Abstract
Despite the importance of phosphoinositide 3-kinase (PI3K) in B-cell development, its activation mechanism still remains elusive. In this study, we show that deletion of both BCAP and CD19 leads to an almost complete block of BCR-mediated Akt activation and to severe defects in generation of immature and mature B cells. The YXXM motifs in BCAP and CD19 are crucial for regulating B-cell development in that mutation of these motifs abrogated their ability to induce BCR-mediated Akt activation as well as to promote B-cell development. Furthermore, the developmental defect in CD19−/−BCAP−/− B cells was partly relieved by introducing a constitutively active form of PI3K or PDK1. Together, our data suggest that BCAP and CD19 have complementary roles in BCR-mediated PI3K activation, thereby, at least in part, contributing to B-cell development.
Introduction
Signaling through the B-cell receptor (BCR) evokes multiple signaling pathways that are required for the development and maintenance of B cells.1,2 BCR engagement results in increased tyrosine phosphorylation mediated by nonreceptor protein tyrosine kinases (PTKs) of the Src, Syk, and Tec families.3 The tyrosine residues located within immunoreceptor tyrosine-based activation motifs (ITAMs) of the intracellular domains of Igα/Igβ are phosphorylated by Lyn, a Src family kinase, and/or Syk.4 Then, activated PTKs phosphorylate several substrates including coreceptors and cytoplasmic adaptor proteins such as BLNK and BCAP.5-7 These adaptor and coreceptor proteins are thought to recruit effector enzymes, for instance phospholipase Cγ2 (PLCγ2) and phosphoinositide 3-kinase (PI3K), by protein-protein and protein-lipid interactions, which in turn is essential for B-cell development and activation.
CD19 is a well-known coreceptor that positively modulates BCR signaling and is suggested to set a threshold of BCR signaling.8 In fact, upon BCR stimulation, CD19 undergoes phosphorylation on multiple tyrosine residues and recruits several signaling molecules that further augment transmembrane signaling.9-14 Among these multiple tyrosine phosphorylation sites, the importance of 2 YXXM motifs in the CD19 cytoplasmic region, recruitment sites to the p85α of phosphoinositide 3-kinase (PI3K), has been validated by experiments using transgenic mice.12 However, given that B-cell developmental defects in p85α−/− mice are more severe than those in CD19−/− mice,15-18 this CD19-dependent activation mechanism seems not to fully account for the activation mode of PI3K in B cells. Indeed, the BCR-mediated Akt activation was inhibited in CD19-deficient B cells, but not completely ablated.19 Together, these observations suggest that other signaling molecule(s) could partially neutralize the defective PI3K activation in the absence of CD19. In this regard, BCAP could be a potential candidate.20
BCAP, an adaptor molecule highly expressed in B cells, possesses binding ability to the p85α through its 4 YXXM motifs.20 Indeed, BCAP underwent tyrosine phosphorylation after BCR stimulation, thereby recruiting the p85α of PI3K. However, B cells from BCAP-deficient mice exhibited apparently normal Akt activation, suggesting again that other molecule(s), presumably CD19, could rescue the BCAP function, particularly in the BCAP single-knockout mice.21 Here, by using CD19/BCAP double-knockout mice, we show that BCAP and CD19 have overlapping functions in BCR-mediated PI3K activation, thereby contributing to generation of immature and mature B cells.
Methods
Mice
Antibodies and reagents
The following monoclonal antibodies (mAbs) and reagents were purchased from BD Bioscience (San Jose, CA): biotin–anti–Mac-1, biotin–anti-Gr1, biotin–anti-IgD, biotin–anti-TER119, biotin–anti-F4/80, biotin–anti-CD3, biotin–anti-IgM, biotin–anti-CD23, biotin–anti-CD43, biotin–anti-CD25, biotin–anti-PE, FITC–anti-IgM, FITC–anti-B220, PE–anti-IgD, PE–anti–BP-1, and PerCPCy5.5–anti-IgM mAbs. PE–anti-AA4.1 mAb was purchased from e-Bioscience (San Diego, CA). Anti-Igβ mAb for stimulating B cells was purified from a culture supernatant of the hybridoma HM79.22 Anti-BCAP and anti-CD19 Abs used for Western blotting were produced as described previously.20 Anti–phospho-serine 473 and -threonine 308 Akt Abs were purchased from Cell Signaling Technology (Danvers, MA). Recombinant murine IL-7, stem cell factor (SCF), and Flk2/Flt3 ligand (FL) were purchased from R&D Systems (Minneapolis, MN).
Purification of bone marrow B cells and culture
Bone marrow cells were stained with a mixture of biotin–anti–Mac-1, biotin–anti-F4/80, biotin–anti-Gr1, biotin–anti-TER119, biotin–anti-IgD, and biotin–anti-CD3 mAbs. Labeled cells were incubated with magnetic beads coupled with streptavidin followed by the depletion of labeled cells using AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). In some experiments, recovered cells were labeled with Cy5.5PerCP–anti-IgM, FITC–anti-B220, and PE–anti-CD43 mAbs and sorted for B220+CD43+IgM− cells for purifying pro-B cells. Cells were cultured in IMDM supplemented with 10% FCS, nonessential amino acids (100 μM), sodium pyruvate (2 mM), 2-ME (50 μM), antibiotics, and 20 ng/mL murine recombinant IL-7 or 10% culture supernatant of J558L transfected with murine IL-7 cDNA.
Purification of splenic AA4.1+ B cells
Splenocytes were prepared from wild-type and BCAP−/−CD19−/− mice and stained with PE–anti-AA4.1 mAb. The cells were sequentially labeled with biotin–anti-PE mAb and streptavidin-magnetic beads followed by positive selection using AutoMACS. The cells were then stained with FITC–anti-B220 mAb. AA4.1+B220+ cells were sorted using FACSVantage (BD Bioscience).
Construction and infection of retrovirus vectors
The protocols for introducing mutations into cDNAs for CD19, BCAP, PDK1, and p85α were as described previously.23 Wild-type and mutant forms of these cDNAs were cloned into pMx-ires-GFP and transfected to plat-E packaging cell lines using Fugene 6 (Roche, Indianapolis, IN). Two days after transfection, culture supernatant containing retrovirus was added to cultured B cells for infection as described previously.24 Two days after infection, the cells were recovered and subjected to Western blotting analysis, or cultured for an additional 2 days without IL-7 to induce in vitro differentiation.
Transplantation of bone marrow cells
Bone marrow cells from BCAP−/−CD19−/− mice were depleted of Mac-1+, TER119+, B220+, CD3+, and Gr-1+ cells by AutoMACS. Cells were cultured in DMEM containing 10% FCS, 100 ng/mL FL, and 20 ng/mL SCF for 2 days. Cells were infected with retrovirus vectors carrying cDNAs for BCAP, CD19, and their mutants. A retrovirus vector carrying no cDNA was also used for mock transfection. After the additional culture for 1 day, 4 × 105 cells were injected intravenously to lethally irradiated (8.5 Gy) C57BL/6 mice via tail veins. Two months later, mice were killed and the spleens were recovered. Splenocytes were stained with anti-IgM and -IgD mAb and subjected to flow cytometric analysis.
Western blotting analysis
Western blotting was performed as described previously.25 Cells were stimulated using 30 μg/mL anti-Igβ mAb at 37°C. Cell lysates from 106/lane were subjected to Western blotting.
Statistical analysis
In some experiments, the data for each study group were compared using Student t test, and P values were calculated.
Results
B-cell development in BCAP−/−CD19−/− mice
Mice lacking both CD19 and BCAP were generated, and the B-cell populations of wild-type, BCAP−/−, CD19−/−, or BCAP−/−CD19−/− mice were analyzed using multiparameter flow cytometry (Figure 1A). Splenic B-cell numbers were reduced to approximately 20% and approximately 35% in BCAP−/−CD19−/− mice compared with wild-type (P = .002) or CD19−/− controls (P = .001), respectively (Figure 1B). T1/T2 immature B cells were still present in the double-deficient mice, but their numbers were reduced to approximately 40% compared with wild-type mice (P = .009). CD19−/− mice exhibited a similar reduction in T1/T2 immature B cells, like BCAP−/−CD19−/− mice. The proportions and numbers of T3 immature B cells and follicular mature B cells were drastically reduced (Figure 1A). Analysis of peritoneal cavity cells revealed that BCAP−/−CD19−/− mice lacked B1-a (CD5+B220+) and B1-b/B-2 (CD5−B220+) cells. Thus, the combined loss of BCAP and CD19 drastically exacerbates the defects in B-cell development that result from the loss of either molecule alone.
Analysis of subpopulations of B cells from wild-type, BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice. (A) Splenocytes, peritoneal cavity cells (PECs), and bone marrow cells were recovered from wild-type (WT), BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice. The cells were stained with the mAbs as described in each panel and subjected to flow cytometric analysis. Numbers on the panels indicate the percentages of cell populations. (B) Cell numbers of subpopulations in the spleen, and bone marrow cells from 2 tibiae and femurs were calculated by multiplying the total numbers of recovered cells by the percentages of B220+CD43+IgM− (pro-B), B220+CD43−IgM− (pre-B), B220loCD43−IgM+ (immature B), and B220+ (splenic B) cells. Data represent the means of cell numbers plus or minus SD of 3 mice.
Analysis of subpopulations of B cells from wild-type, BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice. (A) Splenocytes, peritoneal cavity cells (PECs), and bone marrow cells were recovered from wild-type (WT), BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice. The cells were stained with the mAbs as described in each panel and subjected to flow cytometric analysis. Numbers on the panels indicate the percentages of cell populations. (B) Cell numbers of subpopulations in the spleen, and bone marrow cells from 2 tibiae and femurs were calculated by multiplying the total numbers of recovered cells by the percentages of B220+CD43+IgM− (pro-B), B220+CD43−IgM− (pre-B), B220loCD43−IgM+ (immature B), and B220+ (splenic B) cells. Data represent the means of cell numbers plus or minus SD of 3 mice.
The reduced number of peripheral mature B cells may suggest a defect in maturation that could also be manifested at early stages of B-cell development. In CD19−/− or BCAP−/− mice, the proportions and numbers of pro-B cells in the bone marrow were not significantly perturbed. In contrast, BCAP−/−CD19−/− mice exhibited an accumulation of large pre-B (BP-1+CD25+) cells accompanied by a reduction in small pre-B (BP-1−CD25+) cells (Figure 1A, see B220+IgM− gated profiles). Immature (IgM+B220lo) and recirculating (IgM+B220hi or CD43−B220hi) B-cell numbers in the bone marrow in BCAP−/−CD19−/− mice were reduced to approximately 30% and approximately 40% (P = .011 and P = .038), respectively, compared with wild-type mice (Figure 1B and data not shown). These observations demonstrate that, in the absence of CD19, BCAP is somehow required for transition from the large pre-B stage as well as for the development of immature B cells.
Defective Akt activation in CD19−/−BCAP−/− B cells
To investigate the mechanism underlying the defects in B-cell development in BCAP−/−CD19−/− mice, we used the previously reported in vitro bone marrow culture system.26-28 Bone marrow pro-B cells from BCAP−/−CD19−/− mice exhibited a greater proliferation capacity in response to IL-7, which is similarly observed in Btk−/− and BLNK−/− B-cell progenitors,26,29 compared with those from wild-type, CD19−/−, or BCAP−/− mice (Figure 2A). After 4 days of culture in the presence of IL-7, essentially pure B-cell populations were obtained (> 95% B220+; data not shown), which consisted primarily of pro-B and late pro-B stage cells. These B-cell populations lacked transitional IgM+IgD+ B cells, though a few cells underwent spontaneous differentiation to become IgM+ B cells. Withdrawal of IL-7 stopped the expansion of wild-type precursor B cells and increased the proportion of immature IgM+ and transitional IgM+IgD+ B cells (Figure 2B, see the profile of WT in the presence or absence of IL-7). The most advanced-staged B cells (IgM+IgD+) in this system are thought to resemble those leaving the bone marrow and entering the spleens in vivo.
Absence of both CD19 and BCAP impairs in vitro B-cell differentiation and BCR-mediated Akt activation. (A) Sorted CD43+B220+IgM− cells from bone marrows (5 × 105) were cultured in the presence of 20 ng/mL murine recombinant IL-7 for 4 days. The numbers of recovered cells were counted by trypan blue exclusion. Data represent the means of recovered cell numbers (± SD) of triplicate cultures. (B) Bone marrow B cells were prepared from wild-type, BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice and cultured for 4 days in the presence of IL-7. The cells were washed with medium twice and cultured in the absence (− IL-7) or presence (+ IL-7) of IL-7 for an additional 2 days. Approximately 50% of cells were recovered from each genotype after 2 days in the absence of IL-7. The recovered cells were stained with anti-IgM and anti-IgD Abs and subjected to flow cytometric analysis. The percentage of IgM+IgD+ and IgM+IgD− cells is indicated in each panel. (C) Bone marrow B cells were cultured for 4 days in the presence of IL-7. The recovered cells were stimulated with 30 μg/mL anti-Igβ mAb for the indicated periods of time. Whole-cell lysates from stimulated cells were subjected to Western blotting with anti–phospho-serine 473, anti–phospho-threonine 308 Akt, or anti-Akt1 antibodies. (D) AA4.1+B220+ B cells were sorted from the splenocytes of wild-type or BCAP−/−CD19−/− mice. The cells were stimulated with anti-Igβ mAb at a concentration of 30 μg/mL for 3 minutes. Total-cell lysates were prepared and subjected to Western blotting analysis.
Absence of both CD19 and BCAP impairs in vitro B-cell differentiation and BCR-mediated Akt activation. (A) Sorted CD43+B220+IgM− cells from bone marrows (5 × 105) were cultured in the presence of 20 ng/mL murine recombinant IL-7 for 4 days. The numbers of recovered cells were counted by trypan blue exclusion. Data represent the means of recovered cell numbers (± SD) of triplicate cultures. (B) Bone marrow B cells were prepared from wild-type, BCAP−/−, CD19−/−, or CD19−/−BCAP−/− mice and cultured for 4 days in the presence of IL-7. The cells were washed with medium twice and cultured in the absence (− IL-7) or presence (+ IL-7) of IL-7 for an additional 2 days. Approximately 50% of cells were recovered from each genotype after 2 days in the absence of IL-7. The recovered cells were stained with anti-IgM and anti-IgD Abs and subjected to flow cytometric analysis. The percentage of IgM+IgD+ and IgM+IgD− cells is indicated in each panel. (C) Bone marrow B cells were cultured for 4 days in the presence of IL-7. The recovered cells were stimulated with 30 μg/mL anti-Igβ mAb for the indicated periods of time. Whole-cell lysates from stimulated cells were subjected to Western blotting with anti–phospho-serine 473, anti–phospho-threonine 308 Akt, or anti-Akt1 antibodies. (D) AA4.1+B220+ B cells were sorted from the splenocytes of wild-type or BCAP−/−CD19−/− mice. The cells were stimulated with anti-Igβ mAb at a concentration of 30 μg/mL for 3 minutes. Total-cell lysates were prepared and subjected to Western blotting analysis.
Two days after removal of IL-7, the fraction of IgM+ cells in BCAP−/−CD19−/− bone marrow cells increased, but differentiation to IgM+IgD+ cells was severely inhibited in BCAP−/−CD19−/− cultures (Figure 2B). In CD19−/−, as shown by a previous report,28 or BCAP−/− bone marrow cells, the differentiation capacity to transitional B cells was somewhat inhibited in this experimental system, though not as severely as in the double-deficient cells. When we used sorted pro-B cells instead of the bone marrow progenitors as used in Figure 2B, essentially the same results were obtained (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results demonstrate that the actions of BCAP and CD19 allow B cells in culture to advance toward a more differentiated state of maturation (IgM+IgD+).
The following 2 lines of evidence prompted us to consider the possibility that BCR-mediated PI3K/Akt activation might be severely impaired in BCAP−/−CD19−/− bone marrow B cells, thereby rendering them incapable of differentiation. First, PI3K p85α−/− mice display similar B-cell developmental defects to those observed in BCAP−/−CD19−/− mice.17,18 Second, although BCR-mediated Akt activation was not significantly affected in B cells from BCAP−/− mice, BCAP could be tyrosine phosphorylated at its PI3K-binding motifs (YXXM), and could recruit p85α after BCR stimulation in the B-cell line, DT40.20,21 As demonstrated in Figure 2C, anti-Igβ mAb stimulation on BCAP−/− or CD19−/− bone marrow B cells induced similar or reduced level of Akt activation compared with wild-type cells. In contrast to other genotypes of mice, bone marrow B cells from BCAP−/−CD19−/− mice scarcely exhibited anti-Igβ–induced Akt activation, though the cells exhibited similar levels of Akt activation in response to oxidative stress30,31 compared with other genotypes of mice (Figure S2). Since expression of Igβ was comparable among the 4 types of B cells (Figure S3), defective Akt activation was not due to expression levels of Igβ. As Igβ associates with both BCR and pre-BCR complexes and cultured bone marrow cells contained a small proportion of BCR+ cells, we performed similar experiments using sorted splenic AA4.1+ B cells expressing the BCR, but not the pre-BCR. As demonstrated in Figure 2D, BCR-mediated Akt activation was profoundly inhibited in BCAP−/−CD19−/− B cells. Thus, both pre-BCR and BCR are likely to require CD19 and BCAP for their Akt activation. Overall protein tyrosine phosphorylation after Igβ stimulation was not significantly altered in BCAP−/−CD19−/− cells compared with those of other genotypes (data not shown).
Requirement for PI3K p85α-binding motifs of CD19 or BCAP in its differentiation capability
Having demonstrated the relationship between BCR-mediated Akt activation and differentiation capability, we asked whether YXXM motifs in BCAP and CD19 are critical for BCR-mediated Akt activation and differentiation capability. To do so, we constructed the YF mutants of CD19 and BCAP in which 2 (Y482 and Y513 of mouse CD19) and 4 (Y264, Y420, Y445, and Y460 of mouse BCAP) YXXMs were exchanged for FXXMs, and introduced these mutant molecules into BCAP−/−CD19−/− bone marrow cells via retrovirus vectors. As shown in Figure 3A, introduction of the wild-type form of CD19 or BCAP, but not their mutants, into BCAP−/−CD19−/− bone marrow cells restored BCR-mediated Akt activation. YXXM motifs in BCAP and CD19 indeed function to recruit p85α in this culture system because Flag-tagged wild-type BCAP and CD19 but not their mutants bind to p85α in a BCR-signaling–dependent manner. Even in the absence of BCR stimulation, wild-type BCAP and CD19, but not their mutants, bind to a significant amount of p85α, and induce Akt activation (Figure 3, time point: 0 minutes). This observation may indicate that basal levels of phosphorylation at YXXM motifs of CD19 and BCAP may occur, at least to some extent, resulting in Akt activation without exogenously added stimulants. Correlated with Akt activation and p85α-binding status, in vitro differentiation of BCAP−/−CD19−/− B cells was fully restored by introduction of the wild-type, but not the mutant, forms. When the wild-type form of CD19 or BCAP was introduced, more IgM+IgD+ transitional B cells were generated compared with wild-type bone marrow B cells (Figure 2B); this is probably attributable to overexpression of the introduced CD19 or BCAP.
BCAP and CD19 regulate Akt activation and B-cell differentiation through their binding to p85α subunit of PI3K. Bone marrow B cells were prepared from CD19−/−BCAP−/− mice. The cells were cultured for 2 days with IL-7 and infected with retrovirus vectors carrying the cDNAs of BCAP (BCAP wt), CD19 (CD19 wt), BCAP mutant (BCAP 4YF), or CD19 mutant (2YF). BCAP 4YF and CD19 2YF are mutants in which 4 and 2 YXXM motifs, respectively, were replaced by FXXM. For examining p85α association to CD19, BCAP, and their mutants, Flag-tagged cDNA was introduced via retrovirus vectors into CD19−/−BCAP−/− cells. As a negative control, a retrovirus vector without cDNA (mock) was also used. Infected cells were cultured for an additional 2 days with IL-7. The recovered cells were stimulated with anti-Igβ and subjected to Western blotting analysis as described in Figure 2D. The recovered cells were further cultured for 2 days without IL-7 to induce differentiation and were subjected to flow cytometric analysis as described in Figure 2B. The percentage of IgM+IgD+ cells is indicated in each panel.
BCAP and CD19 regulate Akt activation and B-cell differentiation through their binding to p85α subunit of PI3K. Bone marrow B cells were prepared from CD19−/−BCAP−/− mice. The cells were cultured for 2 days with IL-7 and infected with retrovirus vectors carrying the cDNAs of BCAP (BCAP wt), CD19 (CD19 wt), BCAP mutant (BCAP 4YF), or CD19 mutant (2YF). BCAP 4YF and CD19 2YF are mutants in which 4 and 2 YXXM motifs, respectively, were replaced by FXXM. For examining p85α association to CD19, BCAP, and their mutants, Flag-tagged cDNA was introduced via retrovirus vectors into CD19−/−BCAP−/− cells. As a negative control, a retrovirus vector without cDNA (mock) was also used. Infected cells were cultured for an additional 2 days with IL-7. The recovered cells were stimulated with anti-Igβ and subjected to Western blotting analysis as described in Figure 2D. The recovered cells were further cultured for 2 days without IL-7 to induce differentiation and were subjected to flow cytometric analysis as described in Figure 2B. The percentage of IgM+IgD+ cells is indicated in each panel.
We also examined if YXXMs on BCAP and CD19 are essential for in vivo generation of mature B cells. Bone marrow progenitors from BCAP−/−CD19−/− mice were purified and cultured in the presence SCF and FL. Wild-type or mutant forms of BCAP or CD19 were introduced into the bone marrow progenitor cells via retrovirus vectors. Cells infected with a retrovirus vector without cDNA were also prepared as mock transfectant. Lethally irradiated B6 mice were injected with infected cells and killed 2 months later for examining the reconstitution of the B-cell compartment in spleens. As shown in Figure 4, wild-type but not the mutant of either CD19 or BCAP was able to support the generation of IgMloIgD+ mature B cells in spleens, suggesting that YXXM motifs in BCAP and CD19 play a crucial role for mature B-cell generation. Together, YXXM motifs in BCAP and CD19 are clearly required for BCR-mediated Akt activation and generation of B cells in vitro as well as in vivo.
BCAP and CD19 regulate mature B-cell generation in vivo through their YXXM motifs. Lineage marker–negative bone marrow progenitor cells were cultured and infected with retrovirus vectors carrying BCAP, CD19, and their mutants. Mock transfectant was also prepared using retrovirus vector without cDNA. Infected cells were injected to irradiated C57BL/6 mice. Splenocytes were prepared from the mice after 2 months of injection and stained with anti-IgM and -IgD mAb followed by flow cytometric analysis. Top panels represent expression of GFP in splenocytes. Lines in fluorescence-activated cell sorting (FACS) profiles represent gates settled for discriminating donor-derived cells that are successfully infected retrovirus vectors. Bottom panels represent profiles for IgM and IgD expression on GFP-positive gated cells. The percentage of cells in each quadrant is indicated in panels. Averages of absolute numbers of IgMloIgD+ mature B cells per spleens (± SD) in 3 mice are as follows: 3.0 × 105 ± 0.8 × 105 (BCAP wt→B6), 0.56 × 105 ± 0.19 × 105 (BCAP 4YF →B6), 6.2 × 105 ± 2.6 × 105 (CD19 wt →B6), and 0.33 × 105 ± 0.10 × 105 (CD19 2YF→B6).
BCAP and CD19 regulate mature B-cell generation in vivo through their YXXM motifs. Lineage marker–negative bone marrow progenitor cells were cultured and infected with retrovirus vectors carrying BCAP, CD19, and their mutants. Mock transfectant was also prepared using retrovirus vector without cDNA. Infected cells were injected to irradiated C57BL/6 mice. Splenocytes were prepared from the mice after 2 months of injection and stained with anti-IgM and -IgD mAb followed by flow cytometric analysis. Top panels represent expression of GFP in splenocytes. Lines in fluorescence-activated cell sorting (FACS) profiles represent gates settled for discriminating donor-derived cells that are successfully infected retrovirus vectors. Bottom panels represent profiles for IgM and IgD expression on GFP-positive gated cells. The percentage of cells in each quadrant is indicated in panels. Averages of absolute numbers of IgMloIgD+ mature B cells per spleens (± SD) in 3 mice are as follows: 3.0 × 105 ± 0.8 × 105 (BCAP wt→B6), 0.56 × 105 ± 0.19 × 105 (BCAP 4YF →B6), 6.2 × 105 ± 2.6 × 105 (CD19 wt →B6), and 0.33 × 105 ± 0.10 × 105 (CD19 2YF→B6).
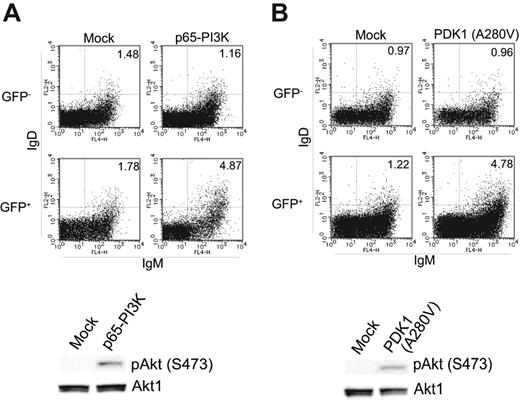
Enhanced PI3K-PDK1 signal restores the defect in B-cell differentiation capacity of BCAP−/−CD19−/− cells
The above data predict that defects in BCAP−/−CD19−/− B cells can be restored by manipulations that enhance PI3K/Akt signaling. To test this prediction, we constructed a deletion mutant of p85α (p65-PI3K) in which the C-terminal residues 571 to 724 were deleted, or mutant PDK1 in which alanine 280 was replaced by valine (PDK1 A280V). These mutants have previously been shown to enhance the PI3K-PDK1 signaling pathway constitutively.32,33 We introduced these mutants into BCAP−/−CD19−/− bone marrow cells and examined the in vitro differentiation potential of these cells. As shown in Figure 5, introduction of the constitutively active mutants restored the development of IgD+ cells to some extent, but did not reach the proportion seen in wild-type cells demonstrated in Figure 2B. This suggests that the defects of development in BCAP−/−CD19−/− B cells could, at least partly, be accounted for by a decreased PI3K-PDK1 signal.
Enhancement of PI3K-PDK1 signaling partially restores in vitro differentiation potential of BCAP−/−CD19−/− B cells. Bone marrow B cells from BCAP−/−CD19−/− mice were infected with retrovirus vectors carrying cDNA encoding p65-PI3K (A) or PDK1 (A280V) (B). As a control, a retrovirus vector without cDNA was also infected (mock). Cells were cultured and assayed as in Figure 3A. The percentage of IgM+IgD+ cells is indicated in each panel.
Enhancement of PI3K-PDK1 signaling partially restores in vitro differentiation potential of BCAP−/−CD19−/− B cells. Bone marrow B cells from BCAP−/−CD19−/− mice were infected with retrovirus vectors carrying cDNA encoding p65-PI3K (A) or PDK1 (A280V) (B). As a control, a retrovirus vector without cDNA was also infected (mock). Cells were cultured and assayed as in Figure 3A. The percentage of IgM+IgD+ cells is indicated in each panel.
Discussion
BCR signaling, which is essential for the development of B cells, evokes various signaling pathways. In addition to adaptor molecules, coreceptors also regulate BCR signaling by recruiting effector signaling molecules for integrating multiple signaling pathways. Here, we demonstrated that BCR-mediated Akt activation necessitates actions of BCAP and CD19. Furthermore, the following 3 lines of evidence allow us to conclude that the defective BCR-mediated PI3K/Akt activation accounts, at least partly, for the phenotypes observed in BCAP−/−CD19−/− mice: (1) the similar developmental defect between BCAP−/−CD19−/− and p85α−/− mice; (2) requirement of YXXM motifs of BCAP and CD19 in BCR-mediated Akt activation as well as in B-cell generation; (3) in vitro restoration of the developmental defect in BCAP−/−CD19−/− B cells, although not complete, by an enhanced PI3K-PDK1 signal. One straightforward explanation for the reason why the restoration is not complete is that other signaling pathways, regulated by CD19 and/or BCAP, are necessary to generate B cells in addition to the PI3K pathway. Although we tried to perform in vivo bone marrow reconstitution experiments with retrovirus vectors carrying constitutively active PI3K and PDK1, donor-derived cells could not be efficiently generated under our experimental conditions, probably due to general adverse effects of these proteins. In addition to the BCR, IL-7 receptor and BAFF receptor are known to be critical receptors for B-cell development and survival. But, severe defects in B-cell generation in BCAP−/−CD19−/− mice are probably not due to the anomalies of such cytokine signaling, because we observed normal or even higher response of BCAP−/−CD19−/− B cells to cytokines such as IL-7 (Figure 2A) or BAFF (data not shown).
B-cell development at the pre-B stage is somewhat perturbed in BCAP−/−CD19−/− mice; a significant increase was observed in the bone marrow large pre-B (BP-1+CD25+IgM−) population. Moreover, as reported for Btk−/−, Syk−/−, and SLP65−/− bone marrow cells in the IL-7 culture system,34 BCAP−/−CD19−/− B cells manifest a hyperproliferative response in the presence of IL-7. In addition, like SLP65−/− cells, we observed an increase in the population of pre-BCR+ cells in the bone marrow BCAP−/−CD19−/− B cells (data not shown). Since pre-BCR signaling is known to induce its own extinction during the pre-B-cell transition as well as to limit IL-7–mediated proliferation,26 one of the simple explanations for our data is that pre-BCR signaling is compromised in BCAP−/−CD19−/− pre-B cells. Given the similarity between pre-BCR and BCR signaling, it is reasonable to suggest that pre-BCR–mediated PI3K activation, like BCR, necessitates combined actions of BCAP and CD19, which in turn, contributes to the transition to small pre-B cells.
In contrast to the apparently normal BCR-mediated Akt activation in BCAP−/− B cells, CD19−/− B cells showed a partial block in Akt activation. In this regard, CD19 appears to play a more dominant role in BCR-mediated Akt activation, rather than BCAP, simply suggesting that BCAP functions as a backup molecule for recruitment of PI3K to the plasma membrane. Although our results demonstrate that BCAP together with CD19 participates in BCR-mediated PI3K/Akt activation, this molecule appears to have additional function(s). For instance, peripheral BCAP−/− B cells manifest severe survival defects, despite apparently normal Akt activation, suggesting that BCAP exerts a survival signal in a PI3K-independent manner.21,35 Given the recent evidence that several signaling pathways, for instance PKCδ and IKK activation, are involved in B-cell survival,36-38 it is tempting to speculate that BCAP uses an as-yet-unidentified molecule to exert its survival function through a PI3K-independent manner in B cells. This might be one mechanism explaining why an enhanced PI3K-PDK signal cannot completely restore the developmental defect in BCAP−/−CD19−/− B cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr K. Ikuta and Dr K. Maki at the Institute for Virus Research in Kyoto University for providing J558L IL-7 transfectant.
This work was supported by grants from the Ministry for Education. Culture, Sports, Science and Technology of Japan and the Takeda Science Foundation (T.K.).
Authorship
Contribution: Y.A. designed and performed research; M.K. performed research; T.Y. and T.F.T. contributed analytical tools; T.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomohiro Kurosaki, Laboratory for Lymphocyte Differentiation, RIKEN Research Center for Allergy and Immunology, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan; e-mail: kurosaki@rcai.riken.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal