Abstract
Hydroxyurea, a drug widely used for treating myeloproliferative diseases, has also been approved for the treatment of sickle cell disease by raising fetal hemoglobin (HbF). We have shown that nitric oxide (NO) and the soluble guanylyl cyclase (sGC) pathways are involved in hydroxyurea induction of HbF levels in erythroid progenitor cells (EPCs). We demonstrate now that during erythroid differentiation, endothelial NO synthase mRNA and protein levels decline steadily, as does the production of NO derivatives and cyclic adenosine monophosphate (cAMP) levels, but guanosine 3′,5′-cyclic monophosphate (cGMP) levels are stable. Hydroxyurea increased intracellular cGMP levels and cAMP levels in EPCs. The NO donor, DEANONOate, induced much higher cGMP levels, but reduced cAMP levels. Hydroxyurea (1 mM) induced production of approximately 45 pM cGMP/minute/ng of purified sGC, similar to induction by 1 μM DEANONOate. We found that hydroxyurea and ProliNONOate produced iron-nitrosyl derivatives of sGC. Thus, we confirm that hydroxyurea can directly interact with the deoxy-heme of sGC, presumably by a free-radical nitroxide pathway, and activate cGMP production. These data add to an expanding appreciation of the role of hydroxyurea as an inducer of the NO/cGMP pathway in EPCs. These mechanisms may also be involved in the cytostatic effects of hydroxyurea, as well as the induction of HbF.
Introduction
Hydroxyurea is a cytostatic drug that has been used to treat a variety of myeloproliferative diseases but has recently been shown to be effective in elevating fetal hemoglobin (HbF) as a therapy for individuals with sickle cell disease.1,2 An intracellular signaling pathway including soluble guanylyl cyclase (sGC) and guanosine 3′,5′-cyclic monophosphate (cGMP)–dependent protein kinase (PKG) was reported to induce the expression of the gamma-globin gene (HBG1) of HbF in human erythroid cell lines and primary cells.3 We demonstrated that hydroxyurea effects include the nitric oxide (NO)–dependent activation of sGC in cultured CD34+ human erythroid precursor cells.4 Hydroxyurea has been reported to significantly increase NO and cGMP in the blood of patients with sickle cell anemia.5 In addition, cGMP levels were found to be significantly higher in the red blood cells (RBCs) of patients with sickle cell anemia than those of healthy individuals, and were further increased in RBCs of patients with sickle cell anemia on hydroxyurea therapy.6 The cyclic adenosine monophosphate (cAMP)–dependent pathway, which is independent of the mitogen-activated protein kinase (MAPK) pathways, appears to play a negative role in gamma-globin gene expression in K562 erythroleukemia cells.7
It is established that hydroxyurea inhibits ribonucleoside diphosphate reductase, thereby blocking DNA synthesis and repair.8 Hydroxyurea is converted to a free radical nitroxide in vivo and transported by diffusion into cells, where it quenches the tyrosyl free radical at the active site of ribonucleotide reductase, inactivating the enzyme.9 Transient nitroxide-like radicals from hydroxy*urea have been detected in the reaction of hydroxyurea with protein R2 from Escherichia coli and mouse, indicating that 1-electron transfer from hydroxyurea to the tyrosyl radical is the dominating mechanism in the inhibitor reaction.10 An electron spin resonance spectroscopic study also demonstrated that NO was generated from hydroxyurea.11 Both NO gas and NO generated by activated macrophage lysates inhibit tumor cell ribonucleotide reductase.12 Cytostasis by activated macrophages and by hydroxyurea appears to have comparable mechanisms, including, but probably not limited to, inhibition of ribonucleotide reductase.13 These results suggest that NO production from hydroxyurea may be the molecular basis of the pharmacologic and antitumor activity of hydroxyurea.14
To define the relationship between hydroxyurea stimulation of erythroid progenitor cells to increase HbF and NO pathways, we investigated the NO production and NO synthase expression during erythroid differentiation. Hydroxyurea elevated intracellular cGMP and cAMP levels of human erythroid progenitor cells. Using purified sGC in solution with GTP, as substrate, we found that hydroxyurea induced production of cGMP. We also measured the formation of the iron-nitrosyl complex as the end product of the reaction of hydroxyurea and the heme of sGC. These results help in elucidate the hydroxyurea role in sGC induction as well as providing a mechanism by which hydroxyurea may generate bioactive NO.
Methods
The 2-phase liquid erythroid cell cultures
Peripheral blood mononuclear cells were isolated from buffy coats of healthy donors. We performed a 2-phase liquid culture protocol previously described.4 Briefly, after incubation in phase I culture in the absence of erythropoietin, CD34+ cells were harvested by centrifugation and purified by negative selection using the StemSep Cell Separation method (Stem Cell Technologies, Vancouver, BC). The CD34+ cells were resuspended in the phase II medium, which contained a mixture of cytokines, including human recombinant erythropoietin (Amgen, Thousand Oaks, CA). The concentration of total proteins and hemoglobins in erythroid cells plated in wells of a 96-well microplate (1 × 105 cells/well) were assessed using an 8453 UV/Visible Spectrophotometer (Hewlett-Packard, Waldbronn, Germany). Cells were subsequently cultured for 16 hours in 200 μL of fresh medium containing an appropriate concentration of bradykinin (Alexis Biochemicals, San Diego, CA). The activated cells in wells were washed twice with Dulbecco phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA), and then 10 μM diaminofluorescein (DAF-2; Alexis Biochemicals) and 1 mM l-arginine (Sigma, St Louis, MO) were added. After incubation for a further 2 hours, the supernatants (an aliquot of 10 μL of each cell supernatant) were transferred to black microplates, and the fluorescence of NO/DAF-2 interaction was measured with a fluorescence microplate reader calibrated for excitation at 485 nm and emission at 538 nm.
Isolation of total RNA
For isolation of total RNA from erythroid cells we used the RNeasy procedure (Qiagen, Valencia, CA) according to the manufacturer's instructions. The concentration and integrity of total RNA were assessed using an 8453 UV/Visible Spectrophotometer (Hewlett-Packard) and Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). A total of 1 μg of total RNA was reverse transcribed with SuperScript II RNase H− Reverse Transcriptase kit (Invitrogen).
Quantitative PCR
Quantitative real-time polymerase chain reaction (PCR) assay of human inducible NO synthase (iNOS) mRNA transcripts was carried out with the use of gene-specific double-fluorescently labeled probe (5′-CAA GAG CCA GAA GCG CTA TCA CGA AGA T-3′) in a 7700 Sequence Detector (Applied Biosystems, Foster City, CA). The specific primers of iNOS (sense: 5′-AGG TCG AGG ACT ATT TCT TTC AGC-3′; antisense: 5′-CTG TCC TTC TTC GCC TCG TAA G-3′) and TAQMAN probes (synthesized by the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] core oligonucleotide facility) were designed using Primer Express software and prepared on an ABI 394 synthesizer (both from Applied Biosystems). The specific primers of human endothelial NO synthase (eNOS) mRNA transcript and TAQMAN probe were designed as previously described.15 Platinum Quantitative PCR SuperMix-UDG (Invitrogen) was used, containing a final concentration of 200 μM 2′-deoxynucleoside 5′-triphosphates (dNTPs is a mix of dATP, dCTP, dGTP, and dTTP), 0.5 μM Rox reference dye (Invitrogen), 0.2 μM each of TAQMAN probe, sense, and antisense primers. Expression levels were determined using the associated SDS software (ABI Prism; Applied Biosystems) and Microsoft Excel (Redmond, WA).
Ozone-based chemiluminescent determination of nitrite in supernatant of cell culture
For nitrite (NO2−) and total NO (nitrate plus nitrite plus nitrosothiols equals NOx) measurements in supernatant of erythroid cell cultures, erythroleukemic and erythroid cells in 6-well plates were washed once with Dulbecco PBS. After 2 hours, the supernatant was immediately frozen on dry ice and stored at −80°C. NO2− is measured as already described,16 whereas NOx was measured using vanadium (III) chloride in hydrochloric acid at 90°C with the Sievers Model 280 NO analyser (Sievers, Boulder, CO). Helium was bubbled through the reaction mixture. I3−-based chemiluminescence assay was modified with acidified sulfanilamide and HgCl2 as follows: purified sGC (300-350 nM, diluted in PBS without Ca2+ and Mg2+) was treated with hydroxyurea and ProliNONOate (t1/2 = 1.8 seconds at 37°C, 2 M of NO released per mole of NO donor; Alexis Biochemicals) for an incubation time of 10 minutes at 37°C. Samples were transferred on dry ice for freezing. For analysis, samples were thawed at room temperature and acidified sulfanilamide was quickly added (final concentration, 0.5%) as well as HgCl2 (final concentration, 5 mM). Acidified sulfanilamide reacts with nitrite to form a diazonium compound that is not reduced to NO in the I3 chemiluminescent assay (and consequently does not have a signal). NO concentrations from the samples containing acidified sulfanilamide and HgCl2 represent plasma iron-nitrosyl levels only.17 After 2 minutes, samples were transferred on wet ice and measured with NO analyzer.
Immunoblotting
Cell lysates were heated to 90°C for 10 minutes in LDS sample buffer (Invitrogen). Proteins were then separated on 3% to 8% NuPAGE Tris acetate gels and transferred onto polyvinylidene difluoride membranes (Invitrogen), and then probed in immunoblots using eNOS (BD Transduction Laboratories, San Jose, CA) and actin (Santa Cruz Biotechnology, Santa Cruz, CA) monoclonal antibodies according to the protocols provided by the suppliers. The membrane was incubated with a primary antibody overnight at 4°C, and afterward 1 hour at room temperature with a horseradish peroxidase–conjugated sheep anti–mouse IgG. Proteins were visualized by chemiluminescence (ECL plus; Amersham Biosciences, Piscataway, NJ). The intensities of immunoreactive bands in Western blots were analyzed by using the NIH IMAGE program (FluorChem Imaging system; Alpha Innotech, San Leandro, CA).
High-performance liquid chromatography
Erythroid cells were harvested at different time points during erythroid differentiation. To perform high-performance liquid chromatography (HPLC) quantitation of hemoglobin, we separated hemoglobins by cation exchange HPLC of supernatants from cell lysates. The pelleted cells were resuspended, lysed in sterile distilled water, and centrifuged in 0.45 μm filter unit (Millipore, Bedford, MA) for 10 minutes at 4°C. The filtrate was chromatographed on a PolyCAT A 20 × 4.0 mm column (PolyLC, Columbia, MD) fitted to a Gilson HPLC system (Gilson, Middleton, WI) developed with a sodium acetate gradient in 20 mM BisTris buffer (pH 6.55-6.96). Peak areas were integrated with the use of the system software.
Human eNOS immunoassay
Erythroid cells were washed 2 times in PBS, the supernate was discarded, and the cells were lysed at 4°C with cell lysis buffer (R&D Systems, Minneapolis, MN), 1 mL of buffer per 1 × 106 cells. The cells were centrifuged at 300g for 5 minutes, and the supernate was removed and assayed immediately or aliquoted and stored at −20°C or less for up to 24 hours. eNOS protein levels were measured using the human eNOS immunoassay (R&D Systems) according to the protocol of the supplier.
cGMP and cAMP measurements
Erythroid progenitor cells were treated with hydroxyurea or DEANONOate (sodium(Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate; t1/2 = 2 minutes at 37°C, 1.5 M of NO released per mole of NO donor; Alexis Biochemicals) at day 4 of culture and about 1 × 106 cells were harvested at different time points. Cells were pretreated 30 minutes with 3-isobutyl-1-methylxanthine (Sigma) at a final concentration of 0.5 mM to inhibit phosphodiesterase activity. Cyclic nucleotides were measured in cell extract by radioimmunoassay using specific antisera provided by Albert Baukal (National Institute of Child Health and Human Development [NICHD], Bethesda, MD). Mixtures of purified sGC (Alexis Biochemicals), 3 mM MgCl2 (cofactor), and 1 mM GTP (substrate) were treated with hydroxyurea and DEANONOate, and incubated for 10 minutes at 37°C (shaking on 600 rpm). After incubation, mixtures were diluted in 1 mL cold PBS (pH = 7.4; without Mg2+ and Ca2+). A total of 10 μL acidmix (two-thirds triethylamine and one-third acetic anhydride) was added to a 0.5-mL mixture with sGC in glass tubes. After vortexing for 7 to 10 seconds, 0.1 mL of mixture was transferred in new glass tubes. Tubes were covered with Parafilm (American National Can Company, Chicago, IL) and frozen at −80°C for storage and thawed before measurement by radioimmunoassay.
Statistical analysis
The 1-way analysis of variance (ANOVA) and Dunnett post tests were applied using Prism 4 software (GraphPad Software, San Diego, CA).
Results
NOS activity in erythroid cells during differentiation
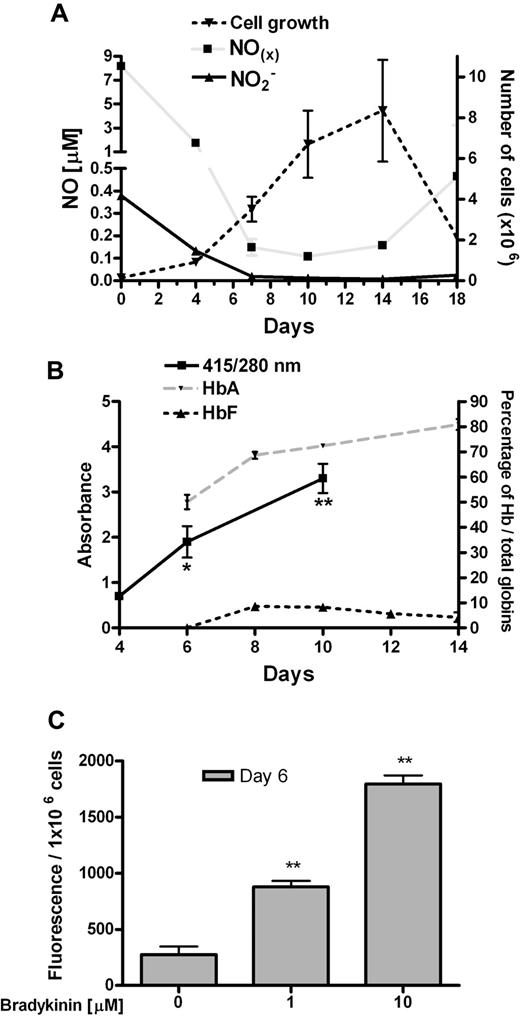
After isolation of CD34+ progenitor cells at day 0 (about 90% purity), erythroid differentiation was stimulated by a mixture of cytokines, including erythropoietin. We observed a fall in both NO2− and NOx levels during differentiation of erythroid cells (Figure 1A). An increase in NOx levels occurred at day 18, which paralleled the marked decrease in cell number and increased apoptosis of erythroid cells at this time. The levels of hemoglobin (primarily HbA), a scavenger of NO, were enriched during erythroid differentiation of CD34+ cells as expected (Figure 1B), as measured by spectrophotometry and HPLC. To show eNOS activity, we treated erythroid progenitor cells with the well-known eNOS inducer bradykinin. The dose-dependent induction of extracellular NO levels was observed during bradykinin treatment of erythroid cells as measured by the fluorescent indicator DAF-2 (Figure 1C). Induction of intracellular NO levels was also revealed during bradykinin treatment as measured by the fluorescent indicator DAF-2 diacetate (data not shown).
Characterization of 2-phase liquid culture of erythroid cells. (A) NO production during erythroid differentiation (per 1 × 106 cells; n = 3). (B) Total globins per total protein levels during erythroid differentiation of CD34+ cells. The heme group of globins gives a maximum absorbance at 415 nm, while 280 nm is the maximum absorbance for most proteins (because of tryptophan, tyrosine, and phenylalanine; n = 3). HPLC analyses of HbA and HbF during erythroid differentiation (n = 4). (C) Detection of extracellular NO levels with fluorescent indicator DAF-2 in erythroid progenitor cells at day 6, during treatment with bradykinin (n = 3). Values are means (± SEM). *P < .05 and **P < .01 compared with cells at day 4 (B) and untreated with bradykinin (C).
Characterization of 2-phase liquid culture of erythroid cells. (A) NO production during erythroid differentiation (per 1 × 106 cells; n = 3). (B) Total globins per total protein levels during erythroid differentiation of CD34+ cells. The heme group of globins gives a maximum absorbance at 415 nm, while 280 nm is the maximum absorbance for most proteins (because of tryptophan, tyrosine, and phenylalanine; n = 3). HPLC analyses of HbA and HbF during erythroid differentiation (n = 4). (C) Detection of extracellular NO levels with fluorescent indicator DAF-2 in erythroid progenitor cells at day 6, during treatment with bradykinin (n = 3). Values are means (± SEM). *P < .05 and **P < .01 compared with cells at day 4 (B) and untreated with bradykinin (C).
NOS levels in erythroid cells during differentiation
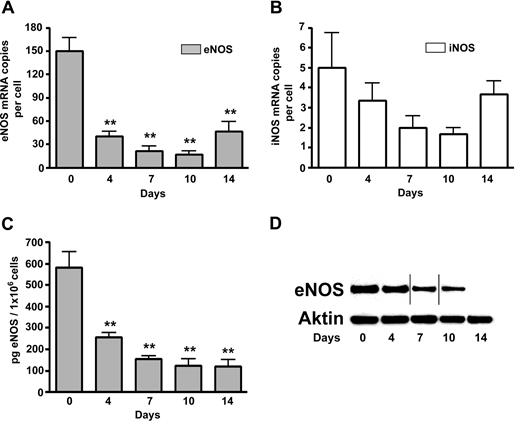
It has been reported that human RBCs express an active and functional eNOS, which is localized in the plasma membrane and the cytoplasm of RBCs.18 According to our experiments, the highest eNOS mRNA levels were detected in CD34+ progenitor cells at day 0. After that, a prominent decline of eNOS mRNA levels in erythroid cells was observed, with a slight elevation at day 14 associated with the start of apoptosis (Figure 2A). Insignificant levels of iNOS mRNA was found during maturation of erythroid cells but with also an apparent slight increase at day 14 (Figure 2B). We did not detect nNOS mRNA and protein in erythroid cells (data not shown). A continuous fall of eNOS protein levels was demonstrated during the maturation of erythroid cells (Figure 2C,D). At day 14, eNOS protein is still detected in erythroid cell cultures by enzyme-linked immunosorbent assay (ELISA; Figure 2C) but no longer observed by the less-sensitive Western blotting technique (Figure 2D).
NOS levels during differentiation of erythroid cells. (A) eNOS mRNA levels during differentiation of erythroid cells. (B) iNOS mRNA levels during differentiation of erythroid cells; (C,D) eNOS protein levels during differentiation of erythroid cells: as measured by ELISA (C) and Western blotting compared with actin protein levels as a control (D). Vertical lines have been inserted to indicate a repositioned gel lane (n = 3). Values are means (± SEM). **P < .01 compared with cells at day 0.
NOS levels during differentiation of erythroid cells. (A) eNOS mRNA levels during differentiation of erythroid cells. (B) iNOS mRNA levels during differentiation of erythroid cells; (C,D) eNOS protein levels during differentiation of erythroid cells: as measured by ELISA (C) and Western blotting compared with actin protein levels as a control (D). Vertical lines have been inserted to indicate a repositioned gel lane (n = 3). Values are means (± SEM). **P < .01 compared with cells at day 0.
Intracellular cyclic nucleotides levels in human erythroid progenitor cells
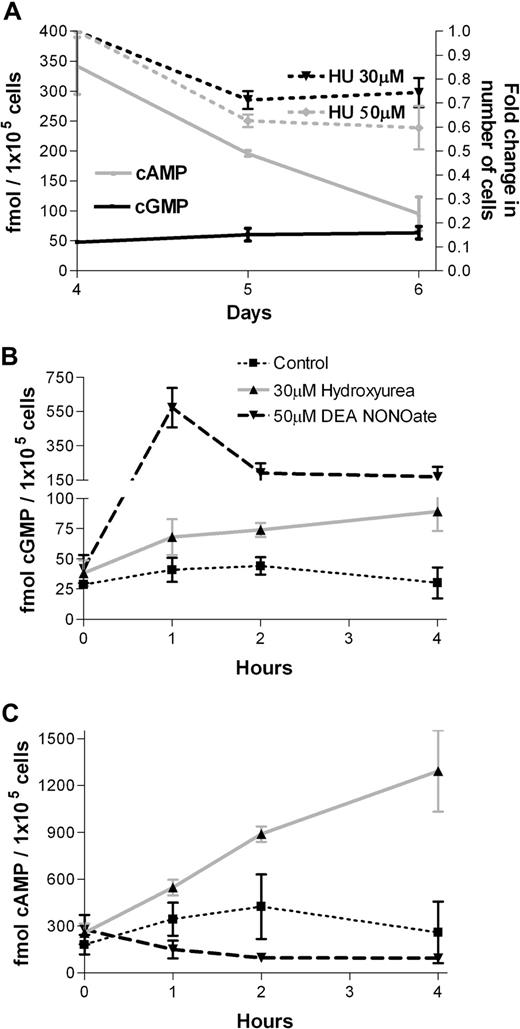
We have previously shown that hydroxyurea and the NO donor CysNO (t1/2 = 30 minutes) increased cGMP levels in erythroid cells.4 Here, we use the cyclic nucleotide–specific radioimmunoassays to compare cGMP and cAMP production. We found a continuous fall in cAMP levels in contrast to steady levels of cGMP during erythroid differentiation (Figure 3A). The cGMP levels in erythroid progenitor cells were similar to primitive CD34+ progenitor cells, whereas both cGMP and cAMP levels were minor in mature RBCs compared with erythroid progenitor cells (data not shown). After 4 hours of incubation, hydroxyurea did not significantly inhibit cell growth by day 4 of erythroid cell culture, whereas hydroxyurea dose-dependently inhibited erythroid cells growth by 30% to 40% after 24 and 48 hours of incubation (Figure 3A). The effects of the 3 cytotoxic agents 5-azacytidine, hydroxyurea, and butyric acid on cell viability in the same erythroid cultures are already reported by our lab.19 Briefly, hydroxyurea (20-100 μM) does not show a consistent and reproducible impact on cell number, whereas both 5-azacytidine (5-20 μM) and butyric acid (0.1-1.0 mM) addition resulted in reduced cell counts on the fourth day after adding erythropoietin to the cultured cells. Hydroxyurea increased intracellular cGMP levels (60-80 fmol/105 cells; Figure 3B) as well as cAMP levels (1000-1800 fmol/105 cells; Figure 3C), thereby reducing the cGMP/cAMP ratio, in human erythroid progenitor cells after 4 hours of incubation. The NO donor, DEANONOate, induced much higher intracellular cGMP levels (600 fmol/105 cells, Figure 3B), but reduced cAMP levels (100-150 fmol/105 cells, Figure 3C). The induced intracellular cGMP levels by hydroxyurea and DEANONOate, corresponded to the increase of extracellular cGMP levels of 30 to 50 fmol/105 cells and 400 to 1600 fmol/105 cells, respectively, after 2 hours of incubation.
Intracellular cGMP and cAMP levels in human erythroid progenitor cells. (A) Intracellular cGMP and cAMP levels in erythroid progenitor cells (left y-axis). Hydroxyurea (HU) dose-dependently inhibits erythroid cells growth after 24 and 48 hours of incubation (right y-axis). (B) Hydroxyurea (30 μM) and DEANONOate (50 μM) increased cGMP levels during 4 hours of incubation of human erythroid progenitor cells at day 4 of phase II liquid culture. (C) Hydroxyurea (30 μM) and DEANONOate (50 μM) increases and inhibits, respectively, cAMP levels during 4 hours of incubation of human erythroid progenitor cells at day 4 of phase II liquid culture (n = 3). Values are means (± SEM).
Intracellular cGMP and cAMP levels in human erythroid progenitor cells. (A) Intracellular cGMP and cAMP levels in erythroid progenitor cells (left y-axis). Hydroxyurea (HU) dose-dependently inhibits erythroid cells growth after 24 and 48 hours of incubation (right y-axis). (B) Hydroxyurea (30 μM) and DEANONOate (50 μM) increased cGMP levels during 4 hours of incubation of human erythroid progenitor cells at day 4 of phase II liquid culture. (C) Hydroxyurea (30 μM) and DEANONOate (50 μM) increases and inhibits, respectively, cAMP levels during 4 hours of incubation of human erythroid progenitor cells at day 4 of phase II liquid culture (n = 3). Values are means (± SEM).
Induction of purified sGC by hydroxyurea and NO donors
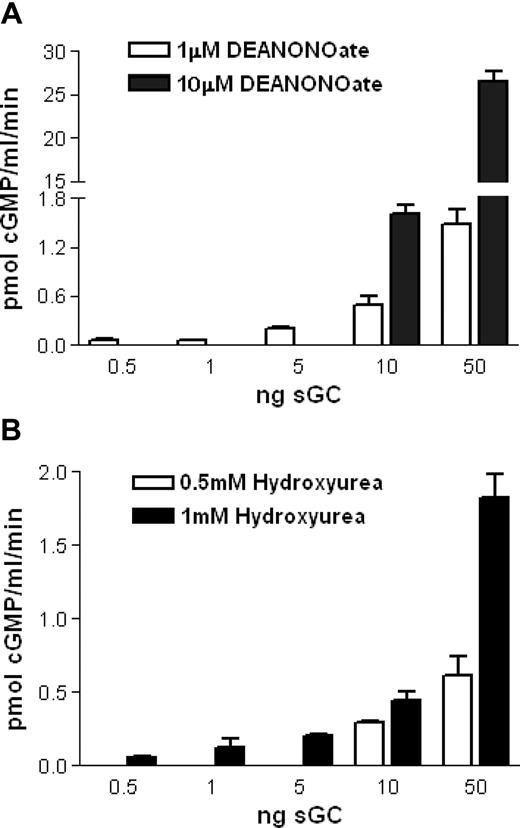
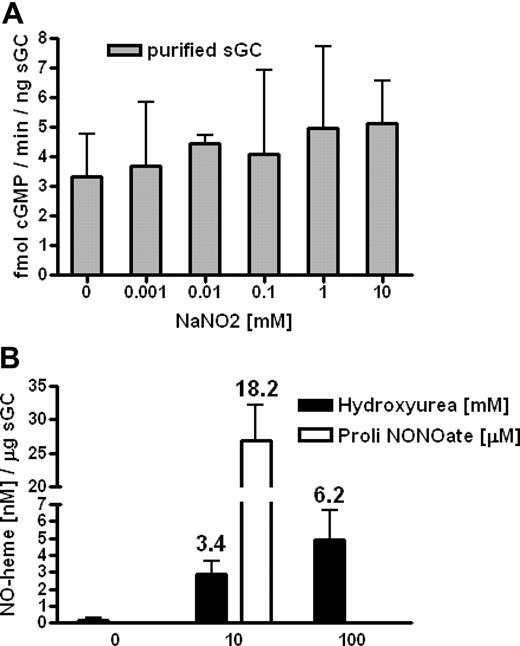
We hypothesized that hydroxyurea could also react with the ferrous heme group of sGC, resulting in iron-nitrosylation and enzymatic activation, in a way similar to the reaction of hydroxyurea and deoxyhemoglobin to form iron-nitrosyl-hemoglobin (HbNO).20 Using purified sGC in solution, we found by radioimmunoassay that 1 mM hydroxyurea induced production of approximately 45 pM cGMP/minute/ng sGC (average value for all presented quantities of sGC), similar to induction by 1 μM of an NO donor, DEANONOate (Figure 4A,B). Also, we demonstrated dose-dependent effects of hydroxyurea and the NO donor on cGMP production: 0.5 mM hydroxyurea incubated with 10 and 50 ng of sGC induces 29 and 12 pM cGMP/minute/ng sGC, respectively, whereas 10 μM DEANONOate induces 160 and 532 pM cGMP/minute/ng sGC, respectively (Figure 4A,B). Under the same conditions, without sGC, hydroxyurea (0.001-100 mM) failed to create NO as measured via nitrite levels by NO analyzer (data not shown). Sodium nitrite (0.001-10 mM) did not induce cGMP production of purified sGC as measured by radioimmunoassay (Figure 5A), which confirmed a primal role of NO (as a part of hydroxyurea molecule) in hydroxyurea induction of sGC.
In vitro induction of purified sGC by hydroxyurea and NO donors.(A) Mixtures of purified sGC (0.5,1, 5, 10, and 50 ng), 3 mM MgCl2, and 1 mM GTP were treated with DEANONOate (1 and 10 μM) during 10 minutes of incubation. (B) Mixtures of purified sGC (0.5, 1, 5, 10, and 50 ng), 3 mM MgCl2 and 1 mM GTP were treated with hydroxyurea (0.5 and 1 mM) during 10 minutes of incubation. Background with 0 ng purified sGC, and the mixtures, was subtracted from the results (n = 3). Values are means ± SEM.
In vitro induction of purified sGC by hydroxyurea and NO donors.(A) Mixtures of purified sGC (0.5,1, 5, 10, and 50 ng), 3 mM MgCl2, and 1 mM GTP were treated with DEANONOate (1 and 10 μM) during 10 minutes of incubation. (B) Mixtures of purified sGC (0.5, 1, 5, 10, and 50 ng), 3 mM MgCl2 and 1 mM GTP were treated with hydroxyurea (0.5 and 1 mM) during 10 minutes of incubation. Background with 0 ng purified sGC, and the mixtures, was subtracted from the results (n = 3). Values are means ± SEM.
In vitro interaction between hydroxyurea and heme of sGC. (A) Sodium nitrite (0.001-10 mM) did not induce cGMP production of purified sGC as measured by radioimmunoassay. (B) Using tri-iodine–based reductive chemiluminescence assay, we measured the formation of the iron-nitrosyl complex during treatment of purified sGC with hydroxyurea and ProliNONOate. The numbers above columns represent percentage of conversion of deoxy-heme to the iron-nitrosyl complex (n = 3). Values are means (± SEM).
In vitro interaction between hydroxyurea and heme of sGC. (A) Sodium nitrite (0.001-10 mM) did not induce cGMP production of purified sGC as measured by radioimmunoassay. (B) Using tri-iodine–based reductive chemiluminescence assay, we measured the formation of the iron-nitrosyl complex during treatment of purified sGC with hydroxyurea and ProliNONOate. The numbers above columns represent percentage of conversion of deoxy-heme to the iron-nitrosyl complex (n = 3). Values are means (± SEM).
In order to test for NO binding to the deoxy-heme of sGC, we measured the formation of the iron–nitrosyl complex with the modified tri-iodine–based chemiluminescence assay (with displacement of NO/thiol groups with HgCl2) following the induction of purified sGC by hydroxyurea.17 We found that 10 mM of hydroxyurea and 10 μM ProliNONOate produced about 3 nM and 30 nM iron-nitrosyl levels per microgram of purified sGC, respectively (100 mM of hydroxyurea induces about 5 nM iron-nitrosyl levels; Figure 5B). These data are consistent with earlier studies showing that hydroxyurea reacts with deoxyhemoglobin in vitro and converts 2% to 6% of the total hemoglobin to HbNO.20 In our studies, hydroxyurea reacted with deoxy-heme of sGC to convert 3% to 6% of the total heme to the iron-nitrosyl complex, whereas ProliNONOate converted 18% of the total heme of sGC to the same complex (Figure 5B). Therefore, we verified the hypothesis that hydroxyurea can directly interact with the deoxy-heme of sGC, generating the iron-nitrosyl complex, and consequently activate cGMP production.
Discussion
Human erythroid progenitor cells contain eNOS mRNA and protein and demonstrate eNOS activity. We observed a fall in eNOS mRNA and protein levels as well as its activity during erythroid differentiation, concomitantly with the elevation of hemoglobin levels. We found a continuous decrease in cAMP high levels in contrast to steady low levels of cGMP during erythroid differentiation. So, the eNOS protein level, as well as cAMP levels, continuously decline during erythroid differentiation. Their activity and presence is parallel to gamma-globin gene expression, which also declines during erythroid differentiation. Hydroxyurea, a drug which stimulates gamma-globin gene expression, increased steady intracellular cGMP levels as well as cAMP levels in human erythroid progenitor cells, whereas the NO donor increased intracellular cGMP levels but reduced cAMP levels. The cAMP signal is adjusted by cAMP degradation determined by phosphodiesterases 3 (PDE3) in erythroid cells, where the PDE3 is inhibited due to activation of sGC.21 Using purified sGC we demonstrated that hydroxyurea could directly interact with deoxy-heme of sGC, generating the iron-nitrosyl complex, and consequently activating cGMP production. These results are all consistent with an NO-dependent sGC mechanism for the elevation of HbF in erythroid progenitor cells.
It has been demonstrated that human RBCs contain iNOS and eNOS as well as calmodulin, suggesting that RBCs may synthesize their own NO.22 This notion was supported by the observation that RBCs have an active eNOS protein.23 However, it was later reported that RBCs possess iNOS and eNOS, but the proteins are without catalytic activity.24 Recent studies revealed eNOS protein in the cytoplasm and in the internal side of RBC membranes as measured by activity.18 However, we demonstrated the continuous decline of eNOS presence and activity throughout erythroid differentiation, suggesting that such activity may be a residual of eNOS production at an earlier stage. Inhibition of NOS partially reversed the hydroxyurea effects on HbF synthesis in erythroid burst-forming unit (BFU-E) colonies,25 but did not decrease NOx production in RBCs during incubation with hydroxyurea.26 In contrast, NOS activity has been reported to be higher in RBCs of healthy individuals and patients with sickle cell disease on hydroxyurea therapy.27 This is in accordance with results that L-arginine increases serum NOx production only in combination with hydroxyurea in patients in steady state.28 It has been reported that hydroxyurea increases NO production in bone marrow endothelial cells.16 The paracrine effect of endothelial cell–derived NO on erythroid cells may be more physiologically relevant in sGC stimulation than hydroxyurea interaction with deoxy-heme of sGC.
Recent studies indicated that hydroxyurea increased HbF levels through the NO-dependent activation of sGC in erythroid cells.4 It has been shown that both sGC activators and cGMP induce gamma-globin gene expression in K562 erythroleukemia cells and primary erythroblasts,3 whereas the cAMP-dependent pathway plays a negative role in gamma-globin gene expression in K562 cells.29 Hydroxyurea increased cGMP levels in human erythroid progenitor cells, while gamma-globin induction by both hydroxyurea and NO donor was prevented by sGC inhibitors.4 cGMP levels correlated with HbF levels in hydroxyurea-treated patients with sickle cell disease.6 Hydroxyurea therapy also concomitantly increased NO, cGMP, and HbF levels in the same patients.5 During erythroid differentiation adenylate cyclase activity, cellular cAMP concentrations and cAMP phosphodiesterase activity have been reported to decline in a synchronized manner.30 In addition to our findings in this and a previous publication,4 there have been 2 reports of cAMP increasing HbF levels. Ikuta's group31 has reported that expression of the gamma-globin gene is induced upon activation of the cAMP pathway, while Dover and colleagues32 found that adenylate-cyclase inhibition markedly decreased HbF induction by hydroxyurea in human erythroid precursor cells. They also reported that hydroxyurea failed to significantly stimulate adenylate-cyclase activity,32 whereas we demonstrated that hydroxyurea stimulated cAMP production in erythroid progenitor cells. While our results are based on studies of erythroid progenitor cells, the above-mentioned studies31,32 are performed on erythroid precursor cells with lower cAMP and elevated hemoglobin levels,4 a potent NO scavenger. NO reduces cAMP levels in erythroid cells, whereas cAMP appears to enhance NO formation.33 The NO-mediated cAMP-reducing mechanisms may operate as a negative feedback in control of cAMP levels. Furthermore, the cGMP response to NO was higher in cells with elevated cAMP levels.34 As already revealed, the cAMP-hydrolyzing PDE isozyme is a cGMP-inhibitable PDE3B in erythroid cells.29 Thus, the cAMP-dependent pathway can be simultaneously triggered since cGMP controls PDE3B activity. Therefore, it appears that hydroxyurea's parallel activation of the linked cGMP- and cAMP-regulatory pathways increases cyclic nucleotide related gamma-globin induction.
The reaction of NO with deoxyhemoglobin produces HbNO.35 Hydroxyurea initially oxidizes deoxyhemoglobin to methemoglobin and then reduces methemoglobin to deoxyhemoglobin during HbNO formation, progressing by inner-sphere mechanisms, where the NO group derives from the −NHOH portion of hydroxyurea.20,36 In contrast, addition of NO to deoxyHbA quickly produces only HbNO. It has been also observed that reaction products of hydroxyurea and deoxyHbA do not arise from the direct reaction of NO.20 sGC permits NO to bind directly to its ferrous deoxy-heme, activating the enzyme.37 Similar to the reaction of hydroxyurea and deoxyhemoglobin to form HbNO, we demonstrate that hydroxyurea can also directly iron-nitrosylate sGC and accordingly trigger cGMP production. Erythroid cells, as well as RBCs, contain high levels of sGC subunits representing an alternative locus for hydroxyurea interaction with deoxy-heme.3,38 It has been shown that nitrite ions increase blood flow in the human circulation as well as vasodilatation of rat aortic rings. Formation of both NO and NO-modified hemoglobin results from the nitrite reductase activity of deoxyhemoglobin.39 It has been also reported that deoxygenated myoglobin is an efficient nitrite reductase that generates bioavailable NO.40 Studies of nitrite activation of sGC demonstrated that nitrite alone (at concentration of 0.1 mM) moderately activated sGC in solution.41 In our in vitro studies nitrite failed to induce cGMP, in purified sGC in solution, confirming a major role of the NO molecule in hydroxyurea interaction with sGC. Moreover in a recent study, inorganic nitrite (in the range of 0.01 mM-1 mM) also failed to increase sGC activity, but in combination with ascorbate increased sGC activity.42 A total of 2 distinct mechanisms have been proposed to explain in vitro nitrate activation of sGC: nitrate reacts with a sGC cysteine residue to yield NO or activation of sGC by an NO-independent mechanism.43
Our observations provide evidence for hydroxyurea stimulation of the NO/cGC pathway, as well as interaction between hydroxyurea and sGC. In addition to the possibility of some NOS presence and activity in mature RBCs, these data show strong eNOS protein levels and function in more primitive human erythroid progenitor and precursor cells, where control of gene expression occurs. The correlation between the levels of cyclic nucleotides and hydroxyurea induction illustrate their participation in hydroxyurea effects on erythroid and endothelial cells. Hydroxyurea stimulation of NO and cyclic nucleotide production may be involved in the therapeutic activity of hydroxyurea as both a cytostatic agent and a pharmacologic means to elevate fetal hemoglobin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH and NIDDK and by grant from the Serbian Ministry of Science and Environment (145048B).
Authorship
Contribution: V.P.C. designed and performed research, analyzed data, and wrote the paper. S.A.A. performed research and analyzed data. S.S.S. and C.T.N. analyzed data and wrote the paper. A.N.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan N. Schechter, Molecular Medicine Branch, NIDDK, NIH, 10 Center Dr, Bldg 10, Rm 9N307, Bethesda, MD 20892-1822; e-mail: aschecht@helix.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal