Abstract
The in vitro priming of tumor-specific T cells by dendritic cells (DCs) phagocytosing killed tumor cells can be augmented in the presence of antitumor monoclonal antibody (mAb). We investigated whether DCs phagocytosing killed lymphoma cells coated with tumor-specific antibody could elicit antitumor immunity in vivo. Irradiated murine 38C13 lymphoma cells were cocultured with bone marrow–derived DCs in the presence or absence of tumor-specific mAb. Mice vaccinated with DCs cocultured with mAb-coated tumor cells were protected from tumor challenge (60% long-term survival), whereas DCs loaded with tumor cells alone were much less effective. The opsonized whole tumor cell–DC vaccine elicited significantly better tumor protection than a traditional lymphoma idiotype (Id) protein vaccine, and in combination with chemotherapy could eradicate preexisting tumor. Moreover, the DC vaccine protected animals from both wild-type and Id-negative variant tumor cells, indicating that Id is not a major target of the induced tumor immunity. Protection was critically dependent upon CD8+ T cells, with lesser contribution by CD4+ T cells. Importantly, opsonized whole tumor cell–DC vaccination did not result in tissue-specific autoimmunity. Since opsonized whole tumor cell–DC and Id vaccines appear to target distinct tumor antigens, optimal antilymphoma immunity might be achieved by combining these approaches.
Introduction
Among human cancers, B-cell lymphomas appear among the most susceptible to immunotherapeutic strategies, because of their high rate of response to monoclonal antibodies (mAbs) targeting the B-cell differentiation antigen CD20 and encouraging results from early phase clinical trials of tumor-specific therapeutic vaccines.1 The availability of both passive and active immunotherapeutic agents against B-cell lymphomas has made them an important testing ground for the development of clinically effective immunotherapies in humans.1-3 The best characterized target for active immunotherapy of B-cell lymphoma is tumor-specific immunoglobulin (idiotype, Id).4 Immunization of patients with Id protein derived from their own tumors can elicit humoral and T cell–mediated immune responses associated with improvements in survival and tumor burden.5-8 Traditional Id vaccines consist of Id protein chemically conjugated to the highly immunogenic carrier protein keyhole limpet hemocyanin (KLH) and injected together with an immunologic adjuvant.1 Because of their potent antigen-presenting properties,9 dendritic cells (DCs) have been used to augment lymphoma vaccine effectiveness, and durable tumor regressions have been observed after immunization with Id-loaded DCs.10,11 Granulocyte-macrophage colony-stimulating factor (GM-CSF), a DC growth and maturation factor, has also been used as an effective adjuvant in Id-KLH vaccines.4,7,12
However, despite the elegant nature of the Id vaccine approach, shortcomings of this strategy include the requirement of producing a custom-made protein for each patient and limitation of the antitumor response to a single antigen. In contrast, vaccines using whole tumor cells offer the opportunity to elicit immunity against the entire collection of antigens expressed by the tumor. Pulsed DC vaccination using apoptotic tumor cells or lysates has emerged as a popular strategy for immunization against tumors in a variety of preclinical and human studies. While killed tumor cells in the form of apoptotic bodies or freeze-thaw lysates alone display limited immunogenicity, DCs loaded with these preparations have been found to elicit antitumor immunity in a variety of preclinical models13-16 and early clinical trials.17-21 Other strategies using DCs to present the full repertoire of tumor antigens expressed by tumor cells include fusion with tumor cells22 or pulsing with tumor-derived RNA.23 The goal of these approaches is to achieve processing and presentation of exogenous cell-derived antigenic peptides by professional antigen-presenting cells (ie, “cross-presentation”), thereby evoking a CD8+ T-cell antitumor response.24
One attractive strategy for increasing tumor antigen cross-presentation is the targeting of IgG-complexed antigens into DCs via Fcγ receptors.25 Antigen-antibody complexes internalized via Fcγ receptors at the DC surface efficiently enter both the MHC class I26-28 and class II29,30 antigen-presentation pathways. Several investigators have recently reported that the uptake of killed, mAb-coated tumor cells by DCs via their Fcγ receptors promotes enhanced processing and presentation of multiple tumor antigens to T cells,31-34 thereby offering a strategy for whole tumor cell–DC vaccination. Thus, we carried out an in vivo test of this approach in a syngeneic murine B-cell lymphoma model, and compared it with a traditional Id-KLH lymphoma vaccine. We also sought to ensure that the use of whole tumor cells expressing many normal cellular antigens would not result in autoimmunity, as previously observed in some tumor vaccine models.35-37
Using a well-characterized lymphoma model in which tumor-specific mAbs were available, we demonstrated that vaccination with DCs loaded in vitro with mAb-coated tumor cells can elicit potent protective antilymphoma immunity in vivo. As an immunogen provided by DCs, mAb-coated tumor cells were superior to untreated apoptotic tumor cells or tumor cell lysates. Importantly, the induced immunity was mediated by T cells, and appeared not to be directed at tumor Id. These in vivo findings suggest a therapeutic lymphoma vaccination strategy with potential for clinical translation.
Methods
Mice and cell lines
Six- to 8-week-old female C3Hf/Sed/Kam mice were bred and housed at the UCLA Defined Pathogen Colony according to institutional guidelines. The carcinogen-induced B-cell lymphoma 38C13 expressing a clonal IgM/κ on its surface has previously been described.38 Subcutaneously administered tumor rapidly metastasizes to spleen, lymph nodes, and bone marrow, within 6 to 9 days, resulting in a systemic tumor burden.39 38C13 as well as 38C13-V2, the idiotype negative variant of 38C13,40,41 were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol (cRPMI). The spontaneously arising, C3H-derived fibrosarcoma AG104A42 (kindly provided by Dr Hans Schreiber, University of Chicago, IL) was also grown in cRPMI. All media and supplements were obtained from Invitrogen (Carlsbad, CA).
Generation and loading of DCs with tumor cell antigens
Bone marrow–derived DCs were prepared as previously described43 with minor modifications. Briefly, bone marrow was flushed from the femurs and tibias of mice and depleted of T and B cells by treatment with rat mAbs followed by panning on plates coated with goat anti–rat Ig Abs (Southern Biotechnology Associates, Birmingham, AL). The mAbs used were GK1.5 (anti-CD4), 53-6.7 (anti-CD8) (both from BD Bioscience, San Diego, CA), and RA3-3A1 (anti-B220) (TIB-146; American Type Culture Collection [ATCC], Manassas, VA). Cells were then plated in 6-well culture plates at 3.5 × 105 cells/mL in cRPMI plus 1000 U/mL recombinant murine GM-CSF (BioSource International, Camarillo, CA) and 1000 U/mL recombinant murine IL-4 (R&D Systems, Minneapolis, MN). Medium was replenished after 4 to 5 days. On day 6, nonadherent and loosely adherent cells were harvested by gentle pipetting and were replated at 5 × 105 cells/mL in 25% conditioned cRPMI media with 75% serum-free AIM V medium (Invitrogen) to minimize exposure to FCS antigens, plus cytokines, and cultured overnight in the presence of media alone or tumor cells (± mAbs) at a ratio of 1 DC:3 tumor cells. 38C13 cells were prepared for DC coculture by resuspension in AIM V at 107/mL, gamma irradiation with 30 Gy (Mark I cesium irradiator; Shepherd and Associates, San Fernando, CA) then incubation for 5 hours at 37°C to promote apoptosis. The apoptotic tumor cells were then incubated for 1 hour on ice with mouse anti-38C13 idiotype mAb (S1C5, IgG2a),44 control mouse IgG2a antibody (UPC-10; Sigma-Aldrich, St Louis, MO), or without antibody. S1C5 binds to tumor-specific determinants on the surface IgM of 38C13 lymphoma cells.44,45 The following day, DCs were harvested, washed, and resuspended in HBSS (Invitrogen) for injection.
Evaluation of tumor cell uptake by DCs
Evaluation of tumor cell uptake by DCs was performed as described previously by Dhodapkar et al.32 Briefly, 38C13 cells were dyed red with PKH 26 (Sigma-Aldrich) before irradiation and antibody coating, and DCs were dyed green with PKH 67 (Sigma-Aldrich) before coculture with 38C13 for 0 hours versus overnight at 37°C. Samples were analyzed using a FACScalibur flow cytometer and CELLQuest software (BD Bioscience). DCs phagocytosing tumor cells were visualized as double-positive cells.
Production and modification of Id protein
38C13 Id protein was derived from tumor-myeloma cell hybridoma and coupled 1:1 with KLH (Pierce, Rockford, IL) using glutaraldehyde as previously described.46
Vaccinations
DC immunizations consisted of 106 DCs loaded with antigens as described under “Generation and loading of DCs with tumor cell antigens” and were injected subcutaneously in the inguinal region in 200 μL HBSS twice biweekly, alternating between sides. Idiotype vaccinations consisted of Id-KLH conjugate (50 μg Id) in HBSS subcutaneously twice biweekly. Where indicated, mice received a single dose of 500 ng IL-12 (R&D Systems) subcutaneously in the inguinal region coinjected with Id-KLH or 55 ng GM-CSF on the day of vaccination and at the same site for 3 days after vaccination as adjuvants.
Humoral immune response assessments
Nine to 14 days following the last immunization, blood was collected via retro-orbital puncture. Serum anti-Id antibodies were quantitated by enzyme-linked immunosorbent assay (ELISA) as previously described.46 Briefly, 96-well Maxisorp plates (Nunc, Naperville, IL) coated with 38C13 IgM were incubated with serially diluted immune sera. Bound Abs were detected with HRP-conjugated goat anti–mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA) using ABTS substrate (Sigma), and absorbance was determined at 405 nm with a SPECTRAmax PLUS microplate reader (Molecular Devices, Menlo Park, CA). Affinity-purified S1C5 was used to generate a standard curve. To test for antibodies against nonidiotype surface antigens, 38C13 or 38C13-V2 cells were incubated for 4 hours on ice with 5% sera from mice vaccinated with Id-KLH, DCs loaded with opsonized 38C13 cells, or naive age-matched animals. Bound IgG or IgM was detected with FITC-labeled goat anti–mouse IgG(γ) and PE-labeled goat anti–mouse IgM(μ) antibodies (Southern Biotechnology) using flow cytometry.
Tumor challenge
38C13, 38C13-V2, and AG104A were thawed from common dedicated frozen stocks 3 days before tumor challenge and split the day before use. For tumor challenge, cells were washed twice in HBSS and diluted to appropriate concentrations in HBSS. Challenge inocula consisted of 1000 or 5000 38C13 cells, 5000 38C13-V2 cells, or 7.5 × 105 AG104A cells subcutaneously above the base of the tail, as indicated. Treatment of preexisting tumors was performed as previously described.12,47 Mice were first injected with 1000 or 5000 38C13 cells subcutaneously above the base of the tail. Two hours later and again 15 days later, mice were immunized with 106 opsonized 38C13-loaded DCs or 50 μg Id-KLH subcutaneously. Eight days after tumor inoculation, mice received a single dose of 100 mg/kg cyclophosphamide (Mead Johnson, Princeton, NJ) intraperitoneally in sterile saline, and were followed for survival as described.
In vivo depletion of T-cell subsets
Groups of 12 mice were vaccinated with opsonized 38C13-loaded DCs as described above and challenged with tumor 2 weeks later on day 0. On days −6, −5, −4, and 0, and weekly thereafter with respect to tumor challenge, mice were injected intraperitoneally with 200 μg T cell–depleting or control mAbs from ascites as previously described.43 Antibodies used were the CD4+ T cell–depleting mAb GK1.5 (rat IgG2b) (BioExpress, Lebanon, NH), control rat polyclonal Ig (Sigma-Aldrich), CD8+ T cell–depleting mAb HB129 (mouse IgG2a; ATCC), or control mouse IgG2a mAb UPC-10 (Sigma-Aldrich). On day −1, peripheral blood was collected by retro-orbital puncture from 3 representative mice from each group, and lymphocytes were analyzed by flow cytometry to confirm depletion of T-cell subsets. Staining antibodies 17A2 (anti-CD3; BD Bioscience), CT-CD4 (anti-CD4; Caltag, Burlingame, CA), and 53–5.8 (anti-CD8β.2; BD Bioscience) were not cross-blocked by the depleting antibodies and demonstrated more than 98% depletion of the target T-cell population (data not shown).
In vivo cytotoxicity assay
The in vivo cytotoxicity assay was adapted from that described by Mueller et al.48 Briefly, 38C13 cells were incubated with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) to a final concentration of 2.5 μM. Cells were resuspended to 2 × 108/mL and 2 × 107 labeled cells were injected intravenously into groups of 4 mice previously treated with HBSS, or opsonized 38C13-loaded DCs, with or without depletion of CD8+ cells using mAb HB129. After 4 hours, mice were killed and spleens harvested, pooled among groups, and analyzed by flow cytometry to detect CFSE-labeled target cells.
Assessment of autoimmunity
To determine the effect of the DC vaccine on the B-cell compartment, 90 days after immunization, spleens were removed from groups of 4 mice that had received either opsonized 38C13-loaded DCs and survived challenge with 38C13, DC vaccine without challenge, or naive age-matched control mice. Splenocytes from individual mice were analyzed by flow cytometry to determine the proportion of cells expressing CD19, B220/CD45, IgM, or IgG (all Abs from BD Bioscience).
Statistical analysis
Survival differences among groups of mice were assessed using the Kaplan-Meier method with the log-rank test using Prism software (GraphPad Software, San Diego, CA). P values were considered statistically significant at P less than .05. B-cell compartment data were charted to display the mean plus or minus standard deviation of marker-positive cells for each group. For the splenic compartment and enzyme-linked immunosorbent spot (ELISPOT) data, groups were compared using the paired, 2-tailed Student t test, and differences considered statistically significant at P less than .05.
Results
Bone marrow–derived DCs phagocytose irradiated antibody-coated tumor cells
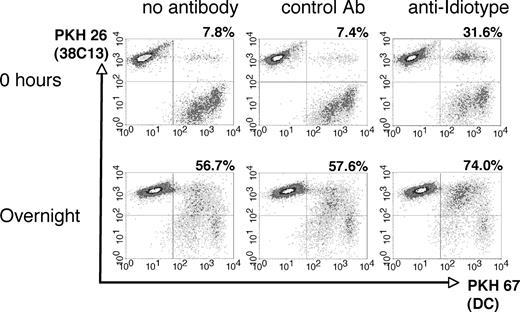
To efficiently introduce the entire set of tumor cell antigens to the host immune system, we chose to coat apoptotic lymphoma cells with a tumor-specific mAb prior to coculture with DCs. After 30 Gy γ-irradiation and incubation in serum-free medium for 5 hours at 37°C, 38C13 lymphoma cells underwent apoptosis as demonstrated by annexin V and propidium iodide staining (data not shown). To opsonize tumor cells for DC coculture, we used the tumor-specific anti-38C13 Id mAb S1C5, as mAbs against other clinically relevant antigens such as murine CD20 were not available. After irradiation, 38C13 cells were bound specifically by S1C5, but not by a control mAb (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and maintained surface expression of IgM for at least 24 hours in the presence of S1C5 (Figure S1B). To study the influence of mAbs on the bulk uptake of apoptotic 38C13 cells by DCs, killed, fluorescent-labeled tumor cells were preincubated with anti-Id mAb, isotype-matched control mAb, or left untreated, and then cocultured with day-6 DCs labeled with a different color fluorochrome (Figure 1). Immediately after initiation of coculture (0 hours), control mAb had no effect on the uptake of tumor cells by DCs, while tumor-specific mAb boosted the uptake of tumor fluorochrome more than 4-fold (Figure 1 top panels). After overnight culture, DC uptake of specific mAb-coated tumor cells remained elevated (74%) compared with control mAb-treated (57.6%) or untreated (56.7%) tumor cells (Figure 1 bottom panels). Uptake of opsonized tumor cells did not result in further up-regulation of class I and II MHC, CD80, CD86, or CD54 beyond that measured on DCs cultured with untreated tumor cells or media alone (data not shown). Thus, in our system, augmentation of DC maturation was not observed, while a modest enhancement of tumor cell uptake was detected in the presence of an opsonizing mAb.
Phagocytosis of irradiated 38C13 lymphoma cells by DCs. Tumor cells were labeled with PKH 26, irradiated (30 Gy), and incubated for 30 minutes on ice with media alone, control mouse IgG2a mAb, or anti-idiotype antibody. The tumor cells were then cocultured for 0 hours or overnight with DCs prelabeled with PKH 67, and analyzed via flow cytometry. The percentage of DCs double-positive for both PKH 26 and PKH 67 is reported in the top right of each panel.
Phagocytosis of irradiated 38C13 lymphoma cells by DCs. Tumor cells were labeled with PKH 26, irradiated (30 Gy), and incubated for 30 minutes on ice with media alone, control mouse IgG2a mAb, or anti-idiotype antibody. The tumor cells were then cocultured for 0 hours or overnight with DCs prelabeled with PKH 67, and analyzed via flow cytometry. The percentage of DCs double-positive for both PKH 26 and PKH 67 is reported in the top right of each panel.
Vaccination with DCs cocultured with antibody-coated tumor cells provides specific protection against 38C13 lymphoma cells
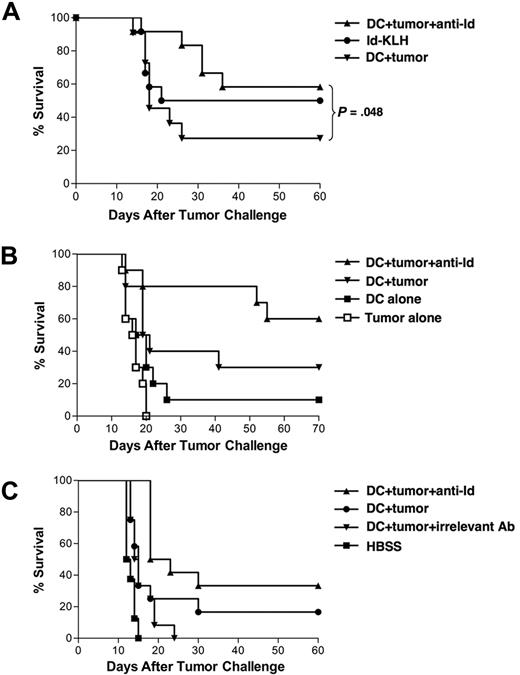
We next tested the in vivo functional activity of tumor cell–loaded DCs. Groups of mice received 2 biweekly vaccinations of either DCs cocultured with mAb-coated 38C13 cells, DCs cocultured with untreated 38C13 cells, Id-KLH protein, DCs alone, or irradiated 38C13 cells alone, and were challenged 2 weeks later with a lethal inoculum of 38C13 cells. DCs loaded with mAb-opsonized tumor cells protected the majority of animals (58%-60%), while DCs loaded with untreated tumor cells protected only 27% to 30% of animals (Figure 2A,B; P < .05 for DCs loaded with mAb-coated vs untreated tumor cells, Figure 2A). However, vaccination with DCs alone or irradiated 38C13 lymphoma cells alone did not provide significant protection against tumor challenge (Figure 2B). Furthermore, immunization with DCs loaded with apoptotic tumor cells coated with an isotype control mAb did not confer protection from lethal tumor challenge (Figure 2C), while DCs loaded with anti-Id opsonized tumor cells protected 33% of mice (P = .001 vs control mAb and P = .038 vs DCs plus untreated 38C13). The antigenic specificity of the protection was demonstrated by challenge with the irrelevant syngeneic tumor AG104a. While DC vaccination offered significant protection against 38C13 tumor challenge (P < .001 compared with HBSS-treated mice), there was no protection against challenge with the irrelevant AG104a tumor (P = .5 vs HBSS; data not shown).
Vaccination with DCs loaded with irradiated, mAb-coated tumor cells protects against a lethal inoculum of 38C13 lymphoma. (A) Groups of mice were vaccinated on days −28 and −14 with DCs cocultured with irradiated, anti-Id mAb-coated 38C13 tumor cells (n = 12), DCs cocultured with tumor cells alone (n = 11), or Id-KLH protein (n = 12). Mice were challenged with 1000 38C13 subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter. (B) Groups of 10 mice were vaccinated on days −28 and −14 with DCs loaded with mAb-coated tumor cells, DCs loaded with untreated tumor cells, DCs alone, irradiated tumor cells alone, or Id-KLH protein. Mice were challenged with 5000 38C13 tumor cells subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter. (C) Groups of mice were vaccinated on days −28 and −14 with DCs cocultured with irradiated, anti-Id mAb-coated 38C13 tumor cells (n = 12), DCs cocultured with tumor cells incubated with irrelevant isotype control antibody (n = 12), DCs cocultured with tumor cells alone (n = 12), or HBSS (n = 8). Mice were challenged with 5000 38C13 subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter.
Vaccination with DCs loaded with irradiated, mAb-coated tumor cells protects against a lethal inoculum of 38C13 lymphoma. (A) Groups of mice were vaccinated on days −28 and −14 with DCs cocultured with irradiated, anti-Id mAb-coated 38C13 tumor cells (n = 12), DCs cocultured with tumor cells alone (n = 11), or Id-KLH protein (n = 12). Mice were challenged with 1000 38C13 subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter. (B) Groups of 10 mice were vaccinated on days −28 and −14 with DCs loaded with mAb-coated tumor cells, DCs loaded with untreated tumor cells, DCs alone, irradiated tumor cells alone, or Id-KLH protein. Mice were challenged with 5000 38C13 tumor cells subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter. (C) Groups of mice were vaccinated on days −28 and −14 with DCs cocultured with irradiated, anti-Id mAb-coated 38C13 tumor cells (n = 12), DCs cocultured with tumor cells incubated with irrelevant isotype control antibody (n = 12), DCs cocultured with tumor cells alone (n = 12), or HBSS (n = 8). Mice were challenged with 5000 38C13 subcutaneously on day 0, followed for tumor growth, and killed when tumors reached 1.4 cm in diameter.
Vaccination with DCs loaded with irradiated, mAb-coated tumor cells provides tumor protection superior to that provided by Id-KLH vaccination
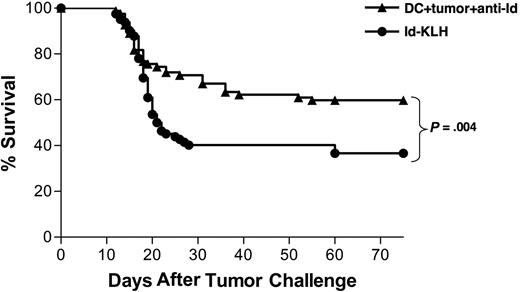
To determine the relative efficacy of the mAb-opsonized tumor cell–DC vaccine and a standard lymphoma Id-KLH vaccine, we combined survival data from 7 individual experiments that included both the Id-KLH protein and the DC vaccines (for example, Figure 2A). All had the same vaccination schedule, tumor challenge protocol, and end point criteria. This composite analysis showed that the protection provided by the DC vaccine was superior to that provided by Id-KLH (P = .004; Figure 3). Therefore, a DC-based vaccine incorporating the collection of antigens in killed whole tumor cells provided stronger antitumor effects than immunization with a single defined protein tumor antigen.
Vaccination with DCs cocultured with irradiated, mAb-coated tumor cells provides protection superior to vaccination with Id-KLH protein. Pooled survival data of mice receiving 2 biweekly injections of either DCs loaded with irradiated, mAb-coated tumor cells (n = 82) or Id-KLH protein alone or with either IL-12 or GM-CSF adjuvant (n = 82) in 7 separate experiments are shown. Two weeks after vaccinations, mice were injected with 38C13 subcutaneously, followed for tumor growth, and killed when their tumors reached 1.4 cm in diameter.
Vaccination with DCs cocultured with irradiated, mAb-coated tumor cells provides protection superior to vaccination with Id-KLH protein. Pooled survival data of mice receiving 2 biweekly injections of either DCs loaded with irradiated, mAb-coated tumor cells (n = 82) or Id-KLH protein alone or with either IL-12 or GM-CSF adjuvant (n = 82) in 7 separate experiments are shown. Two weeks after vaccinations, mice were injected with 38C13 subcutaneously, followed for tumor growth, and killed when their tumors reached 1.4 cm in diameter.
Vaccination with DCs loaded with mAb-coated tumor cells can eradicate preexisting tumor in combination with chemotherapy
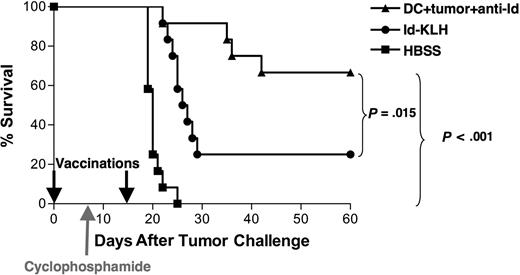
To test the protective efficacy of the opsonized tumor cell–DC vaccine under more stringent conditions, vaccinations were performed in animals bearing preexisting tumor. Mice were inoculated with 38C13 tumor cells on day 0, followed by a single dose of cyclophosphamide on day 8 to cytoreduce the rapidly growing tumor. Vaccinations with DCs loaded with mAb-opsonized 38C13 or Id-KLH were administered on days 0 and 15 after tumor inoculation (Figure 4). Animals treated with HBSS alone plus chemotherapy all died within 3 weeks, whereas tumor was eradicated from 67% of animals vaccinated with DCs loaded with mAb-coated tumor cells (P < .001 vs HBSS). This level of tumor eradication was also statistically superior to that provided by Id-KLH (25%, P = .015). Thus, under the stringent setting of preexisting lymphoma, the DC vaccine provided robust antitumor efficacy that was once again superior to that provided by Id-KLH.
Vaccination with DCs loaded with irradiated, mAb-coated tumor cells can eradicate preexisting tumor in combination with chemotherapy. Groups of 12 mice received 5000 38C13 lymphoma cells subcutaneously on day 0 and were treated on days 0 and 15 with either DCs loaded with irradiated, mAb-coated tumor cells, Id-KLH, or HBSS. On day 8, the animals received 100 mg/kg cyclophosphamide intraperitoneally. Animals were then followed for tumor growth and killed when their tumors reached 1.4 cm in diameter. Data are representative of 2 independent experiments.
Vaccination with DCs loaded with irradiated, mAb-coated tumor cells can eradicate preexisting tumor in combination with chemotherapy. Groups of 12 mice received 5000 38C13 lymphoma cells subcutaneously on day 0 and were treated on days 0 and 15 with either DCs loaded with irradiated, mAb-coated tumor cells, Id-KLH, or HBSS. On day 8, the animals received 100 mg/kg cyclophosphamide intraperitoneally. Animals were then followed for tumor growth and killed when their tumors reached 1.4 cm in diameter. Data are representative of 2 independent experiments.
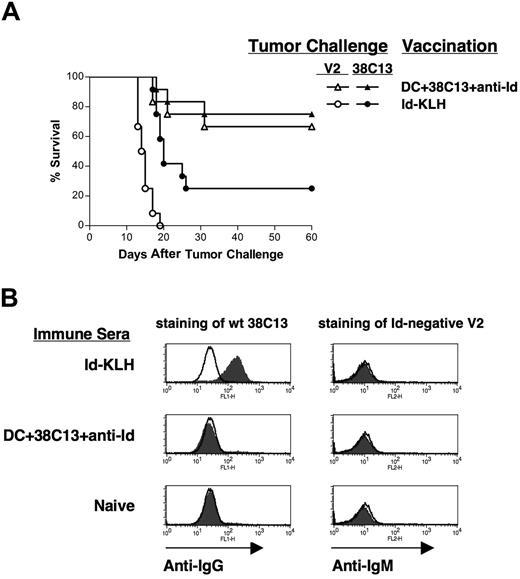
Protective immunity is not dependent on idiotype expression by tumor cells
To determine whether the protective immunity was dependent on surface Id expression by the tumor cells, we used the Id-negative 38C13 variant V2.40,49 38C13-V2 lacks surface expression of IgM due to a frameshift mutation in the leader sequence of the kappa light chain, and, unlike wild-type, 38C13 is insensitive to treatment with anti-Id mAbs.41 Mice were vaccinated with either DCs loaded with mAb-coated 38C13 cells or Id-KLH plus IL-12, then challenged with either wild-type 38C13 or the Id-negative V2 (Figure 5A). While Id-KLH provided some protection against wild-type 38C13 (25%), it provided no protection against V2 (Figure 5A). The DC vaccine, however, provided equal protection against both wild-type 38C13 (75%) and the Id-negative V2 (66.7%) (Figure 5A). Again, the DC vaccine provided significantly better protection against 38C13 than did Id-KLH (P = .01). Thus, the antilymphoma immunity induced by the mAb-opsonized tumor cell–DC vaccine was not dependent on expression of Id at the tumor cell surface.
Protective immunity is not dependent on idiotype expression on the tumor cell surface. (A) Groups of 12 mice were vaccinated subcutaneously on days −28 and −14 with DCs loaded with irradiated, mAb-coated tumor cells, Id-KLH protein plus IL-12, or DCs alone, and challenged on day 0 with either 5000 38C13 or 5000 idiotype-negative variant V2 cells. Animals were then followed for tumor growth and killed when tumors reached 1.4 cm in diameter. (B) Anti-Id antibodies are not induced by vaccination with DCs loaded with mAb-coated tumor cells. Pooled sera from mice vaccinated with Id-KLH, DC loaded with irradiated, mAb-coated tumor cells, or naive control sera were incubated for 4 hours with wild-type 38C13 (left panels) or idiotype-negative V2 cells (right panels). Bound antibodies were detected with FITC-labeled anti-IgG (left panels) or anti-IgM (right panels) antibodies (filled histograms) using flow cytometry; lines indicate isotype control staining.
Protective immunity is not dependent on idiotype expression on the tumor cell surface. (A) Groups of 12 mice were vaccinated subcutaneously on days −28 and −14 with DCs loaded with irradiated, mAb-coated tumor cells, Id-KLH protein plus IL-12, or DCs alone, and challenged on day 0 with either 5000 38C13 or 5000 idiotype-negative variant V2 cells. Animals were then followed for tumor growth and killed when tumors reached 1.4 cm in diameter. (B) Anti-Id antibodies are not induced by vaccination with DCs loaded with mAb-coated tumor cells. Pooled sera from mice vaccinated with Id-KLH, DC loaded with irradiated, mAb-coated tumor cells, or naive control sera were incubated for 4 hours with wild-type 38C13 (left panels) or idiotype-negative V2 cells (right panels). Bound antibodies were detected with FITC-labeled anti-IgG (left panels) or anti-IgM (right panels) antibodies (filled histograms) using flow cytometry; lines indicate isotype control staining.
To further investigate the mechanism of tumor protection after mAb-opsonized tumor cell–DC vaccination, we first sought to determine whether a humoral antitumor immune response was induced. The 38C13 lymphoma is susceptible to control by monoclonal anti-Id antibodies45 and polyclonal anti-Id antibodies induced by Id protein vaccination.43,46,50 Using an ELISA with a sensitivity of less than 0.01 μg/mL, we found no detectable anti-Id antibodies in sera from mice given the DC vaccine (data not shown). We next used flow cytometry to determine whether immune sera contained antibodies against non-Id antigens on the surface of 38C13 cells (Figure 5B). Sera were incubated with wild-type tumor cells and bound IgG antibodies detected with fluorochrome-labeled anti-IgG. While sera from mice vaccinated with Id-KLH readily stained 38C13 lymphoma cells (positive control, Figure 5B upper left panel), immune sera from DC-vaccinated mice contained no measurable antitumor antibodies (Figure 5B middle left panel). Similarly, using the surface Id/IgM-negative V2 variant cells as targets, we found that immune sera also lacked IgM antibodies against non-Id cell surface antigens (Figure 5B right panels).
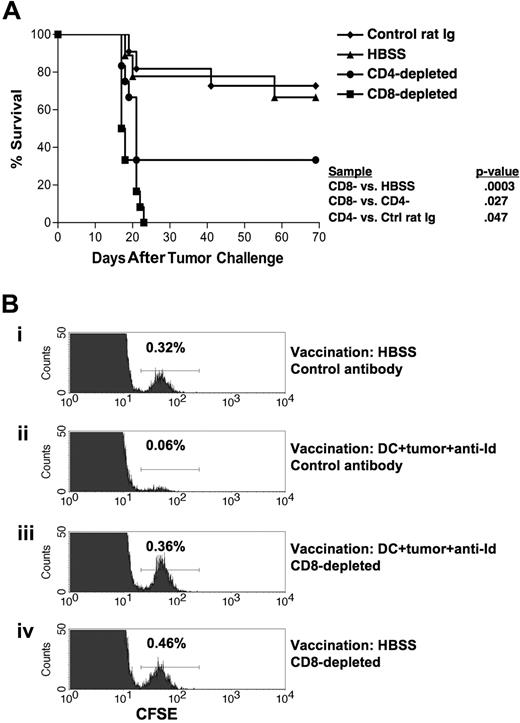
CD8+ T cells are the principal mediators of antitumor immunity following mAb-opsonized whole tumor cell–DC vaccination
The lack of detectable antitumor antibodies suggested that T cells might play the dominant role in mediating tumor protection after vaccination. To test this, we performed tumor challenge experiments in mice depleted of either CD4+ or CD8+ T cells. DC vaccination in CD8 T cell–depleted mice did not provide any protection against tumor challenge (Figure 6A). Vaccination of CD4+ T cell–depleted mice provided an intermediate level of protection that was significantly lower than control-depleted mice (P = .047) yet significantly higher than CD8+ T cell–depleted mice (P = .027; Figure 6A). In 3 independent experiments, the protective antitumor response was critically dependent on CD8+ T cells.
Protective immunity is dependent on CD8+ T cells. (A) Groups of mice were vaccinated on days −28 and −14 with DCs loaded with irradiated, mAb-coated tumor cells subcutaneously. On days −6, −5, −4, and 0 and weekly thereafter the animals were depleted of CD8+ T cells using mAb HB129 (n = 12), depleted of CD4+ T cells using mAb GK1.5 (n = 12), control depleted using control rat Ig (n = 11), or treated with HBSS (n = 9). On day 0, mice were challenged with 5000 38C13 cells subcutaneously. Animals were then followed for survival. Results are representative of 3 individual experiments. (B) In vivo cytotoxicity assay demonstrating the clearance of live tumor cells from vaccinated mice. Groups of 4 mice were vaccinated on days −28 and −14 with either DCs loaded with irradiated, mAb-coated tumor cells (ii,iii) or HBSS (i,iv). On days −6, −5, and −4 animals were depleted of CD8+ T cells using mAb HB129 (iii,iv) or control depleted using isotype-matched mAb UPC10 (i,ii). On day 0, mice were injected with 107 CFSE-labeled 38C13 intravenously. After 4 hours, spleens were harvested, pooled, and analyzed via flow cytometry for loss of 38C13 target cells. Histograms depict CFSE-labeled cells from groups of 4 pooled mice; the percentage target cells recovered is indicated.
Protective immunity is dependent on CD8+ T cells. (A) Groups of mice were vaccinated on days −28 and −14 with DCs loaded with irradiated, mAb-coated tumor cells subcutaneously. On days −6, −5, −4, and 0 and weekly thereafter the animals were depleted of CD8+ T cells using mAb HB129 (n = 12), depleted of CD4+ T cells using mAb GK1.5 (n = 12), control depleted using control rat Ig (n = 11), or treated with HBSS (n = 9). On day 0, mice were challenged with 5000 38C13 cells subcutaneously. Animals were then followed for survival. Results are representative of 3 individual experiments. (B) In vivo cytotoxicity assay demonstrating the clearance of live tumor cells from vaccinated mice. Groups of 4 mice were vaccinated on days −28 and −14 with either DCs loaded with irradiated, mAb-coated tumor cells (ii,iii) or HBSS (i,iv). On days −6, −5, and −4 animals were depleted of CD8+ T cells using mAb HB129 (iii,iv) or control depleted using isotype-matched mAb UPC10 (i,ii). On day 0, mice were injected with 107 CFSE-labeled 38C13 intravenously. After 4 hours, spleens were harvested, pooled, and analyzed via flow cytometry for loss of 38C13 target cells. Histograms depict CFSE-labeled cells from groups of 4 pooled mice; the percentage target cells recovered is indicated.
To further investigate the in vivo mechanisms of antitumor immunity induced by our DC vaccine, an in vivo cytotoxicity assay was performed in mice using CFSE-labeled 38C13 cells as targets for lysis48 (Figure 6B). Using flow cytometry, we were able to quantitate the percentage of target cells recovered from the spleens of experimental mice. Mice that had been vaccinated with opsonized 38C13-loaded DCs and given control antibody injections showed a dramatic reduction in the percentage of labeled 38C13 cells recovered (0.06% vs 0.32% in nonvaccinated mice), representing the in vivo killing of the tumor cells after vaccination (Figure 6Bii vs 6Bi). However, when vaccinated mice had been depleted of CD8+ T cells, the in vivo killing of target 38C13 cells was abolished (0.36% recovered) (Figure 6Biii). Based on these data and the in vivo T-cell depletion experiments, it appears that the tumor immunity generated by our DC vaccine is mediated principally by CD8+ T cells, with a secondary role for CD4+ T cells in providing help to CD8+ effectors.
To further assess the antitumor T-cell response, IFN-γ ELISPOT assays were performed using draining lymph node cells obtained following vaccination with DCs loaded with irradiated, anti-Id opsonized 38C13 cells (Document S1). On day 29 after 2 biweekly vaccinations, draining lymph node cells cocultured with irradiated tumor cells as targets yielded 732 plus or minus 71 spots/2 × 105 effectors versus 594 plus or minus 112 spots/2 × 105 effectors from lymph node cells alone (P = .023). Such high background IFN-γ release was noted in effector cells collected at both 14 and 29 days after immunizations, although naive lymph node and spleen effectors did not respond to stimulation with irradiated 38C13 (3 ± 1 spots). This increased IFN-γ release further supports the antitumor immunity induced by the opsonized tumor cell–DC vaccine being T cell–mediated.
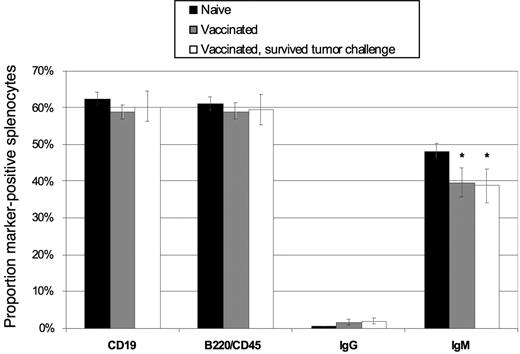
Vaccination with DCs loaded with antibody-coated B-cell lymphoma cells does not deplete the host splenic B-cell compartment
Given our use of whole lymphoma cells in the DC vaccine, the potential existed for induction of autoimmunity against normal B cell–associated antigens. Importantly, at no time in our studies did we observe signs of illness or autoimmunity (weight loss, wasting, behavioral changes) in animals after vaccination, and all survivors appeared healthy up to 75 days after tumor challenge. Nonetheless, we hypothesized that autoimmunity may manifest as a reduction in the normal B-cell compartment after vaccination and/or tumor challenge. Thus, we studied the splenic B-cell compartment in vaccinated mice, comparing animals that had received the mAb-coated tumor cell–DC vaccine and survived a tumor challenge (8 of 12 mice challenged survived), DC vaccine but no challenge (6 mice), and naive age-matched animals (4 mice). Sixty days after challenge, spleens were removed and the percentage of lymphocytes expressing the B-cell markers CD19, B220/CD45, IgG, or IgM was determined in each individual spleen by flow cytometry. There was no statistically significant difference among the percentages of CD19+ and B220/CD45+ cells in the spleens of the 3 groups (Figure 7), indicating that the overall size of the splenic B-cell compartment was not altered by vaccination or tumor challenge. A modest decrease in IgM+ B cells was seen, however (P = .05), matched by a rise in IgG+ cells in the vaccinated groups (P= .04). This was likely indicative of the priming of naive B cells during vaccination, with resultant Ig class switching from IgM to IgG. Thus, despite the use of whole tumor cells, which contain a mixture of both tumor-associated and normal B-cell proteins, no measurable tissue-specific autoimmune effects could be detected.
Vaccination with DCs loaded with irradiated, mAb-coated lymphoma cells does not induce depletion of normal host B cells. Mice were vaccinated with DCs and challenged with 38C13 on day 0 (▭, n = 8), vaccinated with DCs and left without tumor challenge ( , n = 6), or treated only with HBSS (
, n = 6), or treated only with HBSS ( , n = 4) subcutaneously on days −28 and −14. On day 60, splenocytes were removed and analyzed via flow cytometry for surface expression of CD19, B220/CD45, IgG, and IgM. The percentage of cells positive for each marker is shown. Error bars depict the standard deviation among values from individual mice. *P ≤ .05.
, n = 4) subcutaneously on days −28 and −14. On day 60, splenocytes were removed and analyzed via flow cytometry for surface expression of CD19, B220/CD45, IgG, and IgM. The percentage of cells positive for each marker is shown. Error bars depict the standard deviation among values from individual mice. *P ≤ .05.
Vaccination with DCs loaded with irradiated, mAb-coated lymphoma cells does not induce depletion of normal host B cells. Mice were vaccinated with DCs and challenged with 38C13 on day 0 (▭, n = 8), vaccinated with DCs and left without tumor challenge ( , n = 6), or treated only with HBSS (
, n = 6), or treated only with HBSS ( , n = 4) subcutaneously on days −28 and −14. On day 60, splenocytes were removed and analyzed via flow cytometry for surface expression of CD19, B220/CD45, IgG, and IgM. The percentage of cells positive for each marker is shown. Error bars depict the standard deviation among values from individual mice. *P ≤ .05.
, n = 4) subcutaneously on days −28 and −14. On day 60, splenocytes were removed and analyzed via flow cytometry for surface expression of CD19, B220/CD45, IgG, and IgM. The percentage of cells positive for each marker is shown. Error bars depict the standard deviation among values from individual mice. *P ≤ .05.
Discussion
The principal objective of this study was to carry out an in vivo test of vaccination with DCs loaded with antibody-coated tumor cells in a fully syngeneic model system in order to validate and extend the promising in vitro results obtained using this approach.31-34 We hypothesized that DCs loaded with mAb-opsonized, killed tumor cells would present tumor antigens to T cells more efficiently than DCs loaded with untreated apoptotic cells or tumor cell lysates, and thereby provide superior antitumor immunity in vivo. As observed in several previous in vitro studies,32,33 we found that the addition of antitumor mAb to cocultures of killed tumor cells and DCs resulted in only modestly increased cellular antigen uptake (Figure 1), and was not accompanied by DC maturation. However, pretreatment of the tumor cells with mAb before coculture with DCs resulted in greater antitumor immunity in vivo (Figure 2). Antitumor effects were more marked using mAb-opsonized tumor for DC pulsing in the settings of both tumor challenge (Figure 2) and preexisting tumor (Figure 4). Thus, the enhanced in vitro tumor immunity achieved previously using DCs loaded with mAb-opsonized tumor cells was borne out in our in vivo model.
A second major objective of this study was to compare the efficacy of a mAb-opsonized whole tumor cell–DC vaccine with a traditional lymphoma vaccine (tumor-specific Id protein), and to determine whether this vaccine could elicit immunity against tumor antigens beyond Id. The opsonized whole tumor–DC vaccine was found to offer significantly better tumor protection than Id-KLH vaccination in the prophylactic setting (Figure 3). Importantly, in the more stringent setting of preexisting tumor, the DC vaccine was also more potent than Id-KLH (Figure 4). In these comparative studies, IL-12 and GM-CSF were each used as cytokine adjuvants in some experiments in attempt to achieve improved protection using Id-KLH, yet mAb-opsonized tumor cell–DC vaccines still offered superior tumor immunity in vivo. In 2 independent experiments, we found no survival advantage with GM-CSF as an adjuvant over 38C13 Id-KLH alone (data not shown), and thus in subsequent experiments we used Id-KLH alone or with IL-12.
One explanation for the greater efficacy of the DC–whole tumor cell vaccine may be the more efficient induction of CD8+ T cells recognizing one or more non-Id lymphoma antigens. In the 38C13 model, antibodies play a major role in mediating tumor protection using Id vaccines, even when Id is delivered by DCs.43,46,50 In contrast, the mAb-opsonized 38C13-DC vaccine did not induce detectable antibodies to Id or other cell-surface antigens (Figure 5B). Using both in vivo depletion studies and an in vivo cytotoxic T lymphocyte (CTL) assay, we found that tumor immunity after mAb-opsonized tumor cell–DC vaccination was critically dependent on CD8+ T cells (Figure 6A,B). To determine whether Id-derived class I MHC–binding epitopes could be the targets of these CTLs, we challenged vaccinated mice with the 38C13 Id-negative variant cell line V2, which does not express kappa light chain. Since the DC vaccine provided equal protection to this cell and wild-type 38C13, kappa light chain–derived determinants can be excluded as possible targets of the induced CTLs (Figure 5A). Yet as V2 still expresses the IgM heavy chain, we cannot exclude heavy chain–derived epitopes as targets of the induced CTLs. However, the 38C13 heavy chain lacks predicted H-2k class I–binding epitopes, and immunization with Id-pulsed DCs, Id-encoding plasmid DNA, or Id-encoding recombinant adenovirus all fail to elicit 38C13 Id-specific CD8+ CTLs.43,51,52 Thus we do not believe that the CTL-mediated tumor immunity following mAb-opsonized 38C13-DC vaccination is directed at Id-derived epitopes. Rather, it appears that one or more non-Id antigens serve as targets for the induced antilymphoma immunity.
Vaccination with whole tumor cells or antigens shared by normal tissues rather than a defined tumor-specific antigen carries the risk of inducing autoimmunity.35,37 Thus, a third important objective of our study was to determine whether mAb-opsonized whole tumor–loaded DC vaccines would induce tumor immunity without triggering systemic autoimmunity. Roskrow et al found that immunization with DCs loaded with lymphoma cell–derived peptides together with CD40 ligand and IL-2–expressing fibroblasts resulted in a severe autoimmune syndrome resembling graft-versus-host disease.36 In contrast, we found no evidence for autoimmunity in the current study despite the use of whole autologous lymphoma cells. All vaccinated mice rejecting tumor remained healthy when observed for up to 75 days after tumor inoculation, and there was no evidence for autoimmune damage to the splenic B-cell compartment (Figure 7).
We believe that the general strategy of using mAb-opsonized whole tumor–loaded DC vaccines may be more efficacious than simple pulsing of DCs with apoptotic tumor cells or lysates. This approach is amenable to use with any cancers in which antitumor opsonizing mAbs are available.34 Several other groups have recently investigated vaccination with DCs loaded with antibody-coated killed tumor cells in preclinical murine models. Akiyama et al bound the hapten TNP to the surface of E.G7 thymoma cells expressing the model tumor antigen ovalbumin, and coated irradiated cells with anti-TNP antibody before coculture with DCs.53 Immunization with these DCs led to enhanced induction of tumor-specific CTLs. In a single in vivo tumor challenge experiment, they showed that this DC vaccine could protect 50% of animals from tumor challenge, but comparison with other established vaccination techniques was not performed. More recently, Pilon-Thomas et al have demonstrated the induction of immunity against B16 melanoma using DCs pulsed with tumor cells coated with an anti-CD44 mAb,54 with both resistance to tumor growth after B16 challenge and reduction in lung metastases. Our report adds to the above findings and provides unique information in the following: (1) We have used a tumor-specific antibody for tumor cell opsonization. (2) We have systematically compared the efficacy of the pulsed DC vaccine with a standard, clinically relevant vaccine for lymphomas (Id-KLH). (3) The possibility that the opsonized tumor cell–DC vaccine could induce tissue-specific autoimmunity has been investigated. (4) We have attempted to determine the role for one known tumor antigen (idiotype) as a target for the induced tumor immunity. (5) B-cell lymphomas are amenable to clinical translation with the availability of the antihuman CD20 mAb rituximab.3 Together, the above results suggest that vaccination with DCs loaded with mAb-coated tumor cells appears to represent a safe, potent, and clinically feasible approach to active cancer immunotherapy that is deserving of assessment in patients with B-cell lymphoma.
The strategy described here could be readily translated into humans with B-cell lymphomas, using rituximab-coated lymphoma cells cocultured with immature monocyte-derived DCs that are induced to mature after antigen loading.32 To further explore this technique, we are now extending our preclinical studies using murine lymphoma cells engineered by retroviral transduction to express human CD20. Human CD20 may represent an optimal target for tumor cell opsonization given its stable expression on the cell surface without modulation upon antibody binding. Additional studies in this model system using cytokines and other agents to promote optimal maturation and migration of DCs loaded with mAb-opsonized tumor cells may offer techniques for further enhancement of vaccine potency. Furthermore, since the immunity generated by mAb-opsonized tumor-DC vaccination can target non-Id lymphoma antigens, this approach might be combined with Id protein vaccines to obtain additive or synergistic antilymphoma immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ronald Levy (Stanford University) for helpful comments and suggestions. We thank Brent Gordon and the UCLA ELISPOT Core for the use of their facility.
This work was supported by NIH/NHLBI P01 HL57443 and the Albert Yu and Mary Bechmann Foundation.
Authorship
Contribution: S.N.F. and K.K.S. designed and performed the research, analyzed the data, and wrote the paper. D.J.B. and K.K. characterized the humoral anti-Id responses; R.E.Y. performed ELISPOT assays; and J.M.T. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Timmerman, UCLA Medical Center, Center for Health Sciences, Room 42-121, 10833 LeConte Avenue, Los Angeles, CA 90095-1678; e-mail: jtimmerman@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal