In this issue of Blood, Breunis and colleagues examine the role of genetic variation in the human Fc receptors for IgG in the susceptibility to idiopathic thrombocytopenic purpura (ITP).
The Fcγ receptors contribute to the susceptibility to autoimmune diseases such as ITP in several ways: (1) they modulate dendritic cell antigen processing and presentation, (2) they modulate antibody production by B cells, and (3) they participate in effector cell functions, such as phagocytosis, antibody-dependent cellular cytotoxicity, and mediator release. The key finding in recent years has been the appreciation that the activating Fcγ receptors, encoded by FCGR2A, -2C, -3A, and -3B, compete with the inhibitory Fcγ receptor, encoded by FCGR2B, to determine cellular responses to immune complexes and antibody-coated cells.
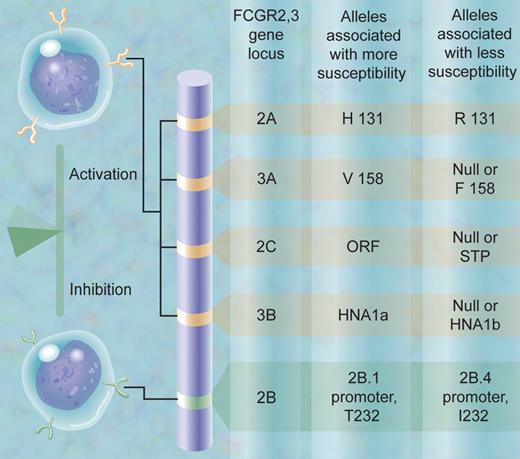
The work by this team, led by Dr Taco Kuijpers, is noteworthy for several reasons. First, the authors have achieved a technical tour-de-force, developing multiplex ligation-dependent polymerase amplification assays (MLPAs) to simultaneously identify both single nucleotide polymorphisms (SNPs) and copy number variations (CNVs) in the 5 gene locus containing the so-called low-affinity Fc receptors for IgG: FCGR2A, -2B, -2C, -3A, and -3B. These genes are highly homologous, and genetic studies of this region are notoriously difficult. The authors typed 8 biologically significant variations at the same time. Second, they discovered a new CNV for the FCGR3A gene. Third, their assays combined the ability to identify an important FCGR2C SNP (ORF, open reading frame, versus STP, stop codon) with the ability to detect deletion or duplication of a block containing the adjacent FCGR2C and FCGR3B genes. Finally, in a study comparing the frequency of these FCGR2,3 gene variations in healthy individuals versus in children and adults with ITP, analyzed separately and as a group, they discovered a significant overrepresentation of the FCGR2C ORF allele in individuals with ITP. The team convincingly confirmed the work by Ernst, Herberman, and colleagues1 that the FCGR2C genotype determines the phenotype, with respect to protein expression and function. Since FCGR2C is an activating receptor in monocytes/macrophages and natural killer (NK) cells, the findings are consistent with the current paradigm in the field that the balance of activating versus inhibitory Fcγ receptors contributes to susceptibility to autoimmune disease (see figure).
The SNP and CNV alleles in the human FCGR2,3 gene locus at chromosome 1q23 that contribute to susceptibility to autoimmune disease are shown schematically, including the genetic variation in FCGR2C in the study on ITP by Breunis et al. (The alleles are defined in that article.) The balance of activation and inhibition contributes to greater or lesser susceptibility to autoimmune disease.
The SNP and CNV alleles in the human FCGR2,3 gene locus at chromosome 1q23 that contribute to susceptibility to autoimmune disease are shown schematically, including the genetic variation in FCGR2C in the study on ITP by Breunis et al. (The alleles are defined in that article.) The balance of activation and inhibition contributes to greater or lesser susceptibility to autoimmune disease.
Where does the field go from here? These 8 genetic variations are not in linkage disequilibrium, but individuals will be endowed in this region of chromosome 1q23 with a haplotype of FCGR2,3 SNPs and CNV that can influence the susceptibility to ITP and other auto- or allo-immune disorders. In the figure, the hypothesized set of alleles conferring greater susceptibility is contrasted to the set conferring lesser susceptibility. Further technical advances and larger numbers of patients and controls, especially those of diverse ethnic groups, will be needed to test the potential clinical value of assaying genetic differences in susceptibility. Equally exciting, however, is the prospect that these genetic variations contribute to differences in the response to therapeutics for ITP. Modulation of Fcγ receptor activating and inhibitory functions are mainstays of ITP treatment.2-4 We may even go so far as to link responses to a broader set of antibody therapeutics for human disease to the haplotype of FCGR2,3 genetic variations.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal