Abstract
Salmonella typhimurium engineered to deliver cancer/testis antigen NY-ESO-1 through type III secretion (S typhimurium–NY-ESO-1) was shown to be an efficient cancer vaccine construct in mice and to stimulate NY-ESO-1–specific CD8+/CD4+ T cells in vitro in patients with cancer with NY-ESO-1 spontaneous immunity. We also showed that individuals without spontaneous immunity to NY-ESO-1 had specific CD4+ T-cell precursors with high avidity to NY-ESO-1 under tight control by CD4+CD25+ regulatory T (Treg) cells. We now found that in healthy donors and patients with melanoma without NY-ESO-1 spontaneous immunity, S typhimurium–NY-ESO-1 elicits CD4+ T helper 1 (Th1) cells in vitro recognizing naturally processed antigen from these high-avidity NY-ESO-1–specific naive precursors. In contrast to peptide stimulation, induction of specific Th1 cells with S typhimurium–NY-ESO-1 did not require in vitro depletion of CD4+CD25+ Treg cells, and this prevailing effect was partially blocked by disruption of interleukin-6 or glucocorticoid-induced TNF receptor (GITR) signals. Furthermore, S typhimurium–induced Th1 cells had higher GITR expression than peptide-induced Th1 cells and were resistant to suppression by CD4+CD25+ Treg cells in a GITR-dependent fashion. We propose that S typhimurium–NY-ESO-1 induces antigen-specific T-cell responses that are resistant to suppression by CD4+CD25+ Treg cells.
Introduction
Naturally occurring CD4+CD25+ regulatory T (Treg) cells play an essential role in maintaining immunologic balance and preventing the development of autoimmunity.1-4 Accumulating evidence shows that Treg cell populations are also crucial for controlling antitumor immune responses. In mice, depletion of Treg cell populations enhances spontaneous and vaccine-induced antitumor T-cell responses5-8 and stimulation of CD4+CD25+ Treg cells by immunization with self-antigens enhances the development of chemically induced primary tumors9 and of pulmonary metastases following injection of transplantable tumor cells.10 In humans, the presence of high numbers of CD4+CD25+ Treg cells or a high ratio of CD4+CD25+ Treg cells to CD8+ T cells at the local tumor site is correlated with unfavorable prognosis.11,12 From these results, it is becoming an important priority to find strategies for controlling Treg cells in the cancer vaccine field
NY-ESO-1, a germ-cell protein, was originally found by SEREX (serological identification of antigens by recombinant expression cloning) using the serum of an esophageal cancer patient.13-15 Its expression pattern and the frequent finding of humoral and cellular immune responses against this antigen in patients with cancer with NY-ESO-1–expressing tumors make NY-ESO-1 one of the most intriguing cancer vaccine targets.14-17 In monitoring a large series of patients with cancer, humoral responses to NY-ESO-1 were found to be correlated with the presence of peripheral CD8+ T cells against NY-ESO-1, suggesting the involvement of CD4+ helper T (Th) cells in coordinating these responses.15-18 It was indeed confirmed that effector CD4+ Th cell responses to NY-ESO-1 were only observed in patients with cancer who had antibodies against NY-ESO-1.15,18 However, it has recently been shown that NY-ESO-1–specific CD4+ T-cell precursors are also present in patients with NY-ESO-1–expressing tumors but without NY-ESO-1–specific antibody as well as in healthy individuals, and that CD4+CD25+ Treg cells play a critical role in keeping these NY-ESO-1–specific precursors under control.15,19-21 The preexisting NY-ESO-1–specific CD4+ T-cell precursors of seronegative and healthy individuals are exclusively from a naive (CD4+CD25−CD45RA+) repertoire with high avidity to antigen and are highly sensitive to Treg cells, while spontaneously induced NY-ESO-1–specific CD4+ T cells of seropositive patients are derived from an effector/memory (CD4+CD25−CD45RO+) repertoire with high avidity to antigen but low sensitivity to Treg cells.15,20,21 Vaccinating patients with epithelial ovarian cancer with HLA-DPB1*0401/0402–restricted NY-ESO-1157-170 peptide in the presence of incomplete Freund adjuvant results in the induction of NY-ESO-1-specific CD4+ T cells derived from an effector/memory (CD4+CD25-CD45RO+) repertoire with only low avidity to antigen and low sensitivity to Treg cells.15,21 These peptide vaccine–induced NY-ESO-1–specific T cells do not recognize naturally processed NY-ESO-1, while high-avidity naive NY-ESO-1–specific T-cell precursors are still present in peripheral blood but subject to continuous CD4+CD25+ Treg cell suppression throughout vaccination.15,21 Thus, a strategy to break ongoing suppression on preexisting high-avidity naive T-cell precursors is a critical element for an effective vaccine.
Recently, potential strategies including recombinant interleukin-2 (IL-2) diphtheria toxin conjugate DAB389IL-2 (targeting CD25, known as denileukin diftitax) and chemotherapy were exploited for eliminating CD4+CD25+ Treg cell populations in cancer vaccine therapies.22-25 Although the effect of Treg cell depletion remains to be determined in patients with cancer, a recent report showed an enhancement of vaccine-mediated antitumor immunity.24 However, an inherent risk to such systemic approaches is the emergence of potential autoimmunity. Glucocorticoid-induced TNF receptor (GITR) was originally reported as a molecule with direct functional relevance with regard to CD4+CD25+ Treg cells, and stimulation of GITR on Treg cells abrogates their suppressive activity.26 A recent GITR knockout mouse study has revealed that the reversal of Treg cell suppression by GITR signaling is attributable to the costimulatory activity of agonistic anti-GITR antibody on the responder CD4+CD25− T cells rather than a direct effect on Treg cells.27,28 Recently, a number of studies shed new light on recognition of pathogen-associated molecular patterns through Toll-like receptors (TLRs) to break the suppressive environment present at the tumor local site.29-34 TLR signals are not only able to block the suppressive activity of CD4+CD25+ Treg cells, but are also able to break CD8 tolerance even in the presence of CD4+CD25+ Treg cells.29-33 Based on this evidence, viral and bacterial vectors that are able to stimulate TLR signaling are attracting much attention. We have recently reported that an avirulent recombinant strain of Salmonella typhimurium endowed with the capacity to deliver NY-ESO-1 (S typhimurium–NY-ESO-1) through its type III secretion system efficiently elicits NY-ESO-1–specific CD8+ and CD4+ T-cell responses in vitro from blood of patients with cancer with spontaneous immunity, while immunization with this construct in mice results in the regression of pre-established NY-ESO-1–expressing tumors.35,36 In this report, we focused on the capacity of S typhimurium to overcome the suppressive activity of CD4+CD25+ Treg cells. S typhimurium was able to elicit high-avidity NY-ESO-1–specific CD4+ T-cell responses from naive T-cell precursors in the presence of CD4+CD25+ Treg cells in healthy individuals and in patients with NY-ESO-1–expressing tumors but seronegative for NY-ESO-1. IL-6 and GITR signaling synergistically worked to break the suppressive activity of CD4+CD25+ Treg cells. Furthermore, S typhimurium–induced CD4+ Th1 cells expressed GITR and were resistant to suppression by CD4+CD25+ Treg cells, and this resistance was abrogated by blocking GITR signaling.
Methods
Donor samples
All healthy donors had no history of autoimmune disease. All patients had NY-ESO-1–expressing melanoma except NW1060 with sarcoma. The HLA typing of healthy donors and patients (except NC235, who is HLA-DPB1*0401) was described previously.20 All samples were collected as part of a study approved by the Ethics Committee of Landesärztekammer Hessen (Frankfurt, Germany). Informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
Synthetic peptides NY-ESO-187-98 (LLEFYLAMPFAT), NY-ESO-1121-132 (VLLKEFTVSGNI), NY-ESO-1143-154 (RQLQLSISSCLQ), NY-ESO-1157-170 (SLLMWITQCFLPVF), and HIV P1737-51 (ASRELERFAVNPGLL) were obtained from Bio-Synthesis (Lewisville, TX).20 Recombinant full-length NY-ESO-1 or SSX-2 proteins were prepared using procedures described previously.37 Anti–IL-4 (MP4-25D2, rat IgG1), anti–IL-6 (MQ2-13A5, rat IgG1), anti–IL-12 (C8.6, mouse IgG1), anti–IFN-γ (NIB42; mouse IgG1), anti–TNF-α (MAb1; mouse IgG1), and phycoerythrin (PE)–conjugated anti-CD62L (DREG-56; mouse IgG1) antibodies were purchased from eBioscience (San Diego, CA). Blocking anti-GITRL (109101; mouse IgG1) monoclonal antibody and recombinant IL-6 were purchased from R&D Systems (Minneapolis, MN). PE-conjugated anti-GITRL (EB11-2; mouse IgG1) mAb was purchased from BioLegend (San Diego, CA). PE-conjugated anti-CD25 (M-A251; mouse IgG1), anti-CD44 (515; mouse IgG1), anti-CD154 (TRAP1; mouse IgG1), and anti-CD45RO (UCHL1; mouse IgG2a) antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). Purified anti-CD3 mAb (UCHT1; mouse IgG1) was purchased from BD Biosciences. A plasmid encoding human GITRL was purchased from Invitrogen (Carlsbad, CA). GITRL cDNA was subcloned into pFUSE-Fc, a mammalian expression vector for Fc-fusion protein (Invivogen, San Diego, CA). This plasmid was transfected into COS-7 cells to produce recombinant GITRL-Fc protein in culture supernatant. Then, secreted GITRL-Fc protein was collected and purified with protein A beads according to the manufacturer's protocol (Pierce, Rockford, IL).
S typhimurium infection
The S typhimurium ΔphoP-phoQ strain recombinant for NY-ESO-1 and control strains have been described previously.35,36 S typhimurium was grown as described previously.35,36 Target cells were infected with the different S typhimurium strains for 1 hour at 37°C with at a multiplicity of infection (MOI) of 40. Extracellular bacteria were killed by transferring the target cells to RPMI 1640 medium containing 100 μg/mL gentamicin (Sigma-Aldrich, St Louis, MO) and incubating at 37°C for 1 hour.36 Infected cells were subsequently used as antigen-presenting cells (APCs).
Generation of NY-ESO-1–specific CD4+ T cells
NY-ESO-1–specific CD4+ T cells were elicited as described previously.20 Briefly, CD4+ T cells and CD4+CD25− T cells were isolated from peripheral blood mononuclear cells (PBMCs) using the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec, Auburn, CA). In some experiments, CD4+CD25− T cells were further separated into CD45RO-depleted T cells (CD4+CD25−CD45RA+ T cells) or CD45RA-depleted T cells (CD4+CD25−CD45RO+ T cells) using CD45RO Microbeads or CD45RA Microbeads (Miltenyi Biotec), respectively.20 The purity of selected populations was confirmed to be greater than 96%.20 Non-CD4+ cells retained in the magnetic-activated cell sorter (MACS) separation column were flushed out and were used to prepare APCs. These non-CD4+ cells were allowed to adhere to tissue culture plates (Corning Glass, Corning, NY) for 2 hours, and nonadherent cells were removed by washing. The adherent cells pulsed with 10 μM of 1 or 2 peptides overnight or infected with S typhimurium were used as APCs. After irradiation, 5 × 105 APC were added to round-bottom 96-well plates (Corning) containing 1 to 5 × 105 unfractionated CD4+, CD4+CD25−, CD4+CD25−CD45RO+, or CD4+CD25−CD45RA+ T cells and were fed with 10 U/mL IL-2 (Roche Molecular Biochemicals, Indianapolis, IN). Subsequently, one-half of medium was replaced by fresh medium containing IL-2 (20 U/mL) twice per week.
ELISPOT assay
The number of IFN-γ–secreting antigen-specific CD4+ T cells was assessed by enzyme-linked immunospot (ELISPOT) assays as described previously.18,20,38 Briefly, flat-bottomed, 96-well nitrocellulose-coated microtiter plates (Millipore, Bedford, MA) were coated with anti–IFN-γ antibody (1-D1K; MABTECH, Stockholm, Sweden). The presensitized T cells and phytohaemagglutinin (PHA HA15; Murex Diagnostics, Dartford, United Kingdom)–activated CD4+ T cells,38 or dendritic cells (DCs) pulsed with 10 μM or indicated amount of peptides, or 25 μg/mL protein overnight were added to each well and incubated for 24 hours. Spots were developed using biotinylated anti–IFN-γ antibody (7-B6-1-biotin; MABTECH), alkaline phosphatase–conjugated streptavidin (Roche Diagnostics, Mannheim, Germany), and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Sigma-Aldrich) and counted with C. T. L. Immunospot analyzer and software (Cellular Technologies, Cleveland, OH).
Preparation of monocyte-derived DCs
Monocyte-derived DCs were generated from PBMCs as described previously.21,39 Briefly, CD14+ monocytes were enriched by positive selection using CD14 Microbeads (Miltenyi Biotec) according to the manufacturer's instruction. Monocytes were cultured in the presence of 1000 U/mL GM-CSF (Immunex, Thousand Oaks, CA) and 20 ng/mL IL-4 (R&D Systems) in X-VIVO-15 serum-free medium (CAMBREX, Walkersville, MD). Medium was replaced by fresh medium containing cytokines 3 days later. On day 6, DCs were harvested and pulsed overnight with 10 μM peptide or 25 μg/mL protein.
CFSE labeling
Presensitized CD4+ T cells were labeled for 7 minutes at 37°C with 1 μM CFSE (Molecular Probes, Eugene, OR) and were washed 3 times with RPMI 1640 containing 10% fetal bovine serum. CFSE-labeled CD4+ T cells were cultured with irradiated CD3-depleted PBMCs pulsed with 10 μM peptide for 6 days. CFSE-labeled cells were analyzed for fluorescence intensity using a flow cytometer (FACSCalibur; BD Biosciences).
Proliferation assay
A total of 5 × 104 presensitized T cells were cultured in the presence or absence of 5 × 104 Treg cells with 5 × 104 irradiated CD3-depleted PBMCs pulsed with 10 μM of peptides. CD4+ T cells were enriched from PBMCs by negative selection using the CD4+ Isolation Kit (Miltenyi Biotec), followed by flow cytometric sorting of CD4+CD25high T cells (FACSAria; BD Biosciences). These CD4+CD25high cells were stimulated with 1 μg/mL anti-CD3 mAb–coated plates for 3 days with 10 U/mL IL-2 and used as Treg cells. Proliferation was evaluated by pulsing cells with 0.037 MBq/well (1 μCi/well) 3H thymidine for the last 18 hours of a 72-hour culture. 3H thymidine incorporation was measured by a scintillation counter.
Statistical analysis
Statistical analyses were performed with the Student paired t test. P values less than .05 were considered significant.
Results
NY-ESO-1–specific CD4+ T cells are elicited by S typhimurium–NY-ESO-1 without CD4+CD25+ T-cell depletion
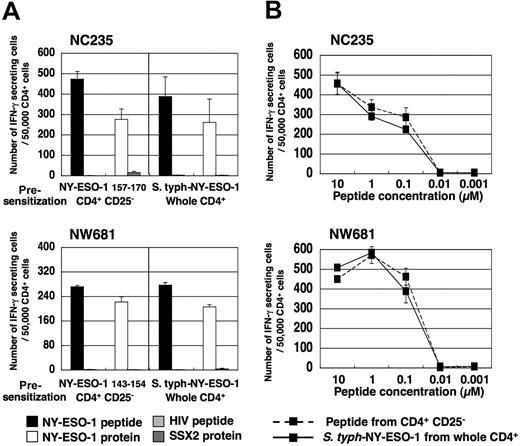
CD4+ T cells and CD4+CD25− T cells were isolated from PBMCs and were cultured with APCs pulsed with a series of HLA class II–restricted NY-ESO-1 peptides or infected with S typhimurium–NY-ESO-1, a S typhimurium ΔphoP ΔphoQ avirulent strain carrying a plasmid that expresses a chimeric protein composed of the first 104 amino acids of the type III secreted protein SopE fused to reporter epitopes and the entire amino acid sequence of the NY-ESO-1 tumor antigen, or with a S typhimurium control strain carrying an equivalent plasmid that expresses only the first 104 amino acids of the type III secreted protein SopE and reporter epitopes but not NY-ESO-1, as reported previously.20,36 At 15 to 20 days later, peptide-specific interferon (IFN)–γ–secreting CD4+ Th1 cell induction was analyzed by ELISPOT and proliferation assays. In accordance with our previous reports using NY-ESO-1 peptide-pulsed APCs,20,21 specific Th1 cells were elicited in a proportion of healthy donors and patients with cancer seronegative for NY-ESO-1, but only after CD4+CD25+ T-cell depletion (Figure 1; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, when using APCs infected with S typhimurium–NY-ESO-1, specific Th1 cells were elicited from whole CD4+ T cells without the need for CD4+CD25+ T-cell depletion (Figures 1 and S1). This specific T-cell induction was dependent on NY-ESO-1 antigen delivery by S typhimurium because the S typhimurium control strain was not able to induce NY-ESO-1 peptide-specific CD4+ Th1 cells (Figures 1, S1). Interestingly, S typhimurium–NY-ESO-1 only elicited Th1 responses in healthy donors and patients for whom Th1 cells were also elicited by peptide-pulsed APCs following CD4+CD25+ T-cell depletion, namely having NY-ESO-1–specific CD4+ T-cell precursors (Figures 1, S1). Because of limitations in sample amounts, we selected 2 typical donors, NC235 and NW681, for further studies with healthy donors and patients with NY-ESO-1–expressing tumors but seronegative for NY-ESO-1, respectively.
NY-ESO-1–specific CD4+ T cells are elicited by S typhimurium–NY-ESO-1 without the need for CD4+CD25+ T-cell depletion. Presence of NY-ESO-1 peptide-specific CD4+ Th1 cells in whole CD4+ T cells and CD4+CD25− T cells was analyzed by ELISPOT assays following a 15- to 20-day culture with APCs pulsed with indicated NY-ESO-1 peptides (except NC155; NY-ESO-1 157-170) or infected with S typhimurium–NY-ESO-1 (S typh–NY-ESO-1) or S typhimurium control strain (S typh–control strain). Responses were analyzed by specific IFN-γ secretion for recognition of autologous T-APCs pulsed with indicated peptide (except NC155; NY-ESO-1 157-170 [▩], or HIV peptide [□]), in 4 healthy donors and in 4 patients with NY-ESO-1–expressing tumors but without NY-ESO-1 antibody. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
NY-ESO-1–specific CD4+ T cells are elicited by S typhimurium–NY-ESO-1 without the need for CD4+CD25+ T-cell depletion. Presence of NY-ESO-1 peptide-specific CD4+ Th1 cells in whole CD4+ T cells and CD4+CD25− T cells was analyzed by ELISPOT assays following a 15- to 20-day culture with APCs pulsed with indicated NY-ESO-1 peptides (except NC155; NY-ESO-1 157-170) or infected with S typhimurium–NY-ESO-1 (S typh–NY-ESO-1) or S typhimurium control strain (S typh–control strain). Responses were analyzed by specific IFN-γ secretion for recognition of autologous T-APCs pulsed with indicated peptide (except NC155; NY-ESO-1 157-170 [▩], or HIV peptide [□]), in 4 healthy donors and in 4 patients with NY-ESO-1–expressing tumors but without NY-ESO-1 antibody. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells are able to recognize naturally processed NY-ESO-1 protein
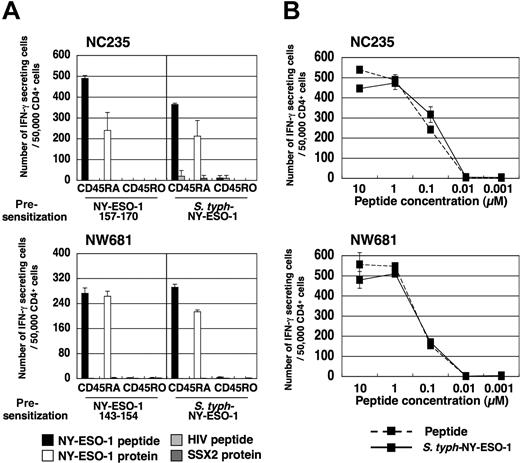
Our previous data have suggested that synthetic peptide vaccination induces CD4+ T cells with lower avidity than preexisting NY-ESO-1–specific CD4+ T-cell precursors and spontaneously induced CD4+ T cells.15,20,21 For this reason, it is interesting to investigate the avidity of CD4+ T cells induced by S typhimurium. First, we assessed whether S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells were able to recognize naturally processed NY-ESO-1 protein. Whole CD4+ T cells derived from healthy donor NC235 and melanoma patient NW681 were presensitized with S typhimurium–infected APCs and analyzed for their capacity to recognize naturally processed antigen by ELISPOT assay using protein-pulsed autologous DCs as APCs. As positive control, we confirmed that NY-ESO-1–specific CD4+ Th1 cells derived from CD4+CD25− T cells and presensitized with APCs pulsed with NY-ESO-1 peptides had high avidity and were able to recognize NY-ESO-1 protein-pulsed DCs (Figure 2), as previously reported.21 Remarkably, NY-ESO-1–specific CD4+ Th1 cells induced by S typhimurium–NY-ESO-1 from whole CD4+ T cells, without the need for CD4+CD25+ T-cell depletion, were similarly able to recognize both NY-ESO-1 peptide-pulsed and protein-pulsed DCs (Figure 2A). Next, specific IFN-γ secretion by these cells was analyzed against PHA-activated CD4+ T cells (T-APCs) pulsed with serial dilutions of peptides by ELISPOT assay. S typhimurium–induced Th1 cells derived from whole CD4+ T cells and peptide-induced Th1 cells derived from CD4+CD25− T cells were both high avidity, and could recognize as little as 0.1 μM of peptide (Figure 2B).
S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells are able to recognize naturally processed NY-ESO-1 protein. (A) Whole CD4+ T cells or CD4+CD25− T cells of NC235 or NW681 were isolated from PBMCs and cultured with APCs infected with S typhimurium–NY-ESO-1 or pulsed with indicated NY-ESO-1 peptide, respectively. At 15 to 20 days later, the capacity of elicited NY-ESO-1–specific Th1 cells to recognize naturally processed NY-ESO-1 protein was analyzed by ELISPOT assay using NY-ESO-1 (NC235, NY-ESO-1 157–170; NW681, NY-ESO-1 143–154) or control HIV peptide-pulsed or NY-ESO-1 or SSX-2 protein-pulsed DCs as APCs. (B) Avidity of induced NY-ESO-1–specific Th1 cells was analyzed by ELISPOT assay using T-APCs pulsed with serial dilutions of peptides. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells are able to recognize naturally processed NY-ESO-1 protein. (A) Whole CD4+ T cells or CD4+CD25− T cells of NC235 or NW681 were isolated from PBMCs and cultured with APCs infected with S typhimurium–NY-ESO-1 or pulsed with indicated NY-ESO-1 peptide, respectively. At 15 to 20 days later, the capacity of elicited NY-ESO-1–specific Th1 cells to recognize naturally processed NY-ESO-1 protein was analyzed by ELISPOT assay using NY-ESO-1 (NC235, NY-ESO-1 157–170; NW681, NY-ESO-1 143–154) or control HIV peptide-pulsed or NY-ESO-1 or SSX-2 protein-pulsed DCs as APCs. (B) Avidity of induced NY-ESO-1–specific Th1 cells was analyzed by ELISPOT assay using T-APCs pulsed with serial dilutions of peptides. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
S typhimurium–NY-ESO-1 activates high-avidity NY-ESO-1–specific CD4+ T-cell precursors from the naive T-cell population
We have reported that high-avidity NY-ESO-1–specific CD4+ T-cell precursors are present in naive CD45RA+ populations, and that their activation is highly suppressed by CD4+CD25+ Treg cells.15,20,21 We investigated whether S typhimurium also would elicit NY-ESO-1–specific CD4+ Th1 cells by activating high-avidity NY-ESO-1–specific CD4+ T-cell precursors from CD45RA+ populations. CD4+CD25− T cells were further separated into naive and effector/memory populations according to typical surface-marker molecules CD45RA and CD45RO, respectively.20 NY-ESO-1–specific Th1 cells were exclusively elicited by S typhimurium–NY-ESO-1 from CD4+CD25−CD45RA+ naive precursors, but not from CD4+CD25−CD45RO+ memory populations of NC235 and NW681. Similar results were obtained in experiments using separated naive and memory populations from whole CD4+ T cells, without depletion of CD25+ T cells (data not shown). These NY-ESO-1–specific CD4+ Th1 cells were able to recognize naturally processed protein antigen. They also had high avidity and could recognize as little as 0.1 μM of peptide, similar to peptide-induced Th1 cells derived from CD4+CD25− T cells (Figure 3).
S typhimurium–NY-ESO-1 activates high-avidity NY-ESO-1–specific CD4+ T-cell precursors from a naive T-cell population (A) CD4+CD25− T cells isolated from PBMCs of NC235 or NW681 were further separated into CD45RA+ or CD45RO+ cells using magnetic beads as described in “Methods.” These T cells were cultured with APCs pulsed with a NY-ESO-1 peptide or infected with S typhimurium–NY-ESO-1. At 15 to 20 days later, specific T-cell elicitation and capacity of elicited NY-ESO-1–specific Th1 cells to recognize naturally processed NY-ESO-1 protein was analyzed by ELISPOT assay using NY-ESO-1 (NC235, NY-ESO-1 157–170; NW681, NY-ESO-1 143–154) or control HIV peptide-pulsed or NY-ESO-1 or SSX-2 protein-pulsed DCs as APCs. (B) Avidity of induced NY-ESO-1–specific Th1 cells was analyzed by ELISPOT assay using T-APCs pulsed with serial dilutions of peptides. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
S typhimurium–NY-ESO-1 activates high-avidity NY-ESO-1–specific CD4+ T-cell precursors from a naive T-cell population (A) CD4+CD25− T cells isolated from PBMCs of NC235 or NW681 were further separated into CD45RA+ or CD45RO+ cells using magnetic beads as described in “Methods.” These T cells were cultured with APCs pulsed with a NY-ESO-1 peptide or infected with S typhimurium–NY-ESO-1. At 15 to 20 days later, specific T-cell elicitation and capacity of elicited NY-ESO-1–specific Th1 cells to recognize naturally processed NY-ESO-1 protein was analyzed by ELISPOT assay using NY-ESO-1 (NC235, NY-ESO-1 157–170; NW681, NY-ESO-1 143–154) or control HIV peptide-pulsed or NY-ESO-1 or SSX-2 protein-pulsed DCs as APCs. (B) Avidity of induced NY-ESO-1–specific Th1 cells was analyzed by ELISPOT assay using T-APCs pulsed with serial dilutions of peptides. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.
Taken together, these data suggest that S typhimurium–NY-ESO-1 is able to elicit NY-ESO-1–specific high-avidity CD4+ Th1 cells in the presence of CD4+CD25+ Treg cells from NY-ESO-1–specific preexisting naive CD4+ T-cell precursors.
IL-6 induced by infection of S typhimurium is required to elicit NY-ESO-1–specific CD4+ Th1 cells
We next asked whether the infection signal of S typhimurium was required to elicit NY-ESO-1–specific CD4+ Th1 cells in the presence of CD4+CD25+ Treg cells. To address this question, APCs infected with a S typhimurium control strain identical to S typhimurium–NY-ESO-1 but lacking expression of the NY-ESO-1 protein were pulsed with NY-ESO-1 peptide and used to stimulate whole CD4+ T cells. As shown in Figure 4A, NY-ESO-1–specific CD4+ Th1 cells were elicited by combining S typhimurium control strain infection and NY-ESO-1 peptide pulse. In contrast, S typhimurium control strain alone was not able to elicit NY-ESO-1–specific CD4+ Th1 cells.
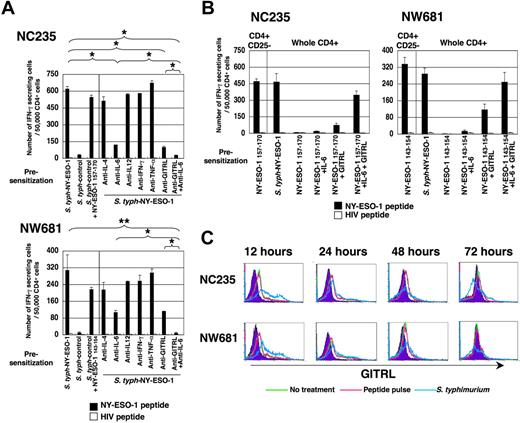
IL-6 and GITR signal is essential for elicitation of NY-ESO-1–specific CD4+ Th1 cells. (A) Whole CD4+ T cells were isolated from PBMCs of NC235 or NW681 and cultured with APCs infected with S typhimurium–NY-ESO-1 or S typhimurium control strain without or with 10 μg/mL of indicated antibodies. At 15 to 20 days later, responses were analyzed by specific IFN-γ secretion for recognition of T-APCs pulsed with NY-ESO-1 (NC235, NY-ESO-1 157-170; NW681, NY-ESO-1 143-154) or control HIV peptide. (B) Whole CD4+ T cells were isolated from PBMCs of NC235 or NW681 and cultured with APCs infected with S typhimurium–NY-ESO-1 or pulsed with indicated NY-ESO-1 peptides with as well as recombinant IL-6 (10 ng/mL), GITRL-Fc (5 μg/mL), or both. At 15 to 20 days later, responses were analyzed by specific IFN-γ secretion for recognition of T-APCs pulsed with NY-ESO-1 (NC235, NY-ESO-1 157-170; NW681, NY-ESO-1 143-154) or control HIV peptide. (C) APCs used for presensitization were prepared from PBMCs of NC235 or NW681 and infected with S typhimurium–NY-ESO-1. The kinetics of GITRL expression after S typhimurium infection was analyzed. These experiments were performed independently at least twice with similar results. Data in panels A and B are expressed as means plus or minus SD. *P < .05; **P < .01.
IL-6 and GITR signal is essential for elicitation of NY-ESO-1–specific CD4+ Th1 cells. (A) Whole CD4+ T cells were isolated from PBMCs of NC235 or NW681 and cultured with APCs infected with S typhimurium–NY-ESO-1 or S typhimurium control strain without or with 10 μg/mL of indicated antibodies. At 15 to 20 days later, responses were analyzed by specific IFN-γ secretion for recognition of T-APCs pulsed with NY-ESO-1 (NC235, NY-ESO-1 157-170; NW681, NY-ESO-1 143-154) or control HIV peptide. (B) Whole CD4+ T cells were isolated from PBMCs of NC235 or NW681 and cultured with APCs infected with S typhimurium–NY-ESO-1 or pulsed with indicated NY-ESO-1 peptides with as well as recombinant IL-6 (10 ng/mL), GITRL-Fc (5 μg/mL), or both. At 15 to 20 days later, responses were analyzed by specific IFN-γ secretion for recognition of T-APCs pulsed with NY-ESO-1 (NC235, NY-ESO-1 157-170; NW681, NY-ESO-1 143-154) or control HIV peptide. (C) APCs used for presensitization were prepared from PBMCs of NC235 or NW681 and infected with S typhimurium–NY-ESO-1. The kinetics of GITRL expression after S typhimurium infection was analyzed. These experiments were performed independently at least twice with similar results. Data in panels A and B are expressed as means plus or minus SD. *P < .05; **P < .01.
Since the signal provided by S typhimurium infection is sufficient to bypass CD4+CD25+ Treg cell suppression and uncover NY-ESO-1–specific Th1 cell precursors, we explored the requirement of several cytokines that could be produced by S typhimurium infection in this process using monoclonal antibody blocking. Among cytokines examined, only blocking of IL-6 partially inhibited the elicitation of NY-ESO-1–specific Th1 cells(Figure 4A; NC235, P < .01; NW681, P = .06). However, IL-6 signaling alone was not sufficient to bypass Treg cell suppression, since no NY-ESO-1 peptide-specific Th1 cells could be detected from whole CD4+ T cells by peptide-pulsed APCs in the presence of recombinant IL-6 (Figure 4B). These data suggest that other mechanism(s) also contribute to the Treg cell counteracting capacity of S typhimurium.
GITR signal is also required for induction of NY-ESO-1–specific CD4+ Th1 cells by S typhimurium
Recently, it has been reported that GITR signaling is important to overcome suppression of CD4+CD25+ Treg cells and that GITR ligand (GITRL) cell-surface expression is up-regulated after viral infection.27,40 We first examined the kinetics of GITRL expression following S typhimurium infection. As shown in Figure 4C, the expression level of GITRL on APCs used for presensitization was up-regulated after S typhimurium infection. The expression was the highest after 12 hours and decreased thereafter to lower levels than the basal expression. As predicted, blocking the GITR signal using anti-GITRL antibody partially inhibited the elicitation of NY-ESO-1–specific CD4+ Th1 cells (Figure 4A; NC235, P < .01; NW681, P = .06). Furthermore, the presence of both anti-GITRL and anti–IL-6 blocking antibodies completely inhibited the elicitation of NY-ESO-1–specific CD4+ Th1 cells by S typhimurium (Figure 4A; NC235, P < .01; NW681, P < .05). Alternatively, providing exogenous GITR signaling with a fusion GITRL-Fc protein was able to partially overcome Treg cell suppression, since NY-ESO-1 peptide-specific CD4+ Th1 cells were elicited from whole CD4+ T cells by peptide-pulsed APCs in the presence of GITRL-Fc (Figure 4B). Induction of NY-ESO-1 peptide-specific CD4+ Th1 cells from whole CD4+ T cells by peptide-pulsed APCs was further enhanced in the presence of both GITRL-Fc and IL-6. These data suggest that IL-6 and GITR signals act independently and synergistically to overcome the suppression of CD4+CD25+ Treg cells.
S typhimurium–induced CD4+ Th1 cells are resistant against suppression of CD4+CD25+ Treg cells
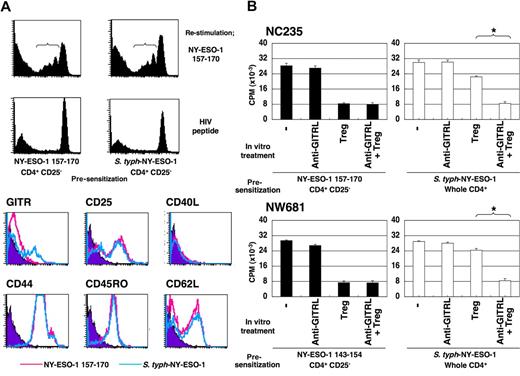
We next examined potential differences between CD4+ Th1 cells induced by NY-ESO-1 peptide after CD4+CD25+ T-cell depletion and S typhimurium–induced CD4+ Th1 cells. To minimize differences in the procedures and avoid potential influence of CD4+CD25+ Treg cells on the phenotype of responder cells, both peptide-induced CD4+ Th1 cells and S typhimurium–induced CD4+ Th1 cells were generated from CD4+CD25− T-cell populations. To identify NY-ESO-1–specific CD4+ T cells, presensitized CD4+ T cells were labeled with CFSE and restimulated with NY-ESO-1 or control peptides. Cells showing CFSE dilution were considered to contain NY-ESO-1–specific CD4+ T cells and were gated. Among surface activation markers tested, only GITR expression was higher on S typhimurium–induced CD4+ Th1 cells than on peptide-induced CD4+ Th1 cells, while other surface activation markers were similarly expressed on specific Th1 cells by both induction methods (Figure 5A). This up-regulation of GITR expression was also observed when S typhimurium–induced CD4+ Th1 cells were elicited from whole CD4+ T cells (data not shown).
S typhimurium–induced CD4+ Th1 cells are resistant against suppression by CD4+CD25+ Treg cells. (A) CD4+CD25− T cells isolated from PBMCs of NC235 were cultured with APCs pulsed with a NY-ESO-1 peptide or infected with S typhimurium–NY-ESO-1 for 20 days. The presensitized T cells were labeled with CFSE as described in “CFSE labeling” and restimulated with T cell–depleted PBMCs pulsed with NY-ESO-1 or control peptides for 6 days. The cells with diluted CFSE staining were gated, and surface activation markers were analyzed as indicated. (B). CD4+CD25+ Treg cells were isolated from PBMCs and preactivated as described in “CFSE labeling.” S typhimurium–induced or peptide-induced CD4+ Th1 cells were cultured with T cell–depleted PBMCs pulsed with cognate NY-ESO-1 peptides in the absence or presence of these preactivated CD4+CD25+ Treg cells and/or 10 μg/mL blocking anti-GITRL antibody. Proliferation was assessed as described in “Proliferation assay.” These experiments were performed independently at least twice with similar results. Data in panel B are expressed as means plus or minus SD. *P ≤ .01.
S typhimurium–induced CD4+ Th1 cells are resistant against suppression by CD4+CD25+ Treg cells. (A) CD4+CD25− T cells isolated from PBMCs of NC235 were cultured with APCs pulsed with a NY-ESO-1 peptide or infected with S typhimurium–NY-ESO-1 for 20 days. The presensitized T cells were labeled with CFSE as described in “CFSE labeling” and restimulated with T cell–depleted PBMCs pulsed with NY-ESO-1 or control peptides for 6 days. The cells with diluted CFSE staining were gated, and surface activation markers were analyzed as indicated. (B). CD4+CD25+ Treg cells were isolated from PBMCs and preactivated as described in “CFSE labeling.” S typhimurium–induced or peptide-induced CD4+ Th1 cells were cultured with T cell–depleted PBMCs pulsed with cognate NY-ESO-1 peptides in the absence or presence of these preactivated CD4+CD25+ Treg cells and/or 10 μg/mL blocking anti-GITRL antibody. Proliferation was assessed as described in “Proliferation assay.” These experiments were performed independently at least twice with similar results. Data in panel B are expressed as means plus or minus SD. *P ≤ .01.
The capacity of S typhimurium–NY-ESO-1 to elicit NY-ESO-1–specific high-avidity CD4+ Th1 cells in the presence of CD4+CD25+ Treg cells prompted us to examine whether S typhimurium–induced CD4+ Th1 cells were resistant to CD4+CD25+ Treg cells. To maintain antigen specificity of CD4+ Th1 cells, we avoided using anti-CD3 antibody for in vitro CD4+CD25+ Treg cell activation during the coculture with effector cells. Rather, CD4+CD25+ Treg cells were isolated from PBMCs and preactivated with anti-CD3 antibody-coated plates for 3 days. Then, CD4+CD25− or CD4+ T cells presensitized with peptide or S typhimurium–NY-ESO-1, respectively, were cocultured with T-cell–depleted PBMCs pulsed with NY-ESO-1 peptides in the presence or absence of preactivated Treg cells. These Treg cells showed suppressive activity against NY-ESO-1–specific proliferation of peptide-induced Th1 cells (Figure 5B). In contrast, S typhimurium–induced CD4+ Th1 cells were able to proliferate in the presence of preactivated Treg cells (Figure 5B), thus displaying relative resistance to Treg cells. Since expression levels of GITR significantly differed between NY-ESO-1 peptide-induced and S typhimurium–induced CD4+ Th1, the effect of blocking GITR signaling was assessed by anti-GITRL antibody (antagonistic anti-GITR blocking reagents are not yet available). As shown in Figure 5B, the treatment completely abrogated the resistance to CD4+CD25+ Treg cells of S typhimurium–induced CD4+ Th1 cells (NC235, P < .01; NW681; P = .01).
Discussion
We have recently engineered a recombinant S typhimurium–NY-ESO-1 strain that uses a specialized type III protein secretion system to inject bacterially produced NY-ESO-1 protein into host cells.36 This strain could elicit NY-ESO-1–specific CD8+ and CD4+ T-cell responses in vitro in patients with cancer with NY-ESO-1 spontaneous immunity and eradicate NY-ESO-1–expressing tumors in vivo in mice.36 In the current study, we examined the capacity of this construct to influence the suppressive activity of CD4+CD25+ Treg cells and elicit NY-ESO-1–specific CD4+ Th1 cells resistant to Treg cell suppression. We found that S typhimurium–NY-ESO-1 could elicit NY-ESO-1–specific CD4+ Th1 cells in vitro in healthy donors as well as in patients with melanoma with NY-ESO-1–expressing tumors but without spontaneous NY-ESO-1 immunity. Stimulation with S typhimurium–NY-ESO-1 was able to elicit NY-ESO-1–specific CD4+ Th1 cells in these patients in the presence of CD4+CD25+ Treg cells, while peptide stimulation required prior depletion of CD4+CD25+ Treg cells to do so (Figures 1 and S1). Furthermore, S typhimurium–NY-ESO-1 elicited NY-ESO-1–specific CD4+ Th1 cells from high-avidity NY-ESO-1–specific naive CD4+ T-cell precursors (Figures 2,3), which are normally kept under CD4+CD25+ Treg cell tight control.21 As a result, S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells had high avidity and recognized naturally processed protein antigen as efficiently as CD4+ Th1 cells elicited from patients with NY-ESO-1 spontaneous immunity. These data suggest that S typhimurium–NY-ESO-1 can elicit NY-ESO-1–specific CD4+ Th1 cells by inhibiting or resisting the suppressive activity of CD4+CD25+ Treg cells. These S typhimurium–induced NY-ESO-1–specific CD4+ Th1 cells could therefore be closer to CD4+ Th1 readily observed in patients with NY-ESO-1 spontaneous immunity, where they may contribute to B-cell activation for establishing specific antibody and help CD8+ T-cell induction.15,18
Our data also showed that induction of NY-ESO-1–specific CD4+ Th1 cells by S typhimurium–NY-ESO-1 was strictly limited to patients with cancer and healthy donors with high-avidity NY-ESO-1–specific naive CD4+ T-cell precursors (Figures 1,S1). These data suggest that we may predict which individuals are better responders to vaccination with S typhimurium–NY-ESO-1 and elicit NY-ESO-1–specific CD4+ T cells, using in vitro assays to examine the presence of high-avidity NY-ESO-1–specific naive CD4+ T-cell precursors.
The capacity of S typhimurium to overcome the suppressive activity of CD4+CD25+ Treg cells was dependent on IL-6 but not on other inflammatory cytokines tested (Figure 4A). IL-6, a proinflammatory cytokine that plays an important role in host defense against infections, is also considered to have an important role for the inhibition of CD4+CD25+ Treg cells.30,41 IL-6 secretion from APCs following bacterial infection, including S typhimurium infection, is considered to be the consequence of TLR stimulation by pathogen-associated molecules such as lipopolysaccharide.29,42 Our results are in accordance with a previous report showing that IL-6 is critical for overcoming suppression of CD4+CD25+ Treg cells via TLR signaling.30 However, other mechanisms were also predicted from our data to be involved in the capacity of S typhimurium to control Treg suppression, since (1) blocking by anti–IL-6 antibody only had a partial effect (Figure 4A), and (2) addition of recombinant IL-6 alone was not sufficient to elicit NY-ESO-1 peptide-specific CD4+ Th1 cells in the presence of CD4+CD25+ Treg cells (Figure 4B). Therefore, it is possible that responses stimulated by S typhimurium other than those dependent on TLR receptors may also influence its ability to suppress CD4+CD25+ Treg cells. For example, S typhimurium is capable of inducing a significant reprogramming of gene expression in infected cells in a manner that is dependent on the function of its type III secretion systems.43 More studies will be required to investigate this possibility.
Disrupting GITR signaling using anti-GITRL blocking antibody also decreased the capacity of S typhimurium to overcome the suppressive activity of CD4+CD25+ Treg cells (Figure 4A). As previously reported for virus infections and TLR signaling,27,40 we observed that GITRL expression on APCs was up-regulated after S typhimurium infection (Figure 4C). It was shown using GITR knockout mice that T cell–mediated suppression is abrogated by GITR engagement on CD4+CD25− effector T cells, but not CD4+CD25+ Treg cells as originally thought.26-28 It is plausible that S typhimurium–induced GITRL on APCs engages with GITR on activated effector T cells and makes them resistant to suppression by CD4+CD25+ Treg cells. This hypothesis is supported by the following data: (1) S typhimurium–induced CD4+ Th1 cells were able to proliferate in the presence of activated CD4+CD25+ Treg cells (Figure 5B); (2) GITR expression was maintained in S typhimurium–induced CD4+ Th1 cells, but not peptide-induced CD4+ Th1 cells (Figure 5A); and (3) addition of GITRL-Fc had a partial effect on the induction of NY-ESO-1 peptide-specific Th1 response in the presence of Treg cells (Figure 4B). Recently, it has been reported that flagellin, a TLR5 ligand present in Salmonella, can directly enhance the suppressive capacity of CD4+CD25+ Treg cells, but has an opposite indirect effect in the presence of APCs, where it overrides suppression.44,45 In our study, while a direct effect of S typhimurium on Treg cells cannot be excluded, a mechanism for overcoming suppressive activity is more likely to be the engagement of GITR on responder effector cells rather than on CD4+CD25+ Treg cells themselves, because addition of blocking anti-GITRL antibody did not enhance the suppressive activity of CD4+CD25+ Treg cells for peptide-induced CD4+ Th1 cells (Figure 5B).
Although both anti–IL-6 and anti-GITRL antibodies blocked the capacity of S typhimurium to inhibit the suppressive activity of CD4+CD25+ Treg cells, these molecules seem to exploit different mechanisms, since (1) addition of both anti–IL-6 and anti-GITRL antibodies worked together to completely abrogate the effect of S typhimurium (Figure 4A), and (2) addition of an agonistic GITRL fusion molecule was able to elicit NY-ESO-1–specific Th1 cells from whole CD4+ T cells without the need for depletion of CD4+CD25+ Treg cells, whereas addition of IL-6 alone could not (Figure 4C). Furthermore, in the restimulation phase, enhanced GITR expression was observed on Treg cell–resistant S typhimurium–induced CD4+ Th1 cells, but not on Treg cell–sensitive peptide-induced CD4+ Th1 cells, and GITR signal alone was sufficient to overcome the suppressive activity of CD4+CD25+ Treg cells (Figure 5). The question remains whether anti–IL-6 or anti-GITRL antibody treatment affects the inhibition of Th1 induction differently in healthy volunteers and in patients with melanoma. It is possible that a difference exists between individuals or between healthy individuals and patients with cancer in the sensitivity of their Treg cells to IL-6 and/or GITRL. Taken together, it is considered that IL-6 and GITR signaling synergistically act to overcome suppression by CD4+CD25+ Treg cells in the priming phase and to make effector cells resistant to CD4+CD25+ Treg cells. In the restimulation phase, GITR signaling is sufficient to block the suppressive activity of CD4+CD25+ Treg cells.
We show here an additional potential of the GITR-GITRL signal as a critical molecules for Treg cell resistance. The costimulatory activity of the GITR-GITRL signal is still under debate. It has recently been reported that several human tumor cell lines express substantial levels of GITRL, and that this constitutive expression diminishes natural killer (NK)–cell activity against tumor cells.46 On the other hand, it was also shown that plasmacytoid DCs activated with CpG promote NK-cell activity through GITRL.47 Several reports propose that costimulatory or inhibitory effects are dependent on strength of antigen stimulation, type of target cells (CD4+ or CD8+ T cells), or sum of other costimulatory signals.48-50 The cell types expressing GITRL may also be a critical factor. Further studies appear necessary for clinical application of this molecule.
Our strategy to overcome the suppressive function of CD4+CD25+ Treg cells using S typhimurium has an advantage compared with other methods, such as eliminating CD4+CD25+ Treg cells. It has been reported that down-regulation of CD4+CD25+ Treg cell activity and blocking of nonspecific suppressive mechanisms are accompanied by autoimmune diseases.6,51,52 Since S typhimurium–induced Treg cell–resistant Th1 cells are directed to a tumor antigen not expressed in normal somatic tissues, occurrence of autoimmune diseases is less likely. We propose that S typhimurium is a promising cancer vaccine platform for specific immunotherapy that could surmount the obstacle of CD4+CD25+ Treg cell activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs E. Sato and T. Kato for helpful discussion, and C. Brooks, L. Cohen, N. Harada, S. Hori, Y. Orito, J. Suzuki, and D. Yoo for technical support.
This study was supported by the Cancer Vaccine Collaborative and the Cancer Antigen Discovery Collaborative funded by the Cancer Research Institute and Ludwig Institute for Cancer Research; Grants-in-Aid for Young Scientists (start-up) and for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Public Health Service Grants from the National Institutes of Health (J.E.G.); and the Kanae Foundation for the Promotion of Medical Science.
National Institutes of Health
Authorship
Contribution: H.N. and T.T. performed experiments; E.J., G.B., and J.E.G. provided materials for the study; H.N., T.T., G.R., L.J.O., J.E.G., H.S., and S.G. analyzed results and/or made the figures; and H.N., L.J.O., J.E.G., H.S., and S.G. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sacha Gnjatic, Ludwig Institute for Cancer Research, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 32/Rm Z-1502, New York, NY 10065; e-mail: gnjatics@mskcc.org; or Hiroyoshi Nishikawa, Department of Cancer Vaccine, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan; e-mail: nisihiro@clin.medic.mie-u.ac.jp.

![Figure 1. NY-ESO-1–specific CD4+ T cells are elicited by S typhimurium–NY-ESO-1 without the need for CD4+CD25+ T-cell depletion. Presence of NY-ESO-1 peptide-specific CD4+ Th1 cells in whole CD4+ T cells and CD4+CD25− T cells was analyzed by ELISPOT assays following a 15- to 20-day culture with APCs pulsed with indicated NY-ESO-1 peptides (except NC155; NY-ESO-1 157-170) or infected with S typhimurium–NY-ESO-1 (S typh–NY-ESO-1) or S typhimurium control strain (S typh–control strain). Responses were analyzed by specific IFN-γ secretion for recognition of autologous T-APCs pulsed with indicated peptide (except NC155; NY-ESO-1 157-170 [▩], or HIV peptide [□]), in 4 healthy donors and in 4 patients with NY-ESO-1–expressing tumors but without NY-ESO-1 antibody. These experiments were performed independently at least twice with similar results. Data are expressed as means plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/3/10.1182_blood-2007-09-113761/6/m_zh80030814090001.jpeg?Expires=1765884272&Signature=ZKi--UAF43ff9WkWUOYDp2mdZ3E4pe99Ynx5QzDuEJt5bCVH~Xd9Wk7zTpy4k1~xgY7jJCRIh1E7zTiZr1fwg06x-lLUiLF6i8PijRGSbo~axNeH0~J~nCZ5SA25p7mgZacDRssxqqI-xyqMSJiVgRgOsr-GiKnlR6VS2-T2HAiIl7pG-BcA1pwgK7qycWdeWhaxCD7QvYmt~flBAZFtiQ1MGCY8~mP2815SR4OZEoyZmSFk66CTgNubPIevhVA18EgEpa-Lya4~l0Q85W-6th5Tfq3ixdNfDK3LGxuGjwmrvto02HwFx4PvxSzgXRb0Xj6FPanNM~BPcw51wHEB4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal