = .47).
Survival curves. Patients with thrombocytopenia due to tumor infiltration (Rai 4) or immune mediated (IT) at the time of CLL diagnosis are compared with other CLL patients (Others). Log-rank test results were P = .03 between “Others” and “IT at diagnosis”, P = .47 between “Rai 4” and “IT at diagnosis”, P < .001 between “Others” and “Rai 4.”
Survival curves. Patients with thrombocytopenia due to tumor infiltration (Rai 4) or immune mediated (IT) at the time of CLL diagnosis are compared with other CLL patients (Others). Log-rank test results were P = .03 between “Others” and “IT at diagnosis”, P = .47 between “Rai 4” and “IT at diagnosis”, P < .001 between “Others” and “Rai 4.”
Survival data from all patients depending on risk factors at diagnosis
| Risk factors . | Patients . | Death . |
|---|---|---|
| Rai 4 | 103 | 55 |
| IT at diagnosis | 14 | 5 |
| Others | 1160 | 263 |
| Risk factors . | Patients . | Death . |
|---|---|---|
| Rai 4 | 103 | 55 |
| IT at diagnosis | 14 | 5 |
| Others | 1160 | 263 |
Survival curves are shown in Figure 2.
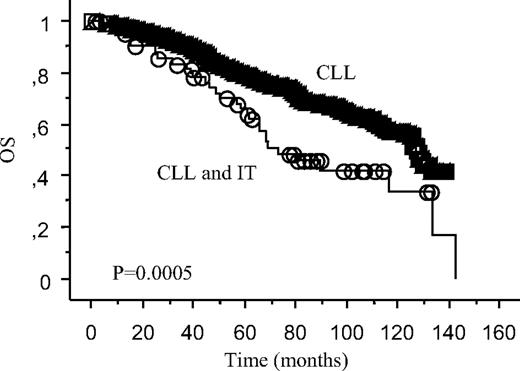
As shown in Figure 3, the occurrence of IT at any time during the observation period was associated with a significantly worst OS (64% vs 82%, P < .001).
OS according to development of IT at any time. Survival curve of the 64 patients with IT and CLL compared with the 1214 patients with CLL not developing IT.
OS according to development of IT at any time. Survival curve of the 64 patients with IT and CLL compared with the 1214 patients with CLL not developing IT.
By means of multivariate analysis, IT occurrence demonstrated an independent prognostic predictive power in terms of OS (hazard ratio 1.71, 95% CI 1.18-2.50; P = .004) when computed in the Cox model with clinical variables that resulted significant at univariate analysis (age > 60, ALC, Rai stage). However, when both clinical and biologic variables were introduced into the multivariate analysis, IT lost its independent predictive power, while only ALC over 30 × 109/L and unmutated IgVh status maintained a significant impact on OS (FISH analysis was not computed in the Cox model because too few patients had been analyzed).
Outcome of CLL patients developing IT
Five- and 10-year OS of patients with IT and CLL was 64% and 42%, respectively (Figure 3). This figure was similar for patients of the 3 participating Institutions (P = .25).
Reduced OS in these 64 patients with IT was significantly related to the occurrence of severe bleeding symptoms (P < .001), chronic responsive or refractory IT (P = .005), shorter time to IT (< 24 months, P = .004), and, unexpectedly, to the absence of concurrent or subsequent AHA (P = .01). Biologic variables were not included in this analysis because there were too few patients with available data to obtain sufficient information.
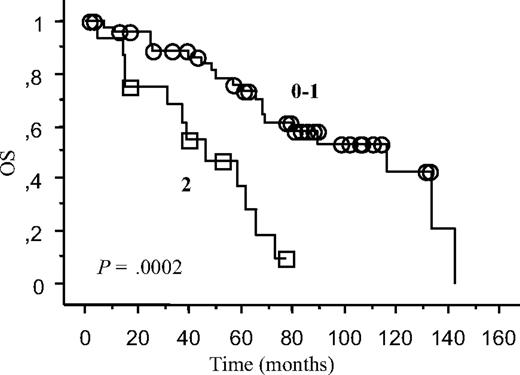
Multivariate analysis revealed chronic or refractory IT (hazard ratio 5, 95% CI 1.57-15.89; P = .006), and IT occurrence less than 24 months from CLL diagnosis (hazard ratio 2.49, 95% CI 1,08-5.76; P = .03) as independent adverse prognostic factors for OS in the 64 patients with CLL and IT. The 16 patients with both of these risk factors (who developed IT less than 24 months from CLL diagnosis, and who had chronic or refractory IT at last follow-up) had a 5-year OS of 30%, significantly inferior to that of patients with 0 or 1 risk factor. Kaplan-Meyer curve is shown in Figure 4. (data in Table 4).
Survival curves. The 64 patients with CLL and IT have been divided according to the presence of 2 or less of the independent risk factors emerging from our Cox's model (IT occurrence less than 24 months from CLL diagnosis, chronic responsive or refractory IT).
Survival curves. The 64 patients with CLL and IT have been divided according to the presence of 2 or less of the independent risk factors emerging from our Cox's model (IT occurrence less than 24 months from CLL diagnosis, chronic responsive or refractory IT).
Survival data from patients with CLL and IT
| Risk factors . | Patients . | Deaths . |
|---|---|---|
| 0-1 | 48 | 20 |
| 2 | 16 | 12 |
| Risk factors . | Patients . | Deaths . |
|---|---|---|
| 0-1 | 48 | 20 |
| 2 | 16 | 12 |
Survival curves are shown in Figure 4.
Discussion
We report the prevalence of IT (5%) in a series of 1278 consecutive patients newly diagnosed with CLL in 3 major hematology institutions in Italy. Our data are the first derived from a systematic review of CLL patients using strict and uniform criteria for IT diagnosis. Prevalence of IT in our series was higher than previously estimated by others (2%-3%).1,2
Studies based on series of incidental CLL cases reported a prevalence of 4% to 8% for CLL-associated AHA,28-30 which is similar to our findings for both AHA and IT. These findings suggest that IT has been underrecognized in previous CLL series, probably due to the absence of standardized clinical diagnostic criteria.
Differently from AHA, which has a very close relationship with CLL activity, IT did not correlate with tumor activity, as already observed by previous reports.10,31 Moreover, while AHA had no independent prognostic effect on survival of patients with CLL,28 IT was a strong adverse prognostic factor. As shown in Figure 2, patients with thrombocytopenia at CLL diagnosis did poorly regardless of the etiology of the low platelet count. At variance with common belief,9,12,17 their prognosis was similar to patients with low platelet count due to tumor infiltration (Rai 4, Binet C). Our multivariate analysis indicated also that the occurrence of IT within 24 months from CLL diagnosis, as well as its refractoriness to treatment, had a further independent pejorative impact on OS.
Impaired survival of our patients with IT might be due to the strong association between IT development and unmutated IgVh status (22 of 27 analyzed patients in this series). In line with this observation and with our Cox model results, IT had no predictive prognostic power in the 100 patients with unmutated IgVh status (P = .22). Because we observed not only a shorter OS, but also a significantly shorter time to tumor progression in our patients with CLL and IT (data not shown), we might consider IT to be a clinical manifestation of unmutated IgVh status, which is well known to discriminate tumors with aggressive behavior. As shown in Table 1, patients with IT were also more often treated with chemotherapy than other CLL patients, confirming a more aggressive clinical behavior. On the other hand, when only clinical prognostic variables were computed in the multivariate analysis, IT occurrence was predictive in terms of OS (hazard ratio 1.71, 95% CI 1,18-2.50; P = .004), indicating once more that IT underscores a different biologic trait of the disease.
A second explanation for the inferior survival of IT patients might be linked to the increased morbidity and mortality due to infections. Immunosuppressive treatments purposely administered for treating IT often included steroids, which have been already reported to increase the prevalence of atypical infections in CLL patients.32 No association emerged between occurrence of IT and “unfavorable” karyotypes or FISH abnormalities, possibly due to the limited number of available patients.
Female to male ratio (0.5), and IT response rates to specific therapies were lower in comparison to the figures generally reported for the idiopathic counterpart (adults' immune thrombocytopenic purpura [ITP]).33 Response to steroids in our patients occurred in 52% of patients, which is in the lower part of the range of responses to steroids reported for ITP (50 to 75%).33,34 On the other hand, while approximately 80% of ITP patients usually respond to IVIg, only half of our patients with IT responded to IVIg alone. However, splenectomy seemed a sufficiently adequate therapeutic approach, with 70% of our patients experiencing a long-term response. There were 14 (22%) patients who were truly refractory, defined as patients who never responded to any of the administered treatments, in our series. This number is greater than what has been reported for adults' ITP (9%).35
The fact that IT had different clinical characteristics and behavior than adults' ITP is not surprising because it probably reflects the altered biologic background and defective immune control caused by the underlying chronic leukemia. IVIg, for instance, which can neutralize autoantibodies and cytokines as well as regulate T- and B-cell function in common autoimmune disorders,36 may not be able to exert their potential because of the altered microenvironment and lymphocyte interactions triggered by the activity of malignant B cells. Also steroids seemed less adequate in the treatment of IT than generally reported for ITP, not only for their possibly hampered immunosuppressive capacity, but also because they facilitated infections, which often contraindicated the use of effective drugs for treating CLL. Hence, the better response on platelet count we observed with the use of cytotoxic drugs may be related to the combined killing of CLL B cells, and of normal lymphocytes producing autoantibodies. In this sense, we believe that rituximab, which is known to be active both in CLL and ITP,14,15 will need to be investigated in this setting.
Unmutated CLL B cells usually carry polyreactive receptors, which bind multiple antigens, including autoantigens, promoting their survival and proliferation. The dynamic balance of negative and positive signals delivered by the B-cell receptor determines the aggressive clinical behavior of unmutated CLL.18 Because not only IT but also a positive direct antiglobulin test was more frequent in unmutated CLL, the preferential occurrence of autoimmune disorders in this subgroup points to the creation of an antigenic loop, which would stimulate B cells when autoantigens are generated in the course of the disease.19
The Vh family gene distribution in our cohort reflected what was previously described in other CLL series.37 We observed a bias toward the Vh1 family in patients with IT (43%) compared with patients not developing IT (21%, P = .01), which seems not surprising in this cohort of mostly unmutated patients. More interestingly, the Vh4 family, which is expected to be found in approximately 20% of both mutated and unmutated CLL,37,38 was found in 22% of our patients with CLL, but in only 4% of patients with CLL and IT (P = .02). This finding of preferential Vh-gene distribution in this subset of CLL leads to the speculation of a possible specific antigenic component involved in the pathogenesis of tumors with a predisposition to autoimmunity.39
In conclusion, we reported a 5% prevalence for IT in a large series of patients with newly diagnosed CLL. Patients developing IT frequently had unmutated IgVh, a positive direct antiglobulin test, and were more prone to AHA. These patients experienced a poor survival rate, especially when IT was refractory to specific treatment or when it occurred in the first 2 years from CLL diagnosis. Their behavior resembled that of Rai 4 patients and unmutated CLL patients. IT response to specific therapy seemed weaker than what has been reported for its adults' idiopathic counterpart. Chemotherapy may be considered the best therapeutic approach for the management of patients with IT and CLL, leaving a role for splenectomy when immunosuppression needs to be avoided.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants of AViLL/AIL (Associazione Vicentina per le Leucemie, i Linfomi e il Mieloma/Associazione Italiana Leucemie) (Vicenza, Italy); Fondazione Progetto Ematologia (Vicenza, Italy); Fondazione Berlucchi (Brescia, Italy) and by the Regione Veneto, through the Ricerca Sanitaria Finalizzata 2006.
We thank Mrs Elena Albiero, BS and Mrs Elisabetta Novella, BS for their skilful technical assistance and Mrs Ivana Frasson for her precious secretarial assistance.
Authorship
Contribution: C.V. designed the project, analyzed the charts, performed the statistical analysis, interpreted the data, and wrote the paper; M.R. designed the project and critically analyzed the charts and the data; R.S. participated in performing the research and revised the paper; I.G. and D.M. performed the laboratory studies; M.L.E., R.Z., A.A., and G.P. performed the research and revised the paper; F.R. designed and supervised the project, revised the paper, and gave final approval for publication. All authors agreed with the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Rodeghiero, MD, Department of Hematology, San Bortolo Hospital, Viale Rodolfi 37, 36100 Vicenza, Italy; e-mail: rodeghiero@hemato.ven.it.