Abstract
Antithymocyte/antilymphocyte globulins are polyclonal antihuman T-cell antibodies used clinically to treat acute transplant rejection. These reagents deplete T cells, but a rabbit antihuman thymocyte globulin has also been shown to induce regulatory T cells in vitro. To examine whether antithymocyte globulin–induced regulatory cells might be functional in vivo, we generated a corresponding rabbit antimurine thymocyte globulin (mATG) and tested its ability to induce regulatory cells in vitro and whether those cells can inhibit acute graft-versus-host disease (GVHD) in vivo upon adoptive transfer. In vitro, mATG induces a population of CD4+CD25+ T cells that express several cell surface molecules representative of regulatory T cells. These cells do not express Foxp3 at either the protein or mRNA level, but do show suppressive function both in vitro and in vivo when adoptively transferred into a model of GVHD. These results demonstrate that in a murine system, antithymocyte globulin induces cells with suppressive activity that also function in vivo to protect against acute GVHD. Thus, in both murine and human systems, antithymocyte globulins not only deplete T cells, but also appear to generate regulatory cells. The in vitro generation of regulatory cells by anti-thymocyte globulins could provide ad-ditional therapeutic modalities for immune-mediated disease.
Introduction
T cell–depleting polyclonal antihuman thymocyte/lymphocyte globulins are currently in use for solid organ transplantation and bone marrow transplantation for graft-versus-host disease (GVHD) prophylaxis.1,2 One such antithymocyte globulin, Thymoglobulin, is generated by immunizing rabbits with human thymocytes and purifying the resulting IgG fraction from the rabbit sera. Thymoglobulin has been shown to recognize a variety of T-cell as well as non–T-cell surface antigens,3-7 but it is also likely to recognize additional surface antigens not yet examined or known. Murine counterpart reagents such as murine antithymocyte or antilymphocyte reagents also deplete T cells in mice and show protective effects in diabetes8,9 and graft-versus-host disease10 models and prevent rejection of pancreas allografts,11 islet xenografts,12 skin allografts cotransplanted with donor bone marrow,13 and allogeneic heart transplants (S.R.N., J.M.W., and Lan Gao, unpublished data, September 2004). Depletion of T cells has been thought to be the primary mechanism of the protection observed in both human transplant patients and animal models14 ; however, given the polyclonal nature of antithymocyte globulins, additional mechanisms of immunosuppression have been suggested.15 One such mechanism is the induction of regulatory T cells based on experiments showing that culture of normal human T cells with Thymoglobulin rapidly induces phenotypic and functional regulatory cells in vitro.16
A variety of regulatory cell populations has been described that limits immune responses.17 These populations include natural regulatory T cells that are CD4+CD25high, in vitro–induced IL-10–producing CD4+ T cells (Tr1), TGF-β producing CD4 T cells (Th3), anergic CD4+ T cells, natural killer T (NKT) cells, CD8+CD28− T cells, and CD3+CD4−CD8− T cells.17 All of these populations have been demonstrated to functionally suppress a variety of immune responses, albeit through different or unknown mechanisms.18 Attempts to define the various regulatory T-cell populations based on surface marker expression and/or cytokine production have suggested that these cells often express surface markers such as CTLA-4, CD62L, GITR, and/or CD103, and secrete suppressive cytokines such as IL-10 and/or TGF-β.17-19 However, the expression of these markers and/or cytokines is neither restricted to one type of regulatory cell nor exclusive to regulatory cells.17-19 The nuclear transcription factor forkhead box protein 3 (Foxp3) thus far appears to be one of the few regulatory T cell–specific markers in mice.20 However, cells possessing immune regulatory function, including Tr1, NKT, and anergic regulatory cells, do not express Foxp3.21,22 Given the variety of regulatory cell populations and lack of specific markers for regulatory cells on the whole, demonstration of functional suppressive activity by regulatory cells in an in vivo setting is critical.
Based on several studies demonstrating a profound protective effect of transferred purified and/or expanded murine regulatory T cells in models of autoimmune disease, transplant rejection, and GVHD,17,23-25 the concept of ex vivo induction and/or expansion of regulatory T cells for clinical use in autoimmune and transplant settings is being pursued.18,22,23,26 Some of the described induction protocols for regulatory T cells include various combinations of T-cell receptor, costimulatory molecule, and cytokine (IL-10, TGF-β) stimulation,22,27 however, there are likely other methods of inducing regulatory T cells. Given that Thymoglobulin has been demonstrated to induce human regulatory cells in vitro,16 we sought to extend these studies by examining whether a murine counterpart of Thymoglobulin could also induce regulatory T cells in vitro that are functional in vivo in a disease setting.
Methods
Mice
Mice used for thymus collections were obtained from Taconic Laboratories (Germantown, NY) and used at 5 to 7 weeks of age. Recombination-activating gene 2–targeted (RAG 2KO) mice on the BALB/c background (C.129S6[B6]-Rag2tmlFwaN12), BALB/c (BALB/cAnNTac), and C57BL/6 (C57BL/6NTac) mice were obtained from Taconic Laboratories and used between 6 and 10 weeks of age. If mice induced for GVHD showed signs of morbidity, butorphanol tartrate (Fort Dodge Animal Health, Fort Dodge, IA) and diazepam (Purepac Pharmaceutical, Elizabeth, NJ) were provided to alleviate discomfort. Mice were housed and maintained in accordance with the Guide for the Care and Use of Laboratory Animals and under American Association for Accreditation of Laboratory Animals Care I accreditation, and all animals protocols used in these studies were approved by the Institutional Animal Care and Use Committee.
Murine ATG and rabbit IgG
Murine ATG was generated by immunizing rabbits with a mixture of thymocytes from 8 different strains of mice (C57BL/6, BALB/c, DBA/2, 129, C3H, SJL, Swiss Webster, ICR). Thymocyte suspensions were prepared from extracted thymuses and New Zealand white rabbits were immunized twice, 2 weeks apart and terminally bled 2 weeks following second immunization (Millbrook Immunoserv, Amherst, MA). Total rabbit IgG from the resulting serum was purified with a process analogous to that used for Thymoglobulin. Control rabbit IgG was similarly purified from whole normal rabbit serum (Sigma-Aldrich, St Louis, MO).
Splenocyte preparations
Single-cell suspensions were generated from harvested mouse spleens by homogenization between frosted glass slides into RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 1% l-glutamine, and 1% penicillin/streptomycin solution (complete medium) (all reagents from Gibco, Grand Island, NY). Red blood cells were lysed by 1- to 2-minute incubation with a red blood cell lysis solution (Sigma-Aldrich), and viable cells were enumerated by trypan blue exclusion on a hemacytometer or ViCell automated counter (Beckman Coulter, Fullerton, CA).
mATG-treated cultures
For mATG treatment of mouse splenocytes, freshly isolated cells were cultured in complete medium with indicated concentrations of either mATG or rabbit IgG and with or without 200 U/mL recombinant human or murine IL-2 (R&D Systems, Minneapolis, MN). On various days following initiation of cultures, supernatants and/or cells were collected for the assays described. Viable cells from the cultures were enumerated as described in “Splenocyte preparations.” For cytokine measurements, supernatants were collected from cultures at various times, and IL-10 and total TGF-β levels were measured using multiplex bead technology (Bioplex; BioRad, Hercules, CA) or enzyme-linked immunosorbent assay (ELISA; R&D Systems), respectively.
Suppression assays
For analysis of suppressive activity of splenocytes treated with mATG or rabbit IgG, cells from 5-day cultures were washed 2 to 3 times with complete media and added at ratios of 1:1, 0.5:1, 0.25:1, and 0.125:1 to normal splenocytes stimulated with 5.0 × 104 anti-CD3/anti-CD28 beads/mL (Dynal Biotech, Oslo, Norway). All cultures were incubated for 3 days and pulsed for 18 hours with 3H thymidine. Cells were then collected using a cell harvester (Tomtec, Hamden, CT), and 3H incorporation was measured with a liquid scintillation counter (PerkinElmer, Waltham, MA)
Flow cytometric analyses
For staining of mATG or rabbit IgG–treated mouse splenocytes, cells were harvested at indicated times following initiation of culture, washed twice with PBS + 2% fetal bovine serum (staining buffer) and incubated with fluorochrome-conjugated antibodies that included antimouse CD4, CD8, CD62L, CD25, CD45RB, GITR, CD103, CD3, NK1.1, CD44, CD19, and CLTA-4 as well as corresponding isotype control antibodies (all antibodies from BD Biosciences, San Diego, CA). For detection of surface TGF-β, cells were first incubated with unlabeled chicken anti–TGF-β or isotype control chicken IgY (both from R&D Systems), washed 2 times with staining buffer, and then incubated with FITC-labeled goat anti–chicken IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Intracellular Foxp3 expression analysis was performed according to the anti-Foxp3 manufacturer's protocol (eBiosciences, San Diego, CA). Briefly, cells were fixed and permeabilized, followed by blocking and incubation with PE-labeled anti-FoxP3. Assessment of proliferation by bromodeoxyuridine (BrdU) incorporation was performed by adding a 1-mM solution of BrdU to the cultures 1 day prior to determination of BrdU uptake. BrdU uptake in specific cell populations was determined by first staining the cells with fluorochrome-conjugated antimouse CD4, CD8, or CD19 followed by BrdU detection using the FITC BrdU Flow Kit (BD Biosciences) according to the manufacturer's instructions. For all staining protocols, after incubation with the antibodies the cells were washed and analyzed by flow cytometry (FACSCanto and FACSDiva software; BD Biosciences).
Quantification of mFoxp3 by Taqman analysis
RNA was generated from mouse splenocytes cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 as described in “mATG-treated cultures” using RNAStat-60 (Tel-Test, Friendswood, TX). RNA was DNase treated and then used in a first-strand cDNA reaction using an oligo-dT primer and Superscript II RNAseH(-) Reverse Transcriptase (Invitrogen, Carlsbad, CA). cDNA was analyzed by Taqman polymerase chain reaction (PCR) using primers and probes supplied in the Applied Biosystems Taqman Gene Expression Assay Kits for mouse Foxp3 and mouse CD3 (Foster City, CA). The standard curve for each assay was serial 2-fold dilutions of a mouse spleen cDNA ranging from 75 ng to 0.15 ng. mFoxp3 and mCD3 were quantified using the relative standard curve method described by Applied Biosystems. mFoxp3 was normalized to mCD3 in each sample.
Acute graft-versus-host disease induction
For induction of acute GVHD, donor splenocytes were prepared as described in “Splenocyte preparations” and then resuspended in RPMI 1640 containing 10 units/mL heparin (Sigma-Aldrich) and filtered through a 70-μm nylon mesh cell strainer (Becton Dickinson, Franklin Lakes, NJ) just prior to injection into recipient mice. C57BL/6 (GVHD) or BALB/c (control) splenocytes (2.5 × 107) were injected intravenously into recipient BALB/c RAG-2 KO mice. In experiments where splenocytes cultured with mATG or rabbit IgG were adoptively transferred, cells were collected from 5-day cultures as described in “mATG-treated cultures,” washed 2 to 3 times with PBS, resuspended in RPMI 1640 containing 10 units/mL heparin (Sigma-Aldrich), and injected intravenously at 2.5 × 107 or 5.0 × 107 cells/mouse 1 day prior to the injection of allogeneic splenocytes. Body weight change assessments over the course of disease were calculated based on initial weight. Serum AST and ALT levels were measured by Alfa Wassermann VetACE clinical chemistry analyzer (West Caldwell, NJ). For analysis of blood cell populations, blood was collected into EDTA containing tubes (Becton Dickinson Vacutainer Systems), red blood cells were lysed with BD PharmLyse solution (BD Biosciences), and cells were surface stained for CD4 and CD8 populations as described in “Flow cytometric analysis.”
Statistical analyses
Significant differences observed in the effects of mATG and rabbit IgG treatment of cells were calculated using a Student t test (Microsoft Excel [Seattle, WA] or Prism Software [GraphPad, San Diego, CA]).
Results
Murine ATG initially depletes splenocytes in vitro, but later stimulates their proliferation
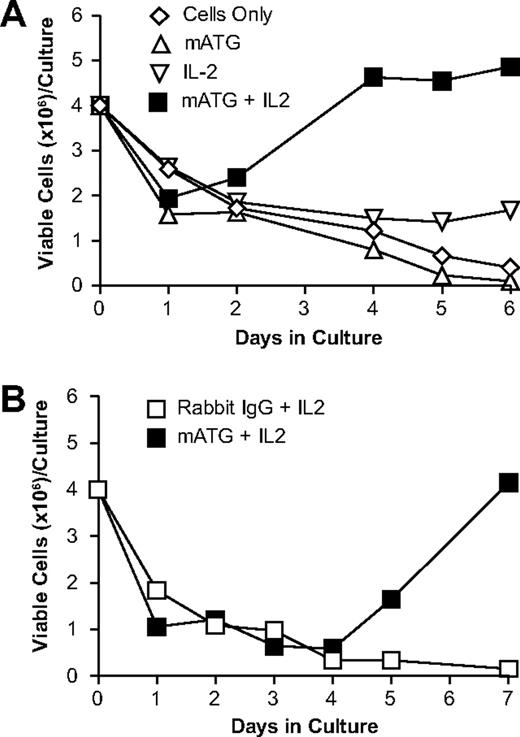
To characterize the effects of mATG on splenocytes isolated from normal mice, we cultured splenocytes from C57BL/6 mice with 100 μg/mL mATG. The addition of exogenous IL-2 to these cultures was also tested because it is well established that murine regulatory T cells require IL-2 for their generation and/or proliferation.28-31 Changes in total cell numbers over the course of the in vitro culture under the various conditions are shown in Figure 1. Splenocytes cultured with mATG, regardless of the presence of IL-2, showed decreased cell numbers at day 1 compared with cells alone, IL-2 alone, or rabbit IgG. However, after 4 to 5 days, mATG cultures containing IL-2 showed greater cell numbers compared with any of the other culture conditions. These cell recoveries with mATG in the presence of IL-2 increased progressively out to day 7, at which point the cultures became overgrown (data not shown). Although IL-2 was required for this observation, we did not see the same proliferative responses in rabbit IgG–treated splenocytes cultured with IL-2 or cells treated with IL-2 alone (Figure 1). Collectively, these results demonstrate that mATG in the presence of IL-2 initially (day 1) depletes cells, but later (days 4-7) stimulates proliferation.
Culture of mouse spleen cells with mATG and IL-2 shows initial depletion at 1 day followed by a proliferative response after 4 days. Normal C57BL/6 mouse splenocytes were cultured alone, with 100 μg/mL mATG, or with rabbit IgG in the presence or absence of 200 U/mL IL-2, and total viable cells were enumerated at various times following initiation of culture as described in “Methods.” (A) Viable cell numbers obtained 1, 2, 4, 5, and 6 days following culture initiation of splenocytes with or without mATG in the presence or absence of exogenous IL-2. (B) Viable cell numbers obtained 1, 2, 3, 4, 5, and 7 days following culture initiation of splenocytes with either mATG or rabbit IgG, both in the presence of exogenous IL-2. Data shown are representative of at least 6 separate experiments.
Culture of mouse spleen cells with mATG and IL-2 shows initial depletion at 1 day followed by a proliferative response after 4 days. Normal C57BL/6 mouse splenocytes were cultured alone, with 100 μg/mL mATG, or with rabbit IgG in the presence or absence of 200 U/mL IL-2, and total viable cells were enumerated at various times following initiation of culture as described in “Methods.” (A) Viable cell numbers obtained 1, 2, 4, 5, and 6 days following culture initiation of splenocytes with or without mATG in the presence or absence of exogenous IL-2. (B) Viable cell numbers obtained 1, 2, 3, 4, 5, and 7 days following culture initiation of splenocytes with either mATG or rabbit IgG, both in the presence of exogenous IL-2. Data shown are representative of at least 6 separate experiments.
T cells are both depleted and induced to proliferate in mATG-treated splenocyte cultures
To further assess the cell types being depleted at early times (day 1) and cells proliferating at late times (day 5), we examined cell subsets at 1 and 5 days following in vitro treatment with mATG or rabbit IgG in the presence of IL-2 (Figure 2). After 1 day of culture, CD8+ and, to a lesser degree, CD4+ T cells were depleted in the presence of mATG compared with rabbit IgG treatment (Figure 2A). CD19+ B-cell, NK-cell, and NKT-cell numbers were all similar to rabbit IgG–treated cultures (Figure 2A). However, by day 5 of culture with mATG, both CD4+ and CD8+ T cells were at much greater absolute numbers than rabbit IgG–treated cultures (Figure 2B). We confirmed that CD4+ and CD8+, but not CD19+, cells were actively proliferating during this late time by BrdU incorporation (Figure 2C). A time course study revealed that decreases in CD4+ and CD8+ T cells observed on day 1 was followed by increases in these cell populations as early as day 2 for CD8+ T cells and day 3 for CD4+ T cells and progressively increased during the remaining culture period (data not shown).
T cells are depleted early but also proliferate late in the cultures with mATG. Cultures of C57BL/6 splenocytes with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 were evaluated for absolute numbers of CD4+, CD8+, CD19+ (B cells), CD3+, NK1.1+ (NKT cells), or CD3−NK1.1+ (classic NK cells) or BrdU incorporation into specific cell populations by flow cytometric analysis described in “Methods.” (A) Cell populations at day 1. (B) Cell populations at day 5. (C) BrdU was added to the cultures on day 4 and evaluated the following day for BrdU incorporation into the CD4+, CD8+ and CD19+ cell populations as described in “Methods.” Data shown are means (± standard deviation [SD]) of 3 replicates from a representative of at least 6 separate experiments.
T cells are depleted early but also proliferate late in the cultures with mATG. Cultures of C57BL/6 splenocytes with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 were evaluated for absolute numbers of CD4+, CD8+, CD19+ (B cells), CD3+, NK1.1+ (NKT cells), or CD3−NK1.1+ (classic NK cells) or BrdU incorporation into specific cell populations by flow cytometric analysis described in “Methods.” (A) Cell populations at day 1. (B) Cell populations at day 5. (C) BrdU was added to the cultures on day 4 and evaluated the following day for BrdU incorporation into the CD4+, CD8+ and CD19+ cell populations as described in “Methods.” Data shown are means (± standard deviation [SD]) of 3 replicates from a representative of at least 6 separate experiments.
Dose ranging studies with mATG demonstrated significant depletion of CD8+ T cells at doses as low as 5 μg/mL, whereas depletion of CD4+ T cells required 20 μg/mL mATG (data not shown), supporting the greater susceptibility of CD8+ T cells to depletion by mATG. Proliferative responses of both CD4+ and CD8+ T cells later in the cultures were detectable at doses of mATG as low as 5 μg/mL (data not shown).
Cells expressing markers indicative of regulatory cells are increased following culture of splenocytes with mATG
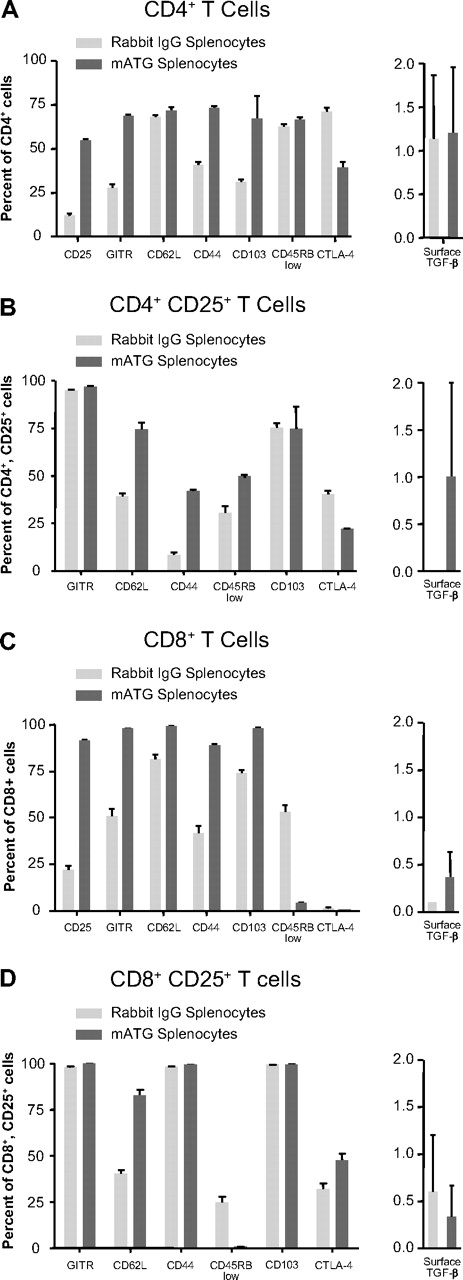
Although no surface marker as of yet has been exclusively or definitively tied to all types of regulatory cells, many cell-surface molecules are observed to a greater degree on regulatory T cells. Increased expression of CD25, CD62L, CD44, CD103, and CTLA-4 as well as low expression of CD45RB (CD45RBlo) have all been described as indicative of certain populations of regulatory cells.32-38 In addition to surface molecules, surface-bound TGF-β can be expressed by regulatory T cells and mediates suppressive effects.39 To examine the phenotype of the proliferating T cells following 5 days of culture with mATG or rabbit IgG, we performed flow cytometric analysis on CD4+ and CD8+ T cells for expression of CD25, CD103, CTLA-4, CD45RB, CD44, CD62L, GITR, and surface TGF-β. We found that 5 days of culture with mATG in the presence of IL-2 resulted in significant increases in CD25 expression on both CD4+ and CD8+ T cells (Figure 3). The percentages of cells expressing GITR, CD62L, CD44, and CD103 were also elevated on total CD4+ and CD8+ T cells and particularly within the CD4+CD25+ population compared with rabbit IgG–treated splenocytes (Figure 3). CD45RBlo cells were increased in the CD4+ and CD4+CD25+ T-cell populations, but were reduced in CD8+ T-cell populations. Percentages of CD4+ or CD4+CD25+ cells expressing CTLA-4 were also reduced by mATG compared with rabbit IgG treatment. Surface TGF-β expression was slightly, but reproducibly, elevated on the CD4+CD25+ T cells. Experiments exploring the time course of CD25 expression revealed that the increase in CD4+CD25+ and CD8+CD25+ T cells was evident as early as 1 day following mATG treatment and remained elevated throughout the cultures (data not shown). Although most regulatory cell markers are increased on T cells from mATG-stimulated splenocytes, CTLA-4 expression was decreased in these cultures, indicating that mATG does not induce a generalized up-regulation of all activation/regulatory markers. In dose-response experiments we continued to see increased percentages of CD4+CD25+ T cells and mATG-induced changes of other markers at concentrations of mATG as low as 1 μg/mL (data not shown). This dose is lower than that required to induce detectable depletion or proliferation of CD4+ and CD8+ T cells in vitro and, thus, indicates a greater sensitivity of regulatory cell marker up-regulation by mATG.
Increases in cell-surface markers indicative of regulatory cells are seen following culture of normal splenocytes with mATG. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 for 5 days. Surface expression of CD25, GITR, CD62L, CD44, CD103, CD45RB, CTLA-4, and TGF-β was evaluated on total CD4+ (A) and CD8+ (C) T cells and all but CD25 expression was evaluated on CD4+CD25+ (B) and CD8+CD25+ (D) T cells as described in “Methods.” Data are means (± SD) of 3 replicates and from a representative of at least 6 separate experiments.
Increases in cell-surface markers indicative of regulatory cells are seen following culture of normal splenocytes with mATG. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 for 5 days. Surface expression of CD25, GITR, CD62L, CD44, CD103, CD45RB, CTLA-4, and TGF-β was evaluated on total CD4+ (A) and CD8+ (C) T cells and all but CD25 expression was evaluated on CD4+CD25+ (B) and CD8+CD25+ (D) T cells as described in “Methods.” Data are means (± SD) of 3 replicates and from a representative of at least 6 separate experiments.
Mouse strain independence of mATG effects
Because different strains of mice show differences in their immune responses, we determined whether the induction of regulatory T-cell markers was observed regardless of the mouse strain used. We cultured splenocytes from C57BL/6, BALB/c, DBA/2, and C3H mice with mATG or rabbit IgG in the presence of IL-2 for 5 days and evaluated the induction of regulatory T-cell surface markers. We found that splenocytes from the 4 different strains of mice tested showed similar up-regulation of CD25 expression on CD4+ (fold increase in percentage over rabbit IgG treatment: C57BL/6 = 3.5, BALB/c = 2.6, DBA/2 = 1.9, C3H = 3.3) or CD8+ T cells (fold increase in percentage over rabbit IgG treatment: C57BL/6 = 3.2, BALB/c = 1.8, DBA/2 = 2.6, C3H = 3.3). Although there are some differences in the levels of regulatory marker induction between strains, there is elevation in all strains and suggests that the ability of mATG to induce regulatory markers is mouse strain independent.
IL-10 and TGF-β levels in culture supernatants from mATG-treated splenocytes
Because regulatory T cells can both be induced by and secrete the immunosuppressive cytokines IL-10 and TGF-β, we examined the culture supernatants of mATG-treated splenocytes for the presence of these cytokines. We were unable to detect TGF-β1 in the supernatants of mATG-treated splenocytes after 7 days of culture (data not shown) but did observe modest and dose-dependent induction of IL-10 in the supernatants of mATG-treated splenocytes (Table 1). We examined multiple time points over the course of the cultures and found that IL-10 levels progressively increased over time with peak levels found at day 6 of culture (data not shown). As shown in Figure 3, we also observed increases in surface-bound TGF-β on the CD4+CD25+ T cells treated with mATG. Given that surface-bound TGF-β can be biologically active in the absence of detectable soluble TGF-β,39 it remains possible that these mATG-treated cells can mediate suppressive effects via surface-bound TGF-β. We are currently investigating the potential role of both IL-10 and TGF-β in mATG-induced regulatory cells. The appearance of surface-bound TGF-β and IL-10 in the supernatants of mATG-treated splenocytes is consistent with the induction or function of regulatory T cells following treatment with mATG.
IL-10 production by mATG-treated cultures
| Treatment . | IL-10 levels, pg/mL . |
|---|---|
| 200 μg/mL rabbit IgG | 74 |
| 5 μg/mL mATG | 115 |
| 10 μg/mL mATG | 116 |
| 20 μg/mL mATG | 123 |
| 50 μg/mL mATG | 237 |
| 100 μg/mL mATG | 334 |
| 200 μg/mL mATG | 451 |
| Treatment . | IL-10 levels, pg/mL . |
|---|---|
| 200 μg/mL rabbit IgG | 74 |
| 5 μg/mL mATG | 115 |
| 10 μg/mL mATG | 116 |
| 20 μg/mL mATG | 123 |
| 50 μg/mL mATG | 237 |
| 100 μg/mL mATG | 334 |
| 200 μg/mL mATG | 451 |
Normal mouse splenocytes were cultured for 6 days with various amounts of mATG or rabbit IgG and 200 U/mL IL-2 and supernatants were assessed for IL-10 levels as described in “Methods.”
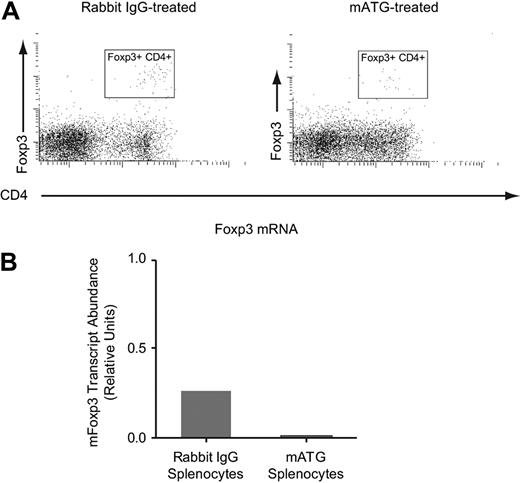
In vitro mATG-stimulated splenocytes do not express Foxp3
It is now well established that several of the regulatory T cells described in the literature express the forkhead box protein transcription factor, Foxp3. This transcription factor has been demonstrated to induce the transcription of many of the surface markers expressed by regulatory T cells and is expressed primarily by regulatory T cells in mice.20 Therefore, we tested whether mATG treatment of murine splenocytes results in up-regulation of this factor in vitro. Using both flow cytometric and quantitative PCR analyses, we were unable to find increased expression of Foxp3 protein or mRNA levels following mATG treatment (Figure 4) compared with rabbit IgG–treated splenocytes, regardless of the time point examined or the dose of mATG used (data not shown). It has been demonstrated that mATG will induce Foxp3-expressing cells in vivo in a model of experimental autoimmune encephalomyelitis,40 and thus, it remains possible that additional factors are present in vivo that allow for mATG induction of Foxp3-positive regulatory cells. However, under the in vitro conditions tested here, it appears that mATG does not induce Foxp3-positive regulatory cells.
mATG does not induce Foxp3 expression in vitro. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2, and 5 days following initiation of culture cells were evaluated for Foxp3 expression by flow cytometric analysis (A) or quantitative RT-PCR (B) as described in “Methods.” Foxp3 mRNA levels were normalized to CD3 mRNA in each sample. The flow cytometric data are representative of at least 6 experiments and the mRNA analysis was performed once.
mATG does not induce Foxp3 expression in vitro. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2, and 5 days following initiation of culture cells were evaluated for Foxp3 expression by flow cytometric analysis (A) or quantitative RT-PCR (B) as described in “Methods.” Foxp3 mRNA levels were normalized to CD3 mRNA in each sample. The flow cytometric data are representative of at least 6 experiments and the mRNA analysis was performed once.
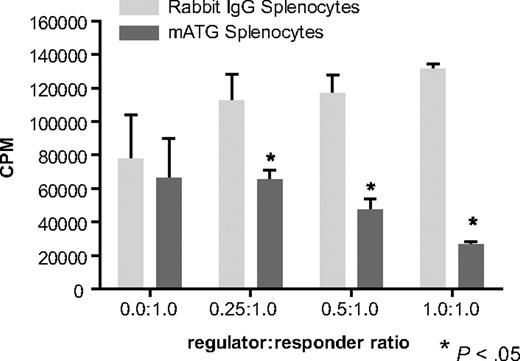
Splenocytes treated with mATG suppress immune responses of normal splenocytes in vitro
Given the up-regulation of regulatory markers on cells cultured with mATG, even in the absence of Foxp3 expression, we examined whether these cells were functionally suppressive. To test this, various concentrations of splenocytes that had been treated with either mATG or rabbit IgG were added to cultures of normal splenocytes stimulated with anti-CD3 plus anti-CD28. We found that addition of increasing concentrations of the mATG-treated splenocytes, but not rabbit IgG–treated splenocytes, resulted in increased inhibition of the proliferation of the normal cells to anti-CD3 plus anti-CD28 (Figure 5). Dose-response studies indicated that suppressive effects could be mediated by cells treated with as little as 5 μg/mL mATG (data not shown). We also find that suppression by the mATG-treated cells increases with increasing length of culture, where peak suppressive activity occurs with cells from day-6 cultures (data not shown), although time points after day 6 have not yet been examined. These data indicate that normal mouse splenocytes cultured with mATG are functionally suppressive.
mATG-treated cells are functionally suppressive in vitro. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2, and 5 days following initiation of culture cells were evaluated for their ability to suppress the proliferative responses of normal splenocytes. Variable numbers of mATG- or rabbit IgG–treated mouse splenocytes were added to normal mouse splenocytes stimulated with anti-CD3/anti-CD28 beads as described in “Suppression assays.” Data are means (± SD) of 3 replicates from a representative of 5 separate experiments.
mATG-treated cells are functionally suppressive in vitro. C57BL/6 mouse splenocytes were cultured with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2, and 5 days following initiation of culture cells were evaluated for their ability to suppress the proliferative responses of normal splenocytes. Variable numbers of mATG- or rabbit IgG–treated mouse splenocytes were added to normal mouse splenocytes stimulated with anti-CD3/anti-CD28 beads as described in “Suppression assays.” Data are means (± SD) of 3 replicates from a representative of 5 separate experiments.
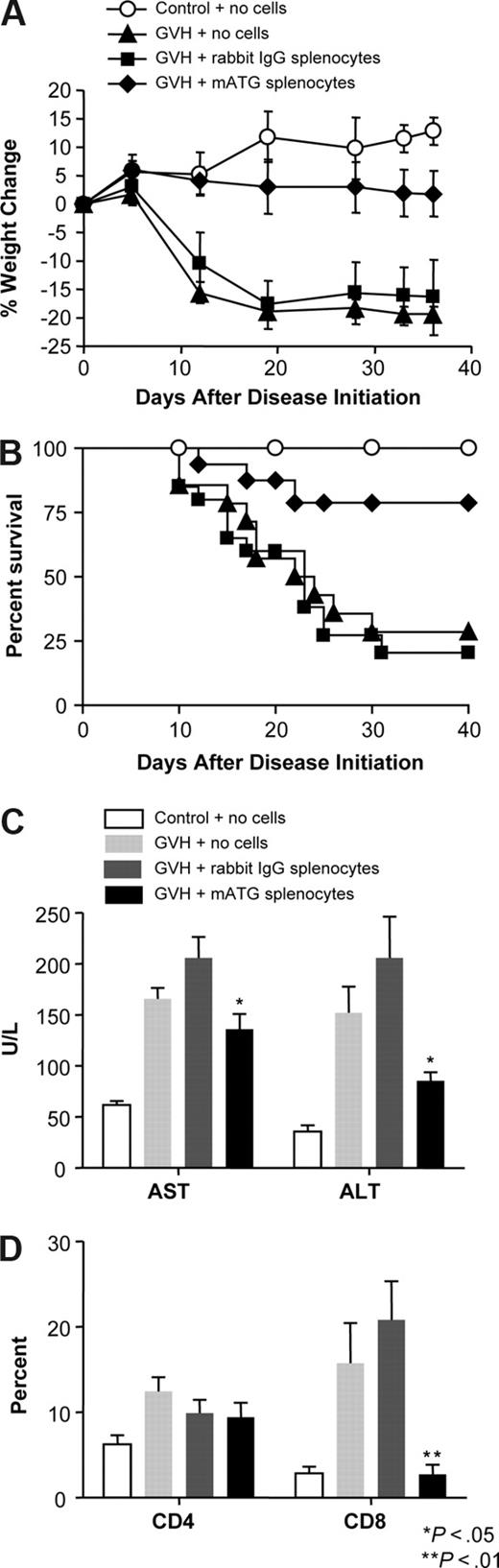
Adoptive transfer of in vitro mATG-treated mouse spleen cells protects against acute GVHD
Because the mATG-treated cells could suppress immune responses in vitro, we were interested in whether these mATG-treated cells could suppress a potent immune response in vivo under disease conditions. We have found that transfer of C57BL/6 splenocytes into BALB/c RAG-2 KO mice (deficient in T and B cells) induces a robust and lethal GVHD (Figure 6) similar to reports that transfer of allogeneic cells into severe combined immunodeficient (SCID) mice results in acute GVHD.41-43 We tested whether the in vitro mATG-treated splenocytes could suppress GVHD by adoptively transferring C57BL/6 splenocytes that had been cultured in the presence of either mATG or rabbit IgG for 5 days into the recipient BALB/c RAG-2KO mice. The following day, mice in all GVHD groups were challenged with normal allogeneic C57BL/6 cells. The mice given mATG-treated splenocytes prior to challenge did show some evidence of disease based on weight loss and mortality, but these parameters were significantly ameliorated compared with mice receiving rabbit IgG–treated cells. Because the liver is one of the target organs in acute GVHD, we examined circulating liver enzyme (AST and ALT) levels and also found significant reductions in the serum liver enzyme levels in mice given mATG-treated splenocytes compared with rabbit IgG–treated splenocytes. This provides additional evidence that the transfer of mATG-treated cells reduces the liver damage observed in acute GVHD.
Adoptive transfer of in vitro mATG-stimulated cells inhibits GVHD and expansion of allogeneic CD8+ T cells. Normal C57BL/6 splenocytes treated with either mATG or rabbit IgG and IL-2 for 5 days were adoptively transferred into recipient RAG-2 KO mice at 2.5 to 5 × 107 cells/mouse 1 day prior to induction of acute GVHD by injection of fresh, normal allogeneic C57BL/6 splenocytes as described in the “Acute graft-versus-host disease induction.” (A) Percentage weight change and (B) percentage survival of the animals at various times following induction disease. (C) Serum ALT and AST levels at 3 weeks of disease. (D) Blood CD4+ and CD8+ T-cell percentages 1 week following disease induction. Data for weight change, serum AST and ALT levels, and blood T-cell populations are expressed as means (± SD). Survival data are pooled from 3 experiments with a total of 20 mice. Data for weight change and serum liver enzymes (AST and ALT) are representative of 3 experiments containing at least 6 mice per group. Statistically significant differences (P < .05) between transfer of mATG-treated splenocytes and control splenocytes was observed in 2 of 3 experiments for weight change and in all 3 experiments for serum liver enzymes. T-cell populations were evaluated in 2 of the 3 experiments with both showing statistically significant differences (P < .01) between groups receiving mATG-treated splenocytes compared with control splenocytes.
Adoptive transfer of in vitro mATG-stimulated cells inhibits GVHD and expansion of allogeneic CD8+ T cells. Normal C57BL/6 splenocytes treated with either mATG or rabbit IgG and IL-2 for 5 days were adoptively transferred into recipient RAG-2 KO mice at 2.5 to 5 × 107 cells/mouse 1 day prior to induction of acute GVHD by injection of fresh, normal allogeneic C57BL/6 splenocytes as described in the “Acute graft-versus-host disease induction.” (A) Percentage weight change and (B) percentage survival of the animals at various times following induction disease. (C) Serum ALT and AST levels at 3 weeks of disease. (D) Blood CD4+ and CD8+ T-cell percentages 1 week following disease induction. Data for weight change, serum AST and ALT levels, and blood T-cell populations are expressed as means (± SD). Survival data are pooled from 3 experiments with a total of 20 mice. Data for weight change and serum liver enzymes (AST and ALT) are representative of 3 experiments containing at least 6 mice per group. Statistically significant differences (P < .05) between transfer of mATG-treated splenocytes and control splenocytes was observed in 2 of 3 experiments for weight change and in all 3 experiments for serum liver enzymes. T-cell populations were evaluated in 2 of the 3 experiments with both showing statistically significant differences (P < .01) between groups receiving mATG-treated splenocytes compared with control splenocytes.
In an effort to understand how mATG-treated splenocytes might be inhibiting the graft-versus-host reaction, we evaluated blood T-cell populations during the course of disease. At 1 week following disease induction, there was a dramatic decrease in CD8+ T-cell percentages in mice given mATG-treated splenocytes compared with mice given rabbit IgG splenocytes (Figure 6D) that remained low throughout the disease course (data not shown). Because cytotoxic CD8+ T cells are important for the direct tissue damage and acute manifestations of GVHD under conditions of disparities in both MHC class I and II loci,44,45 the depletion of or suppression of the expansion and/or activity of CD8+ T cells is beneficial in reducing disease symptoms.44-46 These data indicate that the transfer of in vitro mATG-treated cells results in inhibition of allogeneic CD8+ T-cell expansion. There were no detectable effects of the mATG-treated splenocytes on CD4+ T-cell percentages in the blood at any time point, however, it remains possible that depressed functional activity of CD4 T cells is responsible for limiting the CD8+ T-cell expansion and decreased disease manifestations. Because animals given mATG-treated splenocytes exhibit some clinical symptoms of GVHD, it is possible that chronic GVHD has developed. Whether this is due to a suboptimal dose or intrinsic properties of the suppressive cell type within the mATG-treated splenocytes is currently under investigation in our laboratory. In conclusion, it appears that in vitro mATG treatment of normal mouse splenocytes generates a regulatory cell population that upon adoptive transfer into mice is able to suppress acute GVHD ultimately by suppressing the expansion of allogeneic CD8+ T cells.
Discussion
We have characterized the in vitro treatment of normal murine splenocytes with rabbit mATG and found an initial depletion of T cells (day 1), followed by later expansion of T cells (days 4-7) when cultured in the presence of IL-2. The resulting T cells express many of the cell-surface markers indicative of regulatory T cells, although they do not express the transcription factor, Foxp3. Nevertheless, splenocytes treated with mATG inhibit normal stimulated responder T cells in vitro and, upon adoptive transfer, protect against acute GVHD by reducing CD8+ T-cell expansion in vivo. These results demonstrate that a murine version of Thymoglobulin can induce regulatory function in murine splenocyte cultures that are active not only in vitro but also in vivo under robust immune-mediated disease conditions.
Antithymocyte and antilymphocyte preparations as well as their murine counterparts consist of antibodies against multiple surface antigens expressed on T cells and other cell types.4-7 Antibody reactivities include T cell–specific antigens, such as CD4, CD8, and CD3, adhesion molecules, chemokine receptors, MHC molecules, and costimulatory molecules on both T- and non–T-cell populations.4,6,7 This mixture of antibodies to diverse surface molecules on immune cells delivers a potent immunomodulatory effect in vivo as antithymocyte and antilymphocyte globulins are used frequently as part of conditioning regimens for transplantation and acute rejection episodes.1 Because these reagents readily deplete T cells in vivo, it has been thought that this is the primary mechanism of their immune regulatory effects.14 However, these studies and those of others have shown that antithymocyte or antilymphocyte reagents in both murine and human systems also induce regulatory cells.10,16,47 Lopez et al showed that under nondepleting conditions in vitro, a rabbit antihuman thymocyte reagent (Thymoglobulin) induced human naive CD4+ T cells to become CD25+Foxp3+ and functionally suppressive in a mixed lymphocyte reaction.16 Murine studies have also been conducted with antilymphocyte serum (ALS), generated by immunizing rabbits with murine lymph node cells, showing that adoptive transfer of splenocytes or CD4+CD25+ T cells from ALS-treated nonobese diabetic (NOD) mice prevents diabetes in naive recipient NOD mice.10 Other murine studies have suggested that under conditions of total lymphoid irradiation mATG induces a regulatory CD1-restricted NKT-cell population that inhibits GVHD.47 Given that ligation or blockade of a variety of surface molecules has been shown to affect the generation or activity of regulatory cells,48-55 it is likely that the polyclonal nature of antithymocyte/antilymphocyte globulins not only results in depletion of T cells, but also may engage or block a variety of surface molecule with the end result being the generation of regulatory cells. Although it remains possible that the depletive effects of ATG selectively spare regulatory cells, preliminary studies in our laboratory have demonstrated no evidence of selective sparing by mATG either via complement lysis or apoptotic mechanisms (M.C.R. and Kathleen Neff, unpublished data, May 2007). Together with the data presented here, these studies all suggest that antithymocyte/lymphocyte polyclonal antibodies, in addition to their T cell–depletive effects, can also induce regulatory cell populations that suppress immune responses both in vitro and in vivo.
The present studies clearly demonstrate that mATG induces a functional regulatory cell population, however, the specific type of regulatory cell induced remains to be determined. The lack of Foxp3 expression in the mATG-treated cultures suggests that the regulatory cell type induced is neither of the natural CD4+CD25+ population nor of certain acquired regulatory cells that have been described, such as Th3 regulatory and some CD8+ regulatory T cells.56,57 Although Thymoglobulin has been shown in cultures of human cells to induce Foxp3+CD25+CD4+ regulatory T cells,16 Foxp3 in humans can also be expressed in activated T cells as well as regulatory cells. Because the Foxp3-positive and Foxp3-negative cells were not independently assessed for suppressive activity in these experiments, it remains possible that the suppressive cell type in those studies was actually a Foxp3-negative cell population.
Described regulatory cell populations that do not express Foxp3 include Tr1 cells,22,58 anergic T cells, and NKT cells, any of which could be candidate regulatory cell types induced by mATG in vitro. Tr1 cells appear to be a distinct lineage that are derived from naive T cells in an antigen- and IL-10–dependent manner, with subsequent secretion of IL-10, but not IL-4, as a hallmark.22 Anergic or partially activated cells have also been demonstrated to exhibit regulatory activity and produce IL-10.17,21,59-61 A third possibility includes NKT cells, because under certain conditions these cells have also been found to be important regulators of immune responses.17,62 In addition, other even less well-defined Foxp3-negative regulatory cell populations have recently been described.63,64 Preliminary data from our laboratory actually suggests that multiple regulatory populations may be induced by ATG (M.C.R., J.D., J.M.W., S.R.N., and S.S., unpublished data, January 2006). Nevertheless, given the significant suppressive effects of the mixed population of splenocytes following mATG treatment, the identification and purification of the suppressive populations should yield greater suppressive potency and provide additional understanding of mATG effects.
The role of regulatory cells in suppression of acute GVHD has been well established by adoptive transfer studies.27,65-71 The regulatory cells transferred in these systems were CD4+CD25+ T cells that were either freshly purified or ex vivo–expanded with T-cell mitogens and IL-2.27,67,70 The mechanism by which these transferred regulatory cells mediate their suppressive effects appears to be through inhibition of expansion, activation marker expression, and IFN-γ production by the naive allogeneic cells at early time points.72 Additional studies have demonstrated that depletion of regulatory cells under conditions of chronic GVHD converts the disease to the acute form, coincident with expansion of CD8+ T cells, suggesting that regulatory cells preferentially suppress CD8+ T-cell responses.46 Our results are consistent with these data because transfer of mATG-induced regulatory cells inhibits acute GVHD and is associated with suppressed CD8+ T-cell expansion. Thus, regulatory cell induction and/or transfer might be particularly useful in disease settings mediated or contributed to by CD8+ T cells.
Based on the encouraging murine studies with regulatory T-cell adoptive transfer in GVHD, it has been suggested that ex vivo–expanded regulatory T cells may be feasible for a clinical setting,19,26,73-75 and clinical trials have been proposed to test this approach.18,22 Our studies demonstrate that mATG not only can induce regulatory cells from a whole splenocyte population in vitro but also can stimulate a robust proliferative response and is functionally suppressive as a mixed population. It is likely that increased potency could be obtained upon additional expansion, identification of the suppressive cell population, and/or optimization of the cultures, which are the subjects of current investigations in our laboratory. Thus, in addition to other described methods, in vitro treatment of lymphocytes with Thymoglobulin may be yet another means to generate cells with regulatory function that could be used for therapeutic applications.
It remains possible that antithymocyte globulins mediate their clinical effect exclusively by depleting alloreactive T cells. However, the data provided here demonstrate that murine antithymocyte globulin can also induce regulatory cells in vitro that are functionally immunosuppressive in an in vivo setting. These results also suggest that antithymocyte antibodies, such as Thymoglobulin, could be used to induce and expand potent regulatory T cells for clinical applications.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Russell Bigelow in Process Research for purification of the mATG; Erin Morehouse, Mike Twarog, and the Department of Comparative Medicine for technical assistance; and John McPherson for critical review of the paper.
This work was supported by Genzyme.
Authorship
Contribution: M.C.R. provided intellectual input, designed research, analyzed data, and prepared the paper; J.S.W. designed and performed research, analyzed data, and contributed to paper preparation; D.H. designed and performed research and analyzed data; G.L. designed and performed in vitro suppression assays and analyzed data; J.S. designed and performed Foxp3 mRNA analyses and analyzed data; B.L.R., S.M.R., S.R.N., J.M.W., A.S., J.D., S.S., and R.D.G. designed research and provided intellectual input.
Conflict-of-interest disclosure: All authors are or have been employed by Genzyme, whose commercial product is Thymoglobulin.
Correspondence: Melanie C. Ruzek, Genzyme, 1 Mountain Rd, Framingham MA 01701; e-mail: melanie.ruzek@genzyme.com.

![Figure 2. T cells are depleted early but also proliferate late in the cultures with mATG. Cultures of C57BL/6 splenocytes with 100 μg/mL mATG or rabbit IgG in the presence of 200 U/mL IL-2 were evaluated for absolute numbers of CD4+, CD8+, CD19+ (B cells), CD3+, NK1.1+ (NKT cells), or CD3−NK1.1+ (classic NK cells) or BrdU incorporation into specific cell populations by flow cytometric analysis described in “Methods.” (A) Cell populations at day 1. (B) Cell populations at day 5. (C) BrdU was added to the cultures on day 4 and evaluated the following day for BrdU incorporation into the CD4+, CD8+ and CD19+ cell populations as described in “Methods.” Data shown are means (± standard deviation [SD]) of 3 replicates from a representative of at least 6 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/3/10.1182_blood-2007-08-106526/6/m_zh80030814280002.jpeg?Expires=1764959908&Signature=Hl7bM1hlXG0N9AiAzX6XoKM5O378AkHR5osjfqzLivPLqTXD5Gko2036Crh4BQfV4tzURDgizYQdiAed3J1l-DO-K5yl2f3Ruihu5U4LwerZW0nJZVquVw0jXbfXd-cUIk9vFogo6g7k3tL5rZXF2E7tcOEo~AhUEvwDg~UgyWLFTJ3GDQCXTxmw6UQwhKmIIGRUuqvNGB368uZqh0S2vQIkErMHTz9iVigz8HMwom1Ao4nFf5hwNDFJfzvpvbwnP5-Bzx-j7iSAwf~0VJJ5VtrC4JxCv1yOp0rmbd7VGfkQRWwVKkLFUid-yvvTQ21alKXx57fQ6xzU5qjZf~fQmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal