Abstract
Osteoblasts are a key component in the regulation of the hematopoietic stem cell (HSC) niche. Manipulating osteoblast numbers results in a parallel change in HSC numbers. We tested the activity of strontium (Sr), a bone anabolic agent that enhances osteoblast function and inhibits osteoclast activity, on hematopoiesis. In vitro treatment of primary murine osteoblasts with Sr increased their ability to form bone nodules, and in vivo it increased osteoblast number, bone volume, and trabecular thickness and decreased trabecular pattern factor. However, the administration of Sr had no influence on primitive HSCs, although the number of hematopoietic progenitors was higher than in control cells. When Sr-treated mice were used as donors for HSC transplantation, no difference in the engraftment ability was observed, whereas hematopoietic recovery was delayed when they were used as recipients. Despite the changes in osteoblast numbers, no increment in the number of N-cadherin+ osteoblasts and N-cadherin transcripts could be detected in Sr-treated mice. Therefore, increasing the overall number and function of osteoblasts without increasing N-cadherin+ cells is not sufficient to enhance HSC quantity and function. Our study further supports the notion that N-cadherin+ osteoblasts are fundamental in the hematopoietic niche.
Introduction
Hematopoietic stem cells (HSC) reside in the bone marrow (BM) cavity in a specific microenvironment known as the HSC niche. The hematopoietic niche consists of different stromal cell types such as endothelial cells, fibroblasts, adipocytes and osteoblasts that regulate survival, self-renewal, migration, proliferation and differentiation of HSC.1 Among these stromal cells, osteoblasts have been found to play a crucial role
Osteoblasts have been shown to regulate hematopoiesis by producing a vast array of growth factors and cytokines, important for the maturation of hematopoietic progenitors.2-4 In addition, on the surface of osteoblasts, Angiopoietin 1 regulates HSC number through the activation of Tie-2/Ang-1 signaling pathway,5 whereas the Notch receptor ligand, Jagged 1, modulates HSC self-renewal.6 Furthermore, osteopontin, an osteoblast-secreted protein, participates in HSC location and as a negative regulator of their proliferation.7,8
The first direct involvement of osteoblasts in HSC regulation and maintenance in vivo was reported simultaneously by 2 groups using mouse models. In the first study, a bone morphogenic protein receptor IA (BMPRIA) conditional knockout mouse was used to show that an increase in the number of spindle-shaped N-cadherin+CD45− osteoblastic cells correlates with an increase in the number of HSC.9 In the second study, the use of transgenic mice expressing osteoblast-specific activated parathyroid hormone and parathyroid hormone-related receptors also resulted in a simultaneous expansion in the number of both osteoblasts and HSC in the bone marrow (BM).10 The observation that the conditional ablation of osteoblasts is associated with depletion of hematopoietic cells, including HSC, confirmed the initial results.11 Mutant mice lacking runx2 have defective BM and exhibit extensive extramedullary hematopoiesis, due to defects in osteoblast differentiation and the consequent failure to form bone.12 On the other hand, the generation of new osteoblast-derived ectopic bones resulted in an increase in the number of HSC.13 The manipulation of the number of osteoblasts could thus be an attractive method to alter the number and function of HSC.
Osteoblasts, the bone-forming cells, together with osteoclasts, the bone-resorbing cells, control bone remodeling to durably maintain bone equilibrium and integrity. As a part of this tightly regulated process, osteoblasts regulate osteoclast maturation and proliferation by several factors such as macrophage colony-simulating factor and receptor activator of nuclear factor-κB ligand (RANKL).14,15 Currently, the most effective therapeutic approach to bone loss is the manipulation of bone remodeling by bisphosphonates or parathyroid hormone (PTH) 1-34. Although bisphosphonates inhibit osteoclast-mediated bone resorption, PTH enhances osteoblast function. However, PTH also increases osteoclast activity as a negative feedback, especially when given continuously. More recently strontium (Sr) ranelate has been proposed as a promising therapeutic agent because it simultaneously inhibits preosteoclast differentiation and osteoclast resorbing activity and promotes preosteoblast replication and osteoblast bone-forming activity.16 Evidence suggests that Sr is an agonist of the calcium sensing receptor,17 which has been recently implicated in the localization of the HSC in the BM.18
We hypothesized that, because of this dual action, Sr could be a highly effective agent to improve the capacity of the HSC niche. We observed that, although Sr treatment resulted in an increment in osteoblast numbers, there was no observable increase in the number of N-cadherin+ osteoblasts. The effect of Sr treatment on HSC numbers was biphasic with an increase in the number of hematopoietic progenitor cells, yet the number of the primitive HSC remained unchanged. These observations suggest that either Sr affects an osteoblast subset that interacts with hematopoietic progenitors and/or that the effect on osteoblasts is not sufficient to increase the HSC pool size.
Methods
Cells and cell culture
Primary osteoblasts were isolated from fetal BALB/c mouse calvaria by digestion with 0.1% collagenase and 0.2% dispase as described previously.19 Cells were maintained in α-minimal essential medium (Invitrogen, Paisley, United Kingdom) supplemented with 10% fetal bovine serum (Lonza Nottingham, Nottingham, United Kingdom) and 1% penicillin/streptomycin (Invitrogen; complete medium) in a humidified atmosphere of 5% CO2 at 37°C.
For preparation of bone-associated osteoblasts for real-time reverse transcription (RT)-polymerase chain reaction (PCR), tibias and femurs from Sr-treated for 8 weeks or from untreated mice were cut into small pieces, and BM cells were fully flushed out. Cell lysis buffer was added to the bone fragments before total mRNA extraction using QIAamp RNA Blood Mini kit (QIAGEN, Dorking, United Kingdom).
Bone nodule assay
Cells were seeded in 6-well plates at a density of 1 × 105 cells/well in 2 mL of complete medium plus 5 μg/mL ascorbic acid 2-phosphate (Sigma Chemical, Poole, United Kingdom) until confluent. Cells were then treated with different concentrations of strontium chloride (SrCl2; Sigma) or human synthetic PTH (1-34) (PTH; Sigma) in complete medium supplemented with 100 nmol/L dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, and 10 mmol/L β-glycerol phosphate (Sigma; osteogenic medium) for 21 days. Osteogenic medium was changed twice a week, and fresh Sr or PTH was added with each media change. At the end of the assay, cells were fixed with 10% (vol/vol) formaldehyde and then stained with 40 mmol/L alizarin red S, pH 4.1 (Sigma), as described previously.20
Real-time RT-PCR
Primary osteoblasts were treated with 1 mmol/L Sr or 10 μg/mL PTH in complete medium for the defined times before total mRNA extraction using QIAamp RNA Blood Mini Kit. mRNA was then reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) and oligo (dT)15 primers (Promega, Southampton, United Kingdom). PCR was performed with Platinum SYBR Green qPCR SuperMix (Invitrogen), using the Rotor Gene-6000 apparatus (Corbett Research, Mortlake, Australia). Primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH): GGG TGT GAA CCA CGA GAA AT (forward), ATC CAC AGT CTT CTG GGT GG (reverse); for Runx2: CCG GGA ATG ATG AGA ACT AC (forward), TGT CTG TGC CTT CTT GGT TC (reverse). PCR conditions were: 50°C for 2 minutes and 95°C for 5 minutes, followed by 40 cycles of 3 seconds at 95°C, 10 seconds at 55°C, and 15 seconds at 72°C. For RANKL and N-cadherin mRNA levels, TaqMan Gene Expression Assays (Applied Biosciences, Foster City, CA) and SuperScript III RT/Platinum Taq Mix (Invitrogen) were used and subsequently normalized using the GAPDH TaqMan Gene Expression Assays. Data were analyzed using the ΔΔ threshold cycle (ΔΔCT) relative quantification method, with each sample being normalized to GAPDH. Each sample was run in duplicate and expressed relative to vehicle control for each time point.
In vivo administration of strontium
Six- to 8-week old C57BL/6 female mice, weighing approximately 20 g, were purchased from Olac (Bister, United Kingdom) and treated with 4 mmol/kg/day SrCl2 via the drinking water for 4, 6, 8, and 12 weeks. SrCl2 was used because it easily dissolves in water and has effects similar to those of strontium ranelate in animal studies. This dosage schedule has been previously shown to stimulate bone formation both in mice and rats.21-24 An untreated group of C57BL/6 female mice of the same age and weight was used as a control. At the end of each treatment, mice were killed by CO2 asphyxiation.
Serum osteocalcin measurement
Blood samples were obtained by cardiac puncture from killed animals and incubated at 37°C for 1 hour. Samples were centrifuged at 3600 rpm for 6 minutes and isolated serum was stored at −80°C until use. Osteocalcin concentration was quantified at weeks 4, 6, 8, and 12 of Sr treatment by enzyme-linked immunosorbent assay (ELISA; Biomedical Technologies, Cambridge, MA).
Histomorphometry
Bone histomorphometry was performed on tibias from mice treated with Sr for 8 weeks and from untreated mice according to standard procedures at the University of Aberdeen. In brief, the bones were dissected free of soft tissues, fixed in 4% buffered formalin/saline, pH 7.4, and embedded in methyl methacrylate. Longitudinal sections (4 μm) were then prepared and stained with von Kossa and counterstained with Paragon. Osteoblasts were counted in 3 sections per bone with an intersection distance of 50 μm. Osteoblasts were identified as plump cells lining the bone surface, in the region below the growth place and including all trabecular bone but no cortical bone (using a ×10 objective).
Histomorphometric measurements were made on sections of the proximal metaphysis distal to the epiphyseal growth plate using a 10× objective lens on a Zeiss Axioskop (Carl Zeiss, Welwyn Garden City, United Kingdom) coupled to a Progress C14 digital camera (ISS, Manchester, United Kingdom) using a 1 × C-mount adapter. The camera was connected to an image analysis system running in-house-designed software developed using Aphelion ActiveX Objects (Adcis SA, Caen, France). Bone histomorphometric variables were expressed according to the guidelines of the American Society of Bone and Mineral Research Nomenclature Committee.25
Microcomputed tomography
Microcomputed tomography (CT) analysis was performed by K. Mackenzie at the Histology and EM Core Facility of the University of Aberdeen (United Kingdom). In brief, micro-CT was carried out using a Skyscan 1072 X-ray Microtomograph (Skyscan, Kontich, Belgium). Tibias were placed vertically in the machine's sample holder and images were obtained at 50 kV (197 μA) using a 0.5-mm aluminum filter. For each specimen, a series of 276 projection images were obtained with a rotation step of 0.67° between each image. Magnification used was ×58 (pixel, 5.05 μm; image size, 1024 × 1024).26 The projection images were then reconstructed to give a stack of 2-dimensional images, using Nrecon 1.4.4 (Skyscan). The following bone morphometric parameters27 were obtained: tissue volume (TV), bone volume (BV), tissue surface (TS), bone surface (BS), percentage of bone surface (BS/TV), percentage of bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N) and trabecular pattern factor (Tb.Pf). Tb.Pf is a measure of how well the trabecular network is connected. It is determined by measuring trabecular bone area and perimeter before and after simulated dilation of the trabecula. The difference between the 2 measurements depends on the proportion of concave and convex surfaces. Low values of Tb.Pf suggest a well-connected network; higher values indicate disconnections.28,29
Immunohistochemistry
Skeletal specimens were collected from Sr-treated and untreated mice. Samples were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut at 5 μm thickness on a Leica CM1900 cryostat (Leica Microsystems UK, Milton Keynes, United Kingdom) and were stained with hematoxylin and eosin according to the standard procedures. For N-cadherin staining, fresh femurs specimens were rapidly decalcified in Decal Stat (Quartett Immunodiagnostika Biotechnologie, Berlin, Germany). The 5-μm sections were warmed at 37°C for 1 hour before staining to ensure adhesion to the slides and were fixed at room temperature in acetone for 10 minutes, before being immersed in a 2% H2O2 in methanol bath. Slides were washed in running tap water. Normal swine serum (10%; Serotec, Oxford, United Kingdom) was applied for 10 minutes followed by rabbit polyclonal N-cadherin antibody (Abcam, Cambridge, United Kingdom; 1:750) for 1 hour at room temperature. Slides were washed in Tris-buffered saline (TBS) before the application of biotinylated secondary antibody swine anti-rabbit (Dako UK, Ely, United Kingdom; 1:400) for 35 minutes. Slides were then washed in TBS and avidin-biotinylated enzyme complex was applied (Vector Laboratories, Peterborough, United Kingdom; 1:100) for 35 minutes at room temperature. Diaminobenzidine was applied according to the manufacturer's instructions (Vector Laboratories) for 5 minutes, and slides were counterstained with hematoxylin for 1 minute followed by approximately 10 seconds in 1% acid alcohol.
Colony-forming unit-culture assay
BM cells from treated and untreated mice were assessed for colony-forming unit-culture (CFU-C) frequency in MethoCult media (StemCell Technologies, Vancouver, BC), according to the manufacturer's instructions. Duplicate cultures were prepared for each sample and maintained at 37°C, 5% CO2 in air, and at least 95% humidity. Colonies were scored 12 days after plating.
Long-term culture initiating cells assay
For the stromal preparation, freshly isolated BM cells were seeded in 96-well plates at a final concentration of 3 × 105 cells/well in MyeloCult media supplemented with 10 μmol/L hydrocortisone (both from StemCell Technologies). After 2 weeks, a confluent adherent layer formed and was irradiated with 1500 cGy of γ-irradiation. BM cells suspensions from treated and untreated mice were then added to the stromal layers at 5 successive 2-fold dilutions, the higher being 6 × 104 cells per well in a total of 16 wells per concentration. The cultures were further incubated for 4 weeks and fed weekly by half medium change. Each well was then trypsinized for 10 minutes, washed with phosphate-buffered saline (PBS), and plated in MethoCult media. Twelve days after plating, the wells were scored as positive (≥ 1 CFU) or negative (no CFU) and the long-term culture initiating cell (LTC-IC) frequencies were calculated by the method of maximum likelihood from the proportion of the wells that were negative according to the limiting dilution analysis.30
Flow cytometric analysis
For the immunophenotypical enumeration of HSC, BM cells from treated and untreated mice were stained with PE-conjugated lineage antibodies (anti-CD3, anti-B220, anti-Ter-119, anti-Mac-1 and anti-Gr-1), FITC-conjugated anti-Sca-1, APC conjugated anti-c-kit and PE-Cy5-conjugated anti-Flk2 (BD Pharmingen, San Diego, CA). To assess the cell cycle status of the primitive hematopoietic population Lin−c-kit+Sca-1+ cells were purified from treated and untreated BM cells using the EasySep Mouse Hematopoietic Progenitor Cell Enrichment Kit (Stem Cell Technologies). Cells were washed with PBS, fixed with 2 mL 70% cold ethanol and kept overnight at 4°C. The following day cells were washed twice with PBS before being stained with propidium iodide (DNA dye) and fluorescein isothiocyanate (FITC; protein dye) for 30 minutes at 4°C. FlowJo version 7.0 software was used for the analysis.
Blood counts
Blood was obtained from Sr-treated and untreated mice by tail bleeding in buffer containing heparin to avoid blood clotting. The enumeration of the blood cells was performed using the SYSMEX SE 9000 hematology analyser.
Bone marrow transplantation
For in vivo enumeration of HSC, 2.5 × 106 CD45.2 BM cells were isolated from Sr-treated and untreated C57BL/6 mice and were mixed with 2.5 × 106 CD45.1 cells from CD45.1 congenic mice. Cells were then intravenously injected into CD45.2/CD45.1 recipient mice that had been lethally irradiated with 1500 cGy of γ-irradiation. Four weeks after transplantation, the frequency of the different cell populations was determined in the peripheral blood (PB) by fluorescence-activated cell sorting (FACS) using PE-CD45.1 and FITC-CD45.2 antibodies (BD Pharmingen).
To assess the effect of Sr in the recipients, C57BL/6 mice were treated with SrCl2 for 4 weeks. Each mouse was lethally irradiated with 2 doses of 750 cGy of γ-irradiation within 12 hours and was transplanted with 4 × 106 BM cells from wild-type C57BL/6 donors. Sr administration was continued until the end of the experiment, and cell engraftment was monitored by enumeration of the blood cells in regular time intervals. Eight weeks after transplantation, the mice were killed, and the hemopoietic cell content in the BM was measured by immunophenotypic analysis and CFU-C assay. An untreated group was used as control.
Statistical analysis
Data were analyzed using an unpaired 2-tailed Student t test with the level of significance at P less than .05 and expressed as the mean (± SEM or ± SD, as indicated). For the statistical analysis of the hematopoietic recovery of Sr-treated recipients, one-way analysis of variance was used to test for differences among the 3 independent groups (white blood cells [WBC], red blood cells [RBC], and platelet [PLT] numbers) at each time point. A 2-sided significance level of .05 was used.
Results
Effects of strontium and PTH on osteoblast activity in vitro
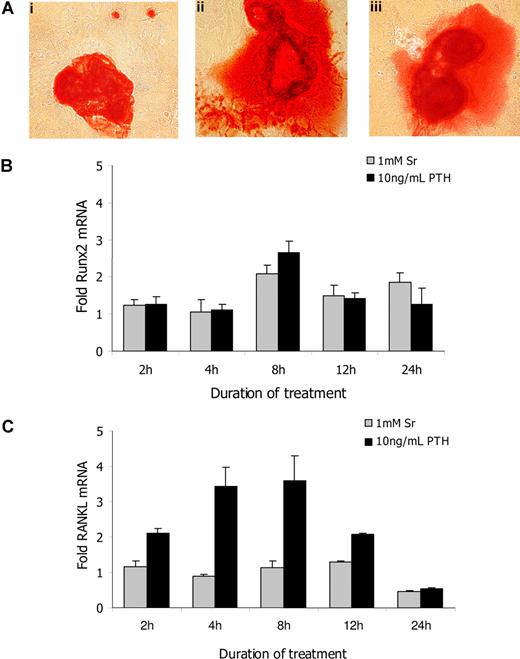
To verify the effects of Sr on osteoblast activity in vitro, we used alizarin red S staining to detect bone nodule formation by primary osteoblasts after 21 days of culture in osteogenic media (Figure 1A). When 1 mmol/L Sr was added to cultures, the mineralization area of the nodules was increased (Figure 1Aii) in a dose dependent manner (data not shown). The addition of 10 ng/mL PTH (Figure 1Aiii) similarly increased bone nodule formation, indicating that both Sr and PTH enhanced osteoblast activity in vitro. The mRNA levels of a series of osteoblast specific genes were tested in response to Sr or PTH after exposure to the compound for 2, 4, 8, 12, and 24 hours. Real-time PCR analysis revealed a 2-fold increase of runx2 mRNA in the presence of 1 mmol/L Sr after 8-hour treatment that returned to baseline levels by 24 hours (Figure 1B). The same pattern of runx2 expression was observed after treatment with 10 ng/mL PTH. However, no significant difference was observed in the expression of osteocalcin, osteopontin, osteonectin, decorin, collagen type 1, alkaline phosphatase, or osterix mRNA levels in the presence of either Sr or PTH when cultures were evaluated at the same time points (data not shown). It is noteworthy that PTH treatment resulted in a 3-fold up-regulation of RANKL mRNA; levels peaked at 4 hours. Sr treatment had no effect (Figure 1C). Therefore, although Sr promoted osteoblast activity in vitro, its effects were not identical to those of PTH.
Strontium promotes osteoblasts' activity in vitro. (A) The mineralization of bone nodules was detected by alizarin red S staining in primary osteoblast cultures that had been incubated for 21 days in osteogenic media alone (i) or osteogenic media containing 1 mmol/L Sr (ii) or 10 ng/mL PTH (iii). The mRNA expression levels of runx2 (B) and RANKL (C) were determined by real-time RT-PCR in primary murine osteoblasts. Cells plated in 6-well plates were treated in complete media with 1 mmol/L Sr or 10 ng/mL PTH and harvested after 2, 4, 8, 12, and 24 hours. The results are reported as fold change in gene expression relative to untreated controls after being normalized to GAPDH (ΔΔCT method), and columns represent mean value (± SEM) of 3 different experiments.
Strontium promotes osteoblasts' activity in vitro. (A) The mineralization of bone nodules was detected by alizarin red S staining in primary osteoblast cultures that had been incubated for 21 days in osteogenic media alone (i) or osteogenic media containing 1 mmol/L Sr (ii) or 10 ng/mL PTH (iii). The mRNA expression levels of runx2 (B) and RANKL (C) were determined by real-time RT-PCR in primary murine osteoblasts. Cells plated in 6-well plates were treated in complete media with 1 mmol/L Sr or 10 ng/mL PTH and harvested after 2, 4, 8, 12, and 24 hours. The results are reported as fold change in gene expression relative to untreated controls after being normalized to GAPDH (ΔΔCT method), and columns represent mean value (± SEM) of 3 different experiments.
Effect of strontium on osteoblast in vivo
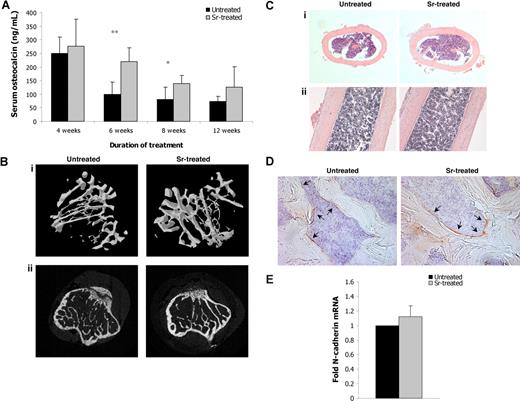
We next investigated whether Sr enhanced osteoblast function in vivo. Administration of Sr for 6 and 8 weeks to C57BL/6 mice resulted in significantly increased levels of serum osteocalcin compared with age-matched untreated control mice (Figure 2A), indicating increased osteoblast activity and number. Indeed, the actual number of osteoblasts, as defined by histomorphometric analysis, was increased by 53.85% in the Sr-treated mice compared with control mice (Table 1). To evaluate differences in bone structure, micro-CT scanning on tibias from mice treated with Sr for 8 weeks and from untreated mice was performed, and the results are summarized in Table 1. TV was 6.23% greater (P = .013) and BV was 36.55% higher (P = .007) in Sr-treated animals, and TS and BS were also elevated. A significant increase of 30.57% in trabecular bone volume as a ratio of total volume (BV/TV, P = .098) and a 10.29% increase in Tb.Th (P = .033) in the Sr-treated tibias were also documented. Further analysis revealed a reduction in the Tb.Pf (P = .001). Tb.Pf represents the trabecular network connectivity, and reduced values are associated with a well-connected network; on the contrary, higher values have been linked to disconnections. The finding that Sr-treated mice have lower Tb.Pf compared with control mice indicates that the trabecula is better connected within the BM cavity (Figure 2B). However, the Tb.Sp, which ultimately represents the “thickness” of the marrow cavities, was comparable between the 2 groups. Thus, although the bone mass was increased, the actual space in the BM cavity was not significantly reduced (Figure 2C). Accordingly, the total BM cell numbers obtained from the tibia of treated and untreated mice were comparable (data not shown). Because N-cadherin has been postulated to be an important component for HSC anchorage in the endosteal niche, we evaluated its presence in Sr-treated bones. The frequency of the N-cadherin+ osteoblasts remained unchanged after 8 weeks of Sr treatment (Figure 2D); likewise, the N-cadherin mRNA abundance in osteoblasts extracted from bones of these mice was comparable with that observed in osteoblasts extracted from control bones (Figure 2E). This shows that Sr treatment did not up-regulate the number of N-cadherin+ osteoblasts present in the marrow cavity.
In vivo administration of strontium increases bone formation. (A) Serum blood was obtained from untreated and treated for 4, 6, 8, and 12 weeks with Sr mice and the concentration of osteocalcin was measured by ELISA. Data are mean (± SEM; n = 8 per group). * P < .05 and ** P < .01. (B) Tibias from mice treated with Sr for 8 weeks were subjected to micro-CT analysis and were compared with ones from untreated age-matched littermates. Representative images of 3D micro-CT reconstruction (i) and 2D microCT scan (ii). (C) Hematoxylin and eosin staining in paraffin-embedded sections of decalcified tibias, vertical (i) and parallel (ii) to the long axis of the tibia sections. Magnifications, ×100. (D) N-cadherin staining in femur sections from mice treated with Sr for 8 weeks (right) and untreated mice (left). Arrows indicate N-cadherin+ osteoblasts. Magnifications, ×200. (E) Osteoblasts were obtained from tibias and femurs of mice treated with Sr for 8 weeks or untreated mice, and the mRNA expression levels of N-cadherin were determined by real-time RT-PCR. The results are reported as fold change in gene expression relative to untreated controls after being normalized to GAPDH (ΔΔCT method), and columns represent the mean value (± SEM) of 2 different experiments (4 mice per group).
In vivo administration of strontium increases bone formation. (A) Serum blood was obtained from untreated and treated for 4, 6, 8, and 12 weeks with Sr mice and the concentration of osteocalcin was measured by ELISA. Data are mean (± SEM; n = 8 per group). * P < .05 and ** P < .01. (B) Tibias from mice treated with Sr for 8 weeks were subjected to micro-CT analysis and were compared with ones from untreated age-matched littermates. Representative images of 3D micro-CT reconstruction (i) and 2D microCT scan (ii). (C) Hematoxylin and eosin staining in paraffin-embedded sections of decalcified tibias, vertical (i) and parallel (ii) to the long axis of the tibia sections. Magnifications, ×100. (D) N-cadherin staining in femur sections from mice treated with Sr for 8 weeks (right) and untreated mice (left). Arrows indicate N-cadherin+ osteoblasts. Magnifications, ×200. (E) Osteoblasts were obtained from tibias and femurs of mice treated with Sr for 8 weeks or untreated mice, and the mRNA expression levels of N-cadherin were determined by real-time RT-PCR. The results are reported as fold change in gene expression relative to untreated controls after being normalized to GAPDH (ΔΔCT method), and columns represent the mean value (± SEM) of 2 different experiments (4 mice per group).
Trabecular morphological parameters (mean ± SEM) in the tibia of untreated and Sr-treated for 8 weeks mice by micro-CT or histomorphometric analysis
| . | Untreated . | Sr-treated . | % Difference . | P . |
|---|---|---|---|---|
| TV (mm3) | 1.63 ± 0.02 | 1.74 ± 0.06 | +6.23% | <.05 |
| BV (mm3) | 0.12 ± 0.02 | 0.17 ± 0.02 | +36.55% | <.01 |
| BV/TV | 7.21 ± 1.11 | 9.73 ± 0.83 | +30.57% | <.01 |
| TS (mm2) | 10.16 ± 0.18 | 10.79 ± 0.35 | +6.01% | <.05 |
| BS (mm2) | 9.44 ± 1.69 | 11.47 ± 1.25 | +20.33% | .1 |
| BS/TV (1/mm) | 5.79 ± 1.09 | 6.58 ± 0.54 | +14.22% | .24 |
| Tb.Th (mm) | 0.05 ± 0.004 | 0.06 ± 0.002 | +10.29% | <0.05 |
| Tb.Sp (mm) | 0.32 ± 0.06 | 0.31 ± 0.03 | +0.83% | .1 |
| Tb.N (1/mm) | 1.40 ± 0.27 | 1.70 ± 0.15 | +20.61% | .1 |
| Tb.Pf (1/mm) | 28.09 ± 1.57 | 21.90 ± 0.88 | −24.69% | <.001 |
| Ob. N* | 23.22 ± 3.36 | 40.33 ± 8.01 | +53.85% | <.05 |
| Ob.N/BS* | 4.71 ± 1.42 | 7.67 ± 0.49 | +47.81% | <.05 |
| . | Untreated . | Sr-treated . | % Difference . | P . |
|---|---|---|---|---|
| TV (mm3) | 1.63 ± 0.02 | 1.74 ± 0.06 | +6.23% | <.05 |
| BV (mm3) | 0.12 ± 0.02 | 0.17 ± 0.02 | +36.55% | <.01 |
| BV/TV | 7.21 ± 1.11 | 9.73 ± 0.83 | +30.57% | <.01 |
| TS (mm2) | 10.16 ± 0.18 | 10.79 ± 0.35 | +6.01% | <.05 |
| BS (mm2) | 9.44 ± 1.69 | 11.47 ± 1.25 | +20.33% | .1 |
| BS/TV (1/mm) | 5.79 ± 1.09 | 6.58 ± 0.54 | +14.22% | .24 |
| Tb.Th (mm) | 0.05 ± 0.004 | 0.06 ± 0.002 | +10.29% | <0.05 |
| Tb.Sp (mm) | 0.32 ± 0.06 | 0.31 ± 0.03 | +0.83% | .1 |
| Tb.N (1/mm) | 1.40 ± 0.27 | 1.70 ± 0.15 | +20.61% | .1 |
| Tb.Pf (1/mm) | 28.09 ± 1.57 | 21.90 ± 0.88 | −24.69% | <.001 |
| Ob. N* | 23.22 ± 3.36 | 40.33 ± 8.01 | +53.85% | <.05 |
| Ob.N/BS* | 4.71 ± 1.42 | 7.67 ± 0.49 | +47.81% | <.05 |
TV indicates tissue volume; BV, bone volume; TS, tissue surface; BS, bone surface; BS/TV, percentage of bone surface; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number; Tb.Pf, trabecular pattern factor; Ob.N, osteoblast number; Ob.N/BS, osteoblast number per bone surface.
Histomorphometric analysis.
Effects of strontium treatment on hematopoiesis
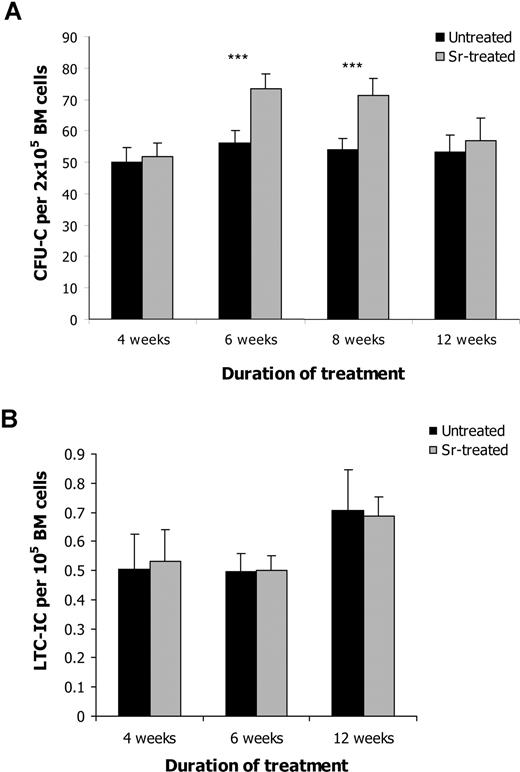
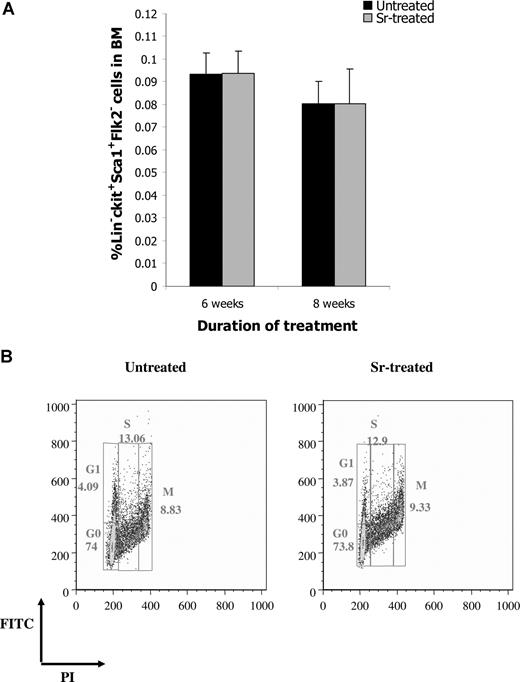
To determine whether the increase in osteoblast activity after Sr administration was paralleled by an expansion of HSC, BM from treated and untreated mice was enumerated for CFU-C, as a general assessment of hematopoietic activity. After 6 or 8 weeks of Sr administration, a statistically significant increase in the frequency of CFU-C was observed (Figure 3A). No difference in the number of CFU-C was detected after 4 or 12 weeks of Sr administration, indicating that Sr required a period of time to exert its effects on the hematopoietic progenitors and that its effect was transient. The increase in the hematopoietic progenitors does not seem to result from a direct effect of Sr on HSC. In fact, when Sr was added directly to CFU-C cultures, no difference in the number of colonies was observed compared with controls (data not shown). The increase in the hematopoietic progenitor population did not affect the number of WBCs, RBCs, or PLTs (data not shown). However, when the frequency of LTC-ICs was analyzed, no difference was detected between the 2 groups at any time point (Figure 3B). The same result was obtained when the frequency of the more primitive HSC in the BM composition of treated and untreated mice was determined by FACS analysis of the lineage-negative (Lin−) Sca-1+ c-kit+ Flk2− cells (Figure 4A). Finally, the proportion of the Lin− Sca-1+ c-kit+ cells that were in G0, G1, M, or S phase was comparable between treated and untreated mice (Figure 4B), indicating that Sr administration did not affect the cell cycle of the primitive HSC.
Strontium alters the hematologic pattern in the BM. BM cells were isolated from control mice and mice that had been treated with Sr for 4, 6, 8, and 12 weeks and were assessed for the CFU-C frequency that identifies the hematopoietic progenitors (n = 12) (A) (*** P < .001) and the LTC-IC frequency that represents the frequency of the functional HSC (n = 8 per group) (B). Values shown are the mean (± SEM).
Strontium alters the hematologic pattern in the BM. BM cells were isolated from control mice and mice that had been treated with Sr for 4, 6, 8, and 12 weeks and were assessed for the CFU-C frequency that identifies the hematopoietic progenitors (n = 12) (A) (*** P < .001) and the LTC-IC frequency that represents the frequency of the functional HSC (n = 8 per group) (B). Values shown are the mean (± SEM).
Strontium does not affect HSC. (A) The frequency of Lin− Sca1+ c-kit+ Flk2− cells in the BM of untreated and Sr-treated mice was determined by FACS analysis. Values are mean (± SD; n = 4 and 8 per group for 6 and 8 weeks of treatment, respectively). (B) The cell- cycle profile of purified Lin− Sca1+ c-kit+ obtained from the BM of mice treated with Sr for 8 weeks and untreated mice was defined by staining with propidium iodide and FITC and FACS analysis (representative analysis from 4 different experiments).
Strontium does not affect HSC. (A) The frequency of Lin− Sca1+ c-kit+ Flk2− cells in the BM of untreated and Sr-treated mice was determined by FACS analysis. Values are mean (± SD; n = 4 and 8 per group for 6 and 8 weeks of treatment, respectively). (B) The cell- cycle profile of purified Lin− Sca1+ c-kit+ obtained from the BM of mice treated with Sr for 8 weeks and untreated mice was defined by staining with propidium iodide and FITC and FACS analysis (representative analysis from 4 different experiments).
Effects of strontium on HSC transplantation
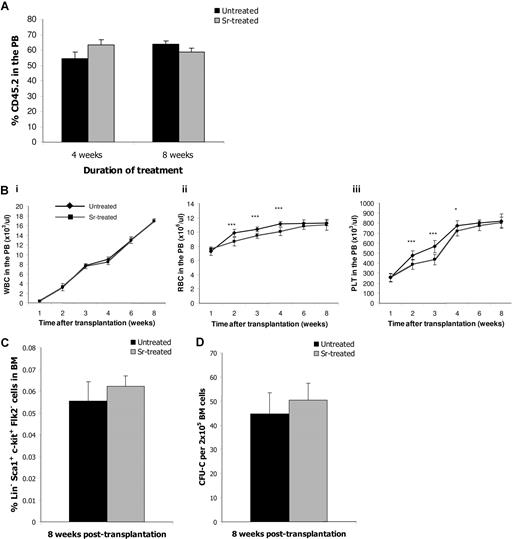
Manipulating the number of HSCs should have a profound impact on clinical HSC transplantation. For this reason, we tested whether the HSC from Sr-treated mice exhibited better engraftment ability, even though their number remained the same. Competitive transplantation experiments revealed that there is no difference in the engraftment ability of BM cells from untreated and treated donors (Figure 5A). However, it is possible that treatment with Sr could enhance the hematopoietic niche and thus provide a facilitating effect on HSC engraftment. Therefore, lethally irradiated recipient mice pretreated with Sr for 4 weeks were transplanted with BM cells from untreated donors, and the hematopoietic reconstitution was monitored by blood counts in the PB (Figure 5B). Sr-treated recipients had reduced numbers of RBCs (Figure 5Bii) and PLTs (Figure 5Biii) in the PB at 2, 3, and 4 weeks after transplantation compared with untreated recipients, thus indicating a delayed recovery. However, the numbers of hematopoietic cells were comparable with control numbers by the sixth week after the transplant. Moreover, the frequencies of (Lin−) Sca-1+ c-kit+ Flk2− HSC (Figure 5C) and CFU-C (Figure 5D) were similar 8 weeks after transplant. The same results were obtained when recipient mice had been pretreated with Sr for 8 weeks (data not shown). When the treatment was commenced after the transplant, the disparity in the hematopoietic recovery between the groups could not be detected (data not shown). Therefore, Sr treatment enhanced neither the engrafting ability of HSC from treated donors nor the ability of recipient osteoblasts to support HSC.
Strontium-treated mice as donors or recipients in HSC transplantation settings. (A) CD45.2 BM cells from control or mice treated with Sr for 4 or 8 weeks were mixed with equal amounts of CD45.1 BM cells and were transplanted into lethally irradiated CD45.2/CD45.1 recipient mice (n = 4). Eight weeks after transplantation, the percentage of the CD45.2 population was determined into the PB of the recipients by FACS analysis. Data are mean (± SD). (B) C57BL/6 mice were treated with Sr for 4 weeks and then received transplants of 4 × 106 BM cells before lethal irradiation. Continuous Sr treatment occurred for 1, 2, 3, 4, 6, and 8 weeks after transplant, and blood cell numbers were measured in the PB. An untreated group was used as controls. The number of white blood cells (i), red blood cells (ii), and platelets (iii) are shown. Eight weeks after transplantation recipient mice were killed, and BM cells were assessed for frequency of the Lin− Sca1+ c-kit+ Flk2− cells (C) and CFU-C frequency (D). Data are mean (± SD; n = 8 per group). *** P < .001; * P < .05.
Strontium-treated mice as donors or recipients in HSC transplantation settings. (A) CD45.2 BM cells from control or mice treated with Sr for 4 or 8 weeks were mixed with equal amounts of CD45.1 BM cells and were transplanted into lethally irradiated CD45.2/CD45.1 recipient mice (n = 4). Eight weeks after transplantation, the percentage of the CD45.2 population was determined into the PB of the recipients by FACS analysis. Data are mean (± SD). (B) C57BL/6 mice were treated with Sr for 4 weeks and then received transplants of 4 × 106 BM cells before lethal irradiation. Continuous Sr treatment occurred for 1, 2, 3, 4, 6, and 8 weeks after transplant, and blood cell numbers were measured in the PB. An untreated group was used as controls. The number of white blood cells (i), red blood cells (ii), and platelets (iii) are shown. Eight weeks after transplantation recipient mice were killed, and BM cells were assessed for frequency of the Lin− Sca1+ c-kit+ Flk2− cells (C) and CFU-C frequency (D). Data are mean (± SD; n = 8 per group). *** P < .001; * P < .05.
Discussion
This study further clarifies the role of osteoblasts in the HSC niche by their pharmacologic manipulation with Sr. Sr, like PTH, is currently used for the treatment of osteoporosis. However, their mode of action is different, because continuous PTH treatment increases both osteoclast and osteoblast activity, whereas Sr enhances osteoblast activity but inhibits that of osteoclasts. PTH signals via the PTH/PTHrP receptor-1, whereas Sr acts via the calcium sensing receptor17 and therefore results in the activation of different signaling cascades.
We observed that Sr enhanced the ability of osteoblasts to form mineralized nodules in vitro (Figure 1A) and increased the abundance of runx2 (Figure 1B), one of the earliest osteoblast-specific transcription factors,31 thus supporting the notion that Sr promotes osteoblast activity in vitro.32,33 The same effects were obtained when using PTH. However, PTH also up-regulated RANKL mRNA expression levels (Figure 1C), indicating that, as previously suggested, PTH can activate the osteoblast-osteoclast interaction leading to osteoclastogenesis.34-36 In contrast, it has been shown that Sr inhibits in vitro osteoclast function37,38 and does not produce any bone loss even after long term treatment.
In vivo administration of Sr increased the overall number of osteoblasts leading to altered bone morphometric parameters (Table 1). Sr-treated mice had increased bone volume and trabecular thickness, whereas the trabecula was found to have more interconnections within the BM cavity (Figure 2B). Despite the increase in bone tissue, the actual size of the BM cavity was not significantly reduced (Figure 2C), and the BM cellularity was comparable between Sr-treated and untreated mice. Although these changes were associated with an increment in the number of hematopoietic progenitors (Figure 3A), the frequency of primitive HSC remained unchanged, as demonstrated by the results of the LTC-IC assay (Figure 3B) and the enumeration of Lin− Sca1+ c-kit+ Flk2− cells (Figure 4A).
N-cadherin is expressed on both early and late osteoblast populations,39 and it has been proposed that the N-cadherin+ subset of osteoblasts is crucial in the hematopoietic niche to support the primitive HSC.5,9 In accord with these findings, we observed that, although Sr-treated mice had a approximately 53% increase in the overall number of osteoblasts, the number of the N-cadherin+ osteoblasts in the BM was comparable between Sr-treated and untreated control mice (Figure 2D,E). Therefore, regardless of the amplification in the overall number of osteoblasts, the HSC pool size was unaffected when this particular osteoblast subset remained the same.
Thus, our findings gives new support to the concept that the N-cadherin+ subset of osteoblast are fundamental in the HSC niche and indicate that a nonselective increase in osteoblast numbers is not sufficient to enhance HSC niche activity. On the contrary, recipient mice treated with Sr before conditioning and HSC transplantation experienced a delayed recovery compared with untreated recipients (Figure 5B). In this respect, Sr might promote the formation of osteoblasts with little niche activity that dilute and/or compete with the function of the N-cadherin+ population. Although we cannot completely exclude a direct effect of Sr on HSC because of its action on the calcium-sensing receptor,18 we did not observe any influence of Sr in vitro on the number of CFU-C (data not shown).
The different profiles of stem versus progenitor cells in the BM may reflect distinct regulatory mechanisms governing specific cell compartments in hematopoiesis.40,41 Several studies have previously reported that osteoblasts contribute to myeloid maturation,2-4 and it has been recently demonstrated that they are both necessary and sufficient for murine B cell commitment and maturation.42 An alternative explanation for our results is that Sr affects a subpopulation of osteoblasts that supports and regulates the fate of progenitors but not primitive HSC, raising the unanswered question of whether HSC and other types of progenitors share the same or different subsets of osteoblasts.
Furthermore, Sr has also been shown to inhibit osteoclast formation from hematopoietic precursors.37,38,43 Mice osteopetrotic because of absent or nonfunctional osteoclasts have a severe reduction in marrow space and exhibit extramedullary hematopoiesis.44 The concept of “available marrow space” has also been shown in allogenic HSC transplantation, where the HSC require sufficient BM space to engraft and expand.45 Likewise, it has recently been shown that active bone remodeling plays an important role in stress-induced mobilization of stem and progenitor cells,46 further supporting a direct role of osteoclasts in hematopoiesis.
In conclusion, we have shown that, although osteoblasts are indeed a key functional component of the niche, HSC number is correlated with the number of a subset but not the overall number of osteoblasts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.L. is a recipient of the State Scholarships Foundation of Greece (Iδρυμα Κρατικων ϒποτροϕιων). This work was supported by the Leukemia Research Fund.
Authorship
Contribution: S.L. designed and performed research, analyzed data, and wrote the manuscript. N.H. and F.D. designed research, analyzed data, and wrote the manuscript. S.M. helped perform research. M.Y.G. analyzed and discussed the data. A.P.C. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Dazzi, Stem Cell Biology Section, Department of Haematology, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; e-mail: f.dazzi@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal