Division and proliferation of dendritic cells (DCs) have been proposed to contribute to homeostasis and to prolonged antigen presentation. Whether abnormal proliferation of dendritic cells causes Langerhans cell histiocytosis (LCH) is a highly debated topic. Transgenic expression of simian virus 40 (SV40) T antigens in mature DCs allowed their transformation in vivo while maintaining their phenotype, function, and maturation capacity. The transformed cells were differentiated splenic CD8 alpha–positive conventional dendritic cells with increased Langerin expression. Their selective transformation was correlated with higher steady-state cycling compared with CD8 alpha–negative DCs in wild-type and transgenic mice. Mice developed a DC disease involving the spleen, liver, bone marrow, thymus, and mesenteric lymph node. Surprisingly, lesions displayed key immunohistologic features of Langerhans cell histiocytosis, including expression of Langerin and absence of the abnormal mitoses observed in Langerhans cell sarcomas. Our results demonstrate that a transgenic mouse model with striking similarities to aggressive forms of multisystem histiocytosis, such as the Letterer-Siwe syndrome, can be obtained by transformation of conventional DCs. These findings suggest that conventional DCs may cause some human multisystem LCH. They can reveal shared molecular pathways for human histiocytosis between humans and mice.
Introduction
Dendritic cells (DCs) are the antigen-presenting cells (APCs) capable of inducing adaptive immune responses and tolerance.1 DCs act as sentinels in peripheral tissues and as APCs in secondary lymphoid organs, filtering their environment and detecting environmental changes that modulate the balance between tolerance and immune response. Following infection and inflammation, DCs induce costimulatory molecules, secrete cytokines, and can change their migration properties.2,3 Several DC subtypes with unique and overlapping functions have been described. Plasmacytoid DCs (pDCs) are the main interferon (IFN) type I–producing cells, whereas several subtypes of conventional DCs (cDCs) are widely distributed.4,5 In skin, 2 DC populations, namely Langerhans cells (LCs) and dermal DCs, localize to the epidermis and dermis, respectively. Upon pathogen encounter, they migrate to the draining LN.6 In skin and spleen, DCs are derived either from blood or from immediate local precursors.7,,,,,–13 DCs are dividing cells rather than terminally differentiated cells.12,,–15 The importance of DC cycling on their homeostasis is currently debated, and DC proli-feration has been recently proposed to extend the duration of antigen presentation.15
The human histiocytic diseases are characterized by abnormal accumulation or proliferation of macrophages or DCs.16 They are divided into LC or non-LC histiocytosis. In the former category, LC histiocytosis (LCH) is best described. LCH can range from a spontaneously regressing single organ disease to a life-threatening or disabling relapsing multisystem disease.16 In the latter category, a genetic deficiency in cytotoxic activity, familial hemophagocytic lymphohistiocytosis (FHL), is the principal type implicating macrophages and lymphocytes. In contrast to LCH and FHL, lesions with proliferative DCs with or without cytologic atypia are less well classified and their prevalence remains controversial.16 Unfortunately, a confusing terminology describing these cases of proliferating DCs persists in the literature as malignant histiocytosis, histiocytic sarcoma, and DC sarcoma.
The diagnosis of histiocytic diseases relies on the morphologic, ultrastructural, and immunohistochemical histiocyte properties. The macrophage, DC, or LC lineage markers allow LC to be distinguished from non-LC histiocytosis. For example, diagnostic criteria for LCH include expression of Langerin or detection of Birbeck granules that are pathognomonic, as well as CD1a and S100, which are less specific. According to the International Lymphoma Study Group, histiocytic sarcoma, LC tumor, and LC sarcoma (LCS) are the terms used to separate cases based on the degree of cytologic atypia and on lineage markers.17 For example, diagnosis of LCS requires the same markers as LCH and the additional presence of cellular and mitotic atypia.
While LCS, LC, and DC tumors are clearly neoplastic, it is vigorously debated whether LCH is a reactive or a neoplastic disease. The first hypothesis focuses on inflammatory factors acting on LCs,18,–20 while the second highlights the importance of intrinsic LC defects, for example their self-renewal capacity.20,,,,–25 The importance of neoplastic transformation in histiocytosis is difficult to determine in the absence of animal models. In this study, we generated transgenic (Tg) mice expressing simian virus 40 (SV40)–derived oncogenes specifically in DCs. In adult mice, transformation, characterized by a dramatic increase in cell proliferation without overt cellular atypia, occurred in non-Langerhans cDCs. Remarkably, these mice developed a disease sharing many features of multisystem LCH. Strikingly, the Langerin-expressing histiocytes, which were also detected in normal human spleen, were CD8α+ cDCs rather than skin-derived LCs. These results open the possibility that a subset of multisystem LCH is caused by cDCs and would make it plausible that genomic screening of the animal model could define target genes of human histiocytosis.
Methods
Generation of Mushi Tg mice
The bicistronic Tg expression cassette coding for T antigens and eGFP was derived from SV40 viral DNA (provided by P. Salmon, Geneva, Switzerland) and MIGR1 vector (provided by S. Marguerat, Geneva, Switzerland), respectively. The gene coding for T antigens was modified by inserting an initiation codon with perfect Kozak consensus and by removing all 5′ and most 3′ untranslated regions (5163-2668 coordinates corresponding to the sequence V01380). The mouse CD11c promoter (−5654 to −2 upstream positions of initiation codon) and a genomic fragment containing a small intron and the poly A addition signal from rabbit β1 globin gene (902-1541 positions of sequence K03256) were derived from a DC-specific Tg construct26 (provided by K. Karjalainen, Basel, Switzerland). The rabbit β globin fragment was modified by inserting FRT and loxP sites followed by the bicistronic Tg expression cassette. A second loxP site was inserted after the polyA addition signal. After removal of vector sequences, the insert named CD11c-GT2 was injected into fertilized (C57BL/6 × DBA2) F2 oocytes.
Mice
DNA from Mushi (multisystem histiocytosis) mice were screened by polymerase chain reaction (PCR) techniques using a CD11c primer (5′-GGCAGCTGTCTCCAAGTTGCTCAG-3′) and a rabbit β1 globin primer (5′-GGGTCCATGGTGATACAAGGG-3′). Experiments were performed with Tg mice backcrossed into the C57BL/6 background (Harlan, Loughborough, United Kingdom) for more than 6 generations. All Tg mice (Mushi, OT-I TCR,27 OT-II TCR28 ) were bred in the C57BL/6 background at the ISREC animal facility under standard SPF conditions. C57BL/6 mice were purchased from Harlan, UK. OT-I RAG−/− mice29 were from The Jackson Laboratory (Bar Harbor, ME). Experiments were approved and controlled by the Swiss federal and cantonal veterinary authorities.
RNA preparation and cDNA synthesis
Total RNA was purified using Trizol (Invitrogen, Basel, Switzerland). PolyA+ RNA was purified from DNAse I (Roche, Rotkreuz, Switzerland)–treated total RNA using Dynabeads mRNA purification kit (Invitrogen). Total or polyA+ RNA was reverse transcribed using random nonamers and Superscript II (Invitrogen).
Reverse-transcription–PCR for Mushi transcripts
cDNA synthesized from polyA+ RNA or control genomic DNA was subjected to PCR using a CD11c primer F 5′-GGCAGCTGTCTCCAAGTTGCTCAG-3′ and a SV40 primer R 5′-GGAACTTCTTTGCCAAAATGATG-3′.
Quantitative reverse transcription–PCR
Quantitative reverse transcription (RT)-PCR was done using SYBR Green and a Light Cycler (Roche) as described.30 Standard curves made of splenocyte cDNA were used to take into account differences in amplification efficiency. Values were normalized to the geometric mean of 4 housekeeping genes TBP, RNA Pol2a, RNA Pol2b, and PBGD. Values of target genes were expressed relative to TBP. Standard curves for each CIITA isoform based on purified amplicons allowed absolute quantification and thus direct comparison among isoforms. Primers for TBP have been described.30 Other primers are as follows: RNA Pol2a F 5′-AGAGAGTGCAGTTCGG-3′; RNA Pol2a R 5′-ACTCGGTCATGTTTCCT-3′; RNA Pol2b F 5′-ACCACCGATTGACCTAC-3′; RNA Pol2b R 5′-CGGAGCAGAATACGTGA-3′; IRF-8 F 5′-CCGAATTGTCCCCGAG-3′; IRF-8 R 5′-GTCAGGGAGGATCTGG-3′; CIITA-I F 5′-CAGGGACCATGGAGACCATAGT-3′; CIITA-I R 5′-CAGGTAGCTGCCCTCTGGAG-3′; CIITA-III F 5′-CTGCATCACTCTGCTCTCTAA-3′; CIITA-III R 5′-GTCATAGAGGTGGTAGAGATGT-3′; CIITA-IV F 5′-CATGCAGGCAGCACTCAGAA-3′; CIITA-IV R 5′-GGGTCGGCATCACTGTTAAGG-3′; PBGD F 5′-GAAGGATGTGCCTACCATACTACCTC-3′; PBGD R 5′-GGTTTTCCCGTTTGCAGATG-3′; IRF-4 F 5′-GCCCAACAAGCTAGAAAG-3′; IRF-4 R 5′-TCTCTGAGGGTCTGGAAACT-3′; Langerin F 5′-ACACTGGATTGGACTTACCAAA-3′; Langerin R 5′-ACACCCTGATATTGGCACA-3′; DC-Sign F 5′-AGTCTACATGGTACTGGGT-3′; DC-Sign R 5′-GATCCAGAATTTCTTGTTAGTACATTTG-3′; CD68 F 5′-GACTACATGGCGGTGGAATAC-3′; CD68 R 5′-AGATGAATTCTGCGCCATGA-3′; Mushi Tg F 5′-CATTTGCTTCTTCTGTGGT-3′; Mushi Tg R 5′-CTTTCTAGAGAATAGGAACTTCGGA-3′.
Cell preparation and purification
Single-cell suspensions were prepared by incubating the indicated organs 25 minutes at 25°C to 0.5 mg/mL collagenase D (Roche) + 40 μg/mL DNAse I (Roche) in RPMI 1640 (Invitrogen) supplemented with 50 μg/mL gentamycin (Invitrogen) and 5% FCS (Sigma-Aldrich, Buchs, Switzerland). Cells were washed twice with 50 mL PBS, 1 × 1% FCS, 2 mM EDTA, and 5 μg/mL DNAse I and then once with 50 mL PBS, 1% FCS, and 2 mM EDTA. Finally, cells were resuspended in PBS, 1% FCS, and 2 mM EDTA and filtered on a 40-μm nylon cell strainer.
Bone marrow cells were flushed out of long bones (tibiae and femur) with 5 to 10 mL PBS, 3% FCS, and 5 mM EDTA using a 5-mL syringe with a 26-G needle. Cells were washed once with PBS, 3% FCS, and 5 mM EDTA.
Epidermal single-cell suspensions were obtained by incubating tail skin for 40 minutes at 37°C to 2 mg/mL Dispase II (Roche) followed by removal of dermis, an additional 10 minutes of incubation, and a final mechanical disruption on a 40-μm nylon cell strainer. Cells were washed twice in PBS, 3% FCS, and 5 mM EDTA.
Splenic DCs were purified using CD11c MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). For quantitative RT-PCR, DCs were further purified by fluorescence-activated cell sorting (FACS) selection for lineage cocktail exclusion (B220, CD19, NK1.1, GR1, TCRαβ, TCRγδ), CD11c+, and CD8α− or CD8α+. B cells were purified from spleen using CD19 MicroBeads (Miltenyi Biotec) from spleen. OT-I Tg CD8+ T cells and OT-II Tg CD4+ T cells were purified from peripheral LN and spleen using CD8α and CD4 MicroBeads, respectively (Miltenyi Biotec). Peritoneal macrophages were magnetically purified (Miltenyi Biotec) by depletion of CD11c+, CD19+ cells followed by magnetic enrichment for F4/80 expression.
Flow cytometry
Cells were incubated with monoclonal antibodies (mAbs) against FcR (2.4G2) to block nonspecific Fc-mediated binding and with PE-, Cy-Chr–, PE-Cy5.5–, and Alexa 647–conjugated mAbs. Streptavidin-PE-Cy5.5 or streptavidin-PE-Cy5 was used with biotinylated antibodies. Analysis was achieved using FACSCalibur or FACSCanto (Becton Dickinson, San Jose, CA).
mAbs were against the following antigens (from eBioscience [San Diego, CA], BD Pharmingen [Basel, Switzerland], Biolegend [San Diego, CA], and Dendritics [Lyon, France]): PE-conjugated Abs: CD3 (clone 145-2C11), CD11c (HL3), CD19 (6D5); PE-Cy5–conjugated Abs: B220 (RA3-6B2), CD3 (145-2D11), CD24 (M1/69); Alexa-647–conjugated Abs: CD3 (17A2), CD8α (53-6.7), CD11b (M1/70), MHC-II I-Ab (1D9); bioltinylated Abs: CD11c (HL3), CD19 (1D3), CD86 (GL-1), F4/80, CD40 (3/23), CD80 (B7-1), CD207 (929F3), MHC-II IA/IE (2G9); PE-Cy7–conjugated Abs: CD8α (53-6.7); and PE-APC-Cy7–conjugated Abs: CD4 (L3T4).
To quantify proliferation, cells were permeabilized using Cytofix/Cytoperm Kit (BD Pharmingen) and stained with 20 μg/mL Hoechst 33342 (Invitrogen) or with Ki-67 Kit (BD Pharmingen). Data acquisition was performed on LSR FACS (Becton Dickinson).
Hematocrit measurement
Blood was collected by tail bleeding, mixed with 2500 IU heparinum (Roche), and centrifuged in microhematocrit capillaries (SIGMA Laborzentrifugen, Seelze, Germany).
Histology and immunohistology
Sections of tissue were obtained from mouse liver lesions and from LN of LCH patients. These 3-μm sections were taken from formalin-fixed paraffin-embedded tissue blocks and underwent deparaffinization followed by antigen retrieval using steam heating in a 10-mM citrate buffer at pH 6.0 for 15 minutes. The tissue sections were then immunoreacted with rabbit polyclonal antibodies (CD207 [Langerin] 1:50 [Vision Biosystem, Newcastle, United Kingdom]; S100 protein 1:200 [Dako, Carpinteria, CA]). Appropriate negative and positive controls for each antibody were performed.31
The images of the slides were aquired with an Olympus BX60 microscope and an Olympus DP71 camera with Olympus software (Tokyo, Japan).
Antigen presentation assay
Magnetically purified DCs were pulsed for 2 hours in either 200 μL RPMI 1640 10% FCS complete (Invitrogen) or medium containing either 10 μM ISQAVHAAHAEINEAGR, SIINFEKL peptides, or 1 mg/mL Ova (Calbiochem, Dietikon, Switzerland). Cells were irradiated with 600 gray and washed twice in complete medium. DCs (4 × 104/mL) were plated into 96-well flat bottom plates (Costar, Corning, NY) and serially diluted into complete medium. Purified TCR Tg T cells (6 × 105) were added per well. After 4 days, 0.5 μCi (0.0185 MBq) 3H-thymidine was added per well, and thymidine incorporation was monitored using Topcount NXT (Perkin Elmer, Boston, MA).
Activation of DCs
Magnetically purified DCs were plated into 48-well plates at 5 × 105 cells/mL (Costar) in IMDM GlutaMAX I (Invitrogen) 10% FCS complete. DC activation was achieved with addition of 8.3 μg/mL poly(I:C) + 170 nM CpG (InvivoGen, San Diego, CA) + 0.1 U/mL IL-4 + 0.1 U/mL IFN-γ (eBioscience). Cell supernatants were collected after 24 hours of incubation.
Detection of cytokines
Cytokines were quantified using OptEIA enzyme-linked immunosorbent assay (ELISA) Sets (BD Biosciences, San Diego, CA), according to the provider's instructions.
Results
DC-specific expression of the SV40 oncogene in 2 Mushi Tg lines
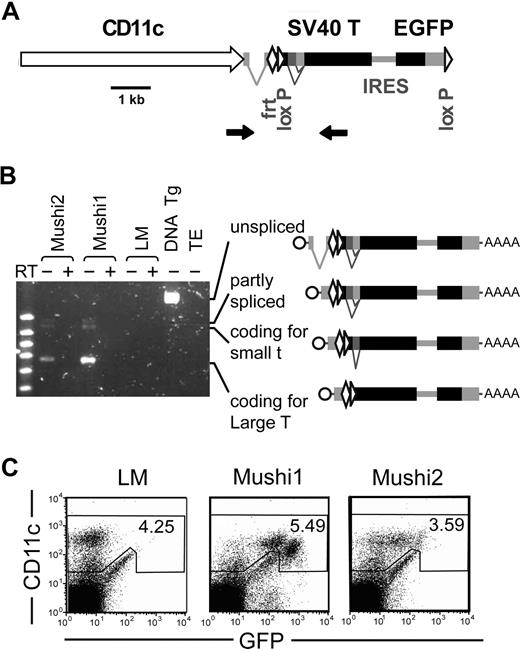
We used the CD11c promoter26 to express the viral SV40 T oncogenes in DCs of Tg mice (Figure 1A). As a reporter, bicistronic expression of GFP allowed monitoring of specificity and levels of Tg expression. The predicted correctly spliced transcripts encoding the SV40 oncogenes were detected by RT-PCR (Figure 1B). We selected 2 Tg lines, Mushi1 and 2 with high or low Tg expression, respectively. By flow cytometry, most CD11c+ cells expressed GFP, while CD11c− and GFPlow cells were rare (0.5%-1.5%) (Figure 1C). The large majority of these latter cells had a cell surface marker profile of follicular and marginal zone B cells (CD19+, CD21+, CD23+ or CD23−, CD24+, CD45R+, CD62L+ or CD62L−, IgM+, MHC class II+) and was negative for the following lineage/stem cell markers: CD3, CD4, CD8, CD11b, CD161, F4/80, Ter119, GR1, and CD117.
Generation and characterization of Mushi Tg mouse lines. (A) Schematic representation of the Tg construct. The Tg consists of the mouse CD11c promoter, the intron and the polyA addition signal from the rabbit β1 globin gene, the gene coding for SV40 T oncogenes, and a second cistron for eGFP. FRT and loxP sites were added to allow generation of single-copy Tg mice and to remove the expression cassette, respectively. Primers (arrows) flanking the introns of rabbit β1 globin and SV40 T oncogenes were used for screening and to monitor splicing of Tg transcripts. (B) Detection of Tg spliced transcripts coding for SV40 large T and small T antigens by RT-PCR (as described in A) on spleen of the independent Tg lines Mushi1 and Mushi2, littermates (LMs), control genomic Tg DNA (DNA TG), and negative control (TE). (C) FACS analysis of Tg expression. CD11c and GFP levels in spleen Tg Mushi1 and Mushi2 and non-Tg littermate (LM) cells.
Generation and characterization of Mushi Tg mouse lines. (A) Schematic representation of the Tg construct. The Tg consists of the mouse CD11c promoter, the intron and the polyA addition signal from the rabbit β1 globin gene, the gene coding for SV40 T oncogenes, and a second cistron for eGFP. FRT and loxP sites were added to allow generation of single-copy Tg mice and to remove the expression cassette, respectively. Primers (arrows) flanking the introns of rabbit β1 globin and SV40 T oncogenes were used for screening and to monitor splicing of Tg transcripts. (B) Detection of Tg spliced transcripts coding for SV40 large T and small T antigens by RT-PCR (as described in A) on spleen of the independent Tg lines Mushi1 and Mushi2, littermates (LMs), control genomic Tg DNA (DNA TG), and negative control (TE). (C) FACS analysis of Tg expression. CD11c and GFP levels in spleen Tg Mushi1 and Mushi2 and non-Tg littermate (LM) cells.
Mushi mice develop systemic disease
Adult Tg mice developed pallor, then rapidly became moribund. The median age for disease development in Mushi1 (4 months) and Mushi2 (13 months) was correlated with Tg expression levels (Figure 2A). Such a dose-dependent disease onset was confirmed with several other independent Tg lines (data not shown). Full disease penetrance was observed in both lines. Hematocrit values decreased dramatically in diseased mice (Figure 2B). During terminal stages, mice showed hepatosplenomegaly and often mesenteric but not peripheral lymphadenopathy. Nodules were observed in liver and spleen, but bone and skin were not involved (Figure 2C).
Mushi1 and 2 mice develop histiocytosis. (A) The high and low Tg-expressing Mushi1 and Mushi2 lines show an early and late development of disease signs (anemia and general symptoms), respectively. (B) Hematocrit values in percentage of healthy control (LM) and diseased Mushi1 mice (Tg). (C) Multiple nodules and organ enlargement in spleen, liver, and mesenteric LN (MLN) from diseased mice (Mushi1) compared with healthy controls (LM). (D) Similar histologic characteristics in human LCH in LN (human) and Mushi1 liver lesions (TG) are detected by hematoxylin & eosin (H&E) and immunostaining for Langerin (CD207) and S100. (E) Human and Mushi lesions show reniform nuclei (enlargement of D left column). (F) Expression of the cell cycle marker Ki-67 was tested by FACS in gated CD11c− and CD11c+ MLN cells from control (LM) and diseased (TG) mice. (G) Normal human spleen stained with anti-CD1a or anti-Langerin antibodies.
Mushi1 and 2 mice develop histiocytosis. (A) The high and low Tg-expressing Mushi1 and Mushi2 lines show an early and late development of disease signs (anemia and general symptoms), respectively. (B) Hematocrit values in percentage of healthy control (LM) and diseased Mushi1 mice (Tg). (C) Multiple nodules and organ enlargement in spleen, liver, and mesenteric LN (MLN) from diseased mice (Mushi1) compared with healthy controls (LM). (D) Similar histologic characteristics in human LCH in LN (human) and Mushi1 liver lesions (TG) are detected by hematoxylin & eosin (H&E) and immunostaining for Langerin (CD207) and S100. (E) Human and Mushi lesions show reniform nuclei (enlargement of D left column). (F) Expression of the cell cycle marker Ki-67 was tested by FACS in gated CD11c− and CD11c+ MLN cells from control (LM) and diseased (TG) mice. (G) Normal human spleen stained with anti-CD1a or anti-Langerin antibodies.
Lesions show characteristics of human LCH
At late stages, histologic examination revealed replacement of the normal parenchyma in spleen and liver (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). At higher magnification, the infiltrating cells were frequently opposed to one another and showed an eosinophilic cytoplasm (Figure 2D left panel). The morphology of pathologic mouse cells was strikingly similar to that of human LCH lesions. In contrast to human disease, accumulations/eosinophils and macrophages were not observed.
Immunohistochemistry for diagnostic markers of LCH, namely Langerin (CD207) and S100, showed diffuse positive cytoplasmic and membranous staining in both human LCH patients and Tg mice (Figure 2D middle and right panels). Reniform nuclei, typically observed in LCH lesions, were also found in mouse cells (Figure 2E). Compared with human LCH, more frequent mitoses were observed in mouse cells and their cytoplasm was less abundant. Importantly, abnormal mitotic figures, indicating malignant LCS lesions, were not observed in mice. Flow cytometry analysis using Ki-67 staining showed that the proportion of cycling cells was specifically increased among CD11c+ cells (Figure 2F). To quantify this infiltration in lymphoid organs, we measured the percentage of GFP+CD11c+ cells by FACS (Table 1; Figure S2). The highest percentages were found in spleen and bone marrow, while minimal infiltration was detected in peripheral LN.
Quantification of DC numbers in different organs of littermate or diseased Mushi1 mice
| . | Littermate . | Sick Mushi1 . | ||
|---|---|---|---|---|
| % DCs . | No. of DCs ×106 . | % DCs . | No. of DCs ×106 . | |
| Spleen | 7.4 ± 1.3 | 5.9 ± 1.6 | 31 ± 15 | 158 ± 34 |
| Bone marrow | 3.6 ± 1.0 | 2.2 ± 2.5 | 40 ± 9.2 | 4.2 ± 2.9 |
| Peripheral LN | 1.6 ± 0.7 | 0.06 | 3.2 ± 0.5 | 0.41 |
| Mesenteric LN | 3.0 ± 0.6 | 0.11 | 11 ± 8.8 | 0.67 |
| Thymus | 0.5 ± 0.4 | 0.27 | 22 ± 18.9 | 0.42 |
| Liver | 7.0 ± 6.2 | 0.25 | 42 | 229 |
| PBL | ND | ND | 5.6 | ND |
| . | Littermate . | Sick Mushi1 . | ||
|---|---|---|---|---|
| % DCs . | No. of DCs ×106 . | % DCs . | No. of DCs ×106 . | |
| Spleen | 7.4 ± 1.3 | 5.9 ± 1.6 | 31 ± 15 | 158 ± 34 |
| Bone marrow | 3.6 ± 1.0 | 2.2 ± 2.5 | 40 ± 9.2 | 4.2 ± 2.9 |
| Peripheral LN | 1.6 ± 0.7 | 0.06 | 3.2 ± 0.5 | 0.41 |
| Mesenteric LN | 3.0 ± 0.6 | 0.11 | 11 ± 8.8 | 0.67 |
| Thymus | 0.5 ± 0.4 | 0.27 | 22 ± 18.9 | 0.42 |
| Liver | 7.0 ± 6.2 | 0.25 | 42 | 229 |
| PBL | ND | ND | 5.6 | ND |
Mean absolute cell numbers derived from 3 mice were calculated by multiplying the total cell numbers by the percentage of DCs within each organ. Standard deviations are indicated.
PBL indicates peripheral blood leukocyte; and ND, not determined.
RNA analysis of cytokine and chemokine expression revealed shared profiles between human LCH and Tg mouse DC samples. IL-2 and IL-12 p40 produced by freshly isolated DCs were not increased, while proinflammatory cytokine levels (IL-1 and TNFα) were enhanced compared with wild-type (WT) CD8α+ DCs. In addition, the levels of the immunoregulatory IL-10 were comparable with WT DCs. CCR6 expression was similar to that in control mice, while CCR7 expression was strikingly decreased as in human LCH (data not shown).
Taken together, these results show that the mouse lesions are very similar to human LCH. However, the high proliferation rate combined with the lack of overt cellular atypia suggests a more aggressive form of LCH. The progressive and severe course of disease, the infiltration of internal organs, and the morphology would be compatible with LCH cases that were previously described under the historical term, Letterer-Siwe disease.32
Proliferating cells are CD8α+ cDCs
Based on surface marker expression, several subtypes of murine CD11c+ DCs can be identified. pDCs (B220+Langerin−), CD8α+ cDCs (B220−CD11b−CD24+Langerin+), CD8α− cDCs (B220−CD11b+CD24−CD4+/−Langerin−), as well as epi-dermal LCs (CD8α−CD11b+Langerin+) and dermal DCs (CD8α−Langerin−) are found in peripheral LNs.
Since expression of Langerin has been reported outside the skin in mouse spleen CD8α+ cDCs,33 we tested for Langerin+ cells in normal human spleen and, indeed, detected them in white pulp periarteriolar sheaths (Figure 2G). This result is in line with Langerin expression being observed in other human organs34 and suggests that Langerin+ human splenocytes are cDCs similar to the ones described in WT and Mushi mice.
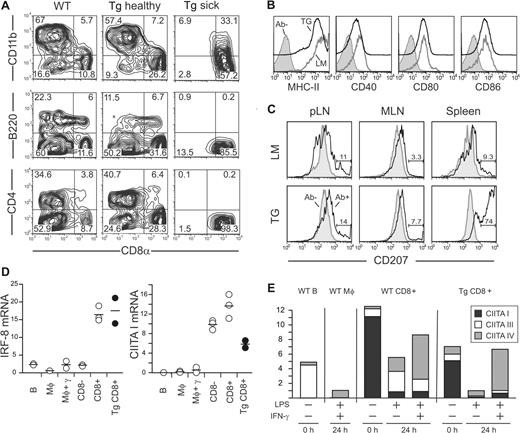
Flow cytometry was performed to determine the subtype and activation status of DCs in Tg mice5,35,36 (Figure 3A). In a healthy Tg spleen, the frequency of CD11c+CD8α+ DCs was increased 2- to 3-fold compared with nontransgenic littermates. The CD11c+ cells from diseased mice were positive for CD8α and negative for B220, CD11b, and CD4. As in their wild-type counterparts, the accumulating cells expressed CD24 and CD205 (DEC-205) (Figure S3). The cell surface levels of MHC class II (MHC-II) and the costimulation molecules CD40, CD80, and CD86 were similar to WT DCs (Figure 3B).
Pathologic cells are CD8α+ cDCs. (A) CD11c+ DCs were magnetically purified from spleen of WT, healthy (2 months old), and sick Mushi1 Tg mice and analyzed by FACS. The percentage of CD11c+ gated cells expressing the indicated DC subtype marker is shown. (B) The indicated activation markers were analyzed on CD11c+ purified cells from littermate (LM) or sick Mushi1 Tg (TG) mice. CD11c+ CD8α+ gated cells are shown with or without antibody for activation marker (Ab−). (C) Langerin was analyzed on skin-draining LN (pLN), MLN, and spleen cells from littermate (LM) or 3.5-month-old Mushi1 Tg mice with splenomegaly (TG). Fixed and permeabilized CD11c+ CD24+ gated cells are shown with (Ab+) or without (Ab−) Langerin antibody. The percentage of Langerin-positive cells is indicated. (D) The indicated mRNAs were quantified by real-time RT-PCR in magnetically purified B cells and macrophages (Mφ) from WT mice, and FACS-purified spleen CD8α− and CD8α+ DC subsets from Mushi1 sick Tg and non-Tg mice. (E) Levels of CIITA isoform mRNA before and after activation with interferon-γ (IFN-γ) and lipopolysaccharide (LPS) or LPS alone were quantified by real-time RT-PCR using an absolute quantification titration curve. The indicated cells were purified as in panel C.
Pathologic cells are CD8α+ cDCs. (A) CD11c+ DCs were magnetically purified from spleen of WT, healthy (2 months old), and sick Mushi1 Tg mice and analyzed by FACS. The percentage of CD11c+ gated cells expressing the indicated DC subtype marker is shown. (B) The indicated activation markers were analyzed on CD11c+ purified cells from littermate (LM) or sick Mushi1 Tg (TG) mice. CD11c+ CD8α+ gated cells are shown with or without antibody for activation marker (Ab−). (C) Langerin was analyzed on skin-draining LN (pLN), MLN, and spleen cells from littermate (LM) or 3.5-month-old Mushi1 Tg mice with splenomegaly (TG). Fixed and permeabilized CD11c+ CD24+ gated cells are shown with (Ab+) or without (Ab−) Langerin antibody. The percentage of Langerin-positive cells is indicated. (D) The indicated mRNAs were quantified by real-time RT-PCR in magnetically purified B cells and macrophages (Mφ) from WT mice, and FACS-purified spleen CD8α− and CD8α+ DC subsets from Mushi1 sick Tg and non-Tg mice. (E) Levels of CIITA isoform mRNA before and after activation with interferon-γ (IFN-γ) and lipopolysaccharide (LPS) or LPS alone were quantified by real-time RT-PCR using an absolute quantification titration curve. The indicated cells were purified as in panel C.
Because Langerin expression was recently shown to be lower in C57BL/6 spleen CD8α+ cDCs than in BALB/c,37 we evaluated its expression in Tg peripheral LN, mesenteric LN (MLN), and spleen by flow cytometry (Figure 3C). In line with this recent report,37 only a part of control CD11c+ CD24+ DCs expressed Langerin. Unexpectedly, we found a dramatic increase in proportion of Langerin+ cells among spleen CD11c+ CD24+ DCs from older Tg mice. No such increase was observed in peripheral LN or MLN.
To confirm the DC lineage and activation status of proliferating DCs, expression of the transcription factors IRF-8/ICSBP and class II transactivator (CIITA) isoforms was measured by quantitative RT-PCR. The interferon regulatory factor IRF-8, which is a transcription factor encoded by the gene Icsbp1, controls development and function of immune cells.38 CD8α+ cDCs and pDCs express higher IRF-8 levels than the other subsets. CIITA functions as a key transactivator for the MHC-II expression. The 3 isoforms of CIITA mRNA derived from distinct promoters are differentially expressed.39 Under steady-state conditions, CIITA form I is the major form in cDCs, and form III, in pDCs, while form IV is inducible by IFN-γ. Following activation, expression of CIITA form I is repressed in DCs and increased in macrophages.40 The combined examination of IRF-8 and CIITA thus allows for the determination of lineage, subset, and activation status of DCs.
The mRNAs were quantified in B cells, monocytes, and DCs from control and CD8α+ DCs from diseased mice (Figure 3D). High IRF-8 expression was found in CD8α+ DCs from both control and sick Tg mice. CIITA form I level was highest in control DCs and in transformed Tg cells. As expected, the CIITA form I sharply decreased after activation with LPS or LPS and IFN-γ (Figure 3E). As reported, the latter condition led to increased CIITA form IV.40 Despite slightly reduced levels of CIITA form I, and a small increase in form IV before activation, transformed DCs showed expected modulation in expression of CIITA isoforms on activation.
mRNA of CIITA-I, Langerin, IRF-4, DC-SIGN, and CD68 was next quantified in spleen CD8α− and CD8α+ cDCs, and LCs (Figure S4). As expected, IRF-8 and CIITA-I were high in control and Tg CD8α+ cDCs, and LCs. Langerin level was highest in LCs, and the increase in CD8α+ cDCs from healthy and sick Tg mice was confirmed. IRF-4, an essential factor in development and function of DCs,41 DC-SIGN, and CD68, which are differentially expressed in myeloid cells were also quantified. The expression of these 3 markers was highest in LCs and CD8α− cDCs. In summary, each of the 3 DC subtypes expressed a specific gene profile, and the RNA profile of accumulating DCs from sick Tg mice was closest to the one of CD8α+ cDCs.
Our results clearly show that the proliferating CD11c+ cells are CD8α+ Langerin+ cDCs with a comparable activation status to nontransgenic freshly isolated splenic DCs. In the numerous diseased mice that underwent analysis, we never observed transformation of other DC subtypes, cell types such as granulocytes, macrophages, natural killer (NK) cells, T cells, B cells, or even CD11c−GFPlow cells (not shown).
Preserved antigen presentation and inducible cytokine secretion
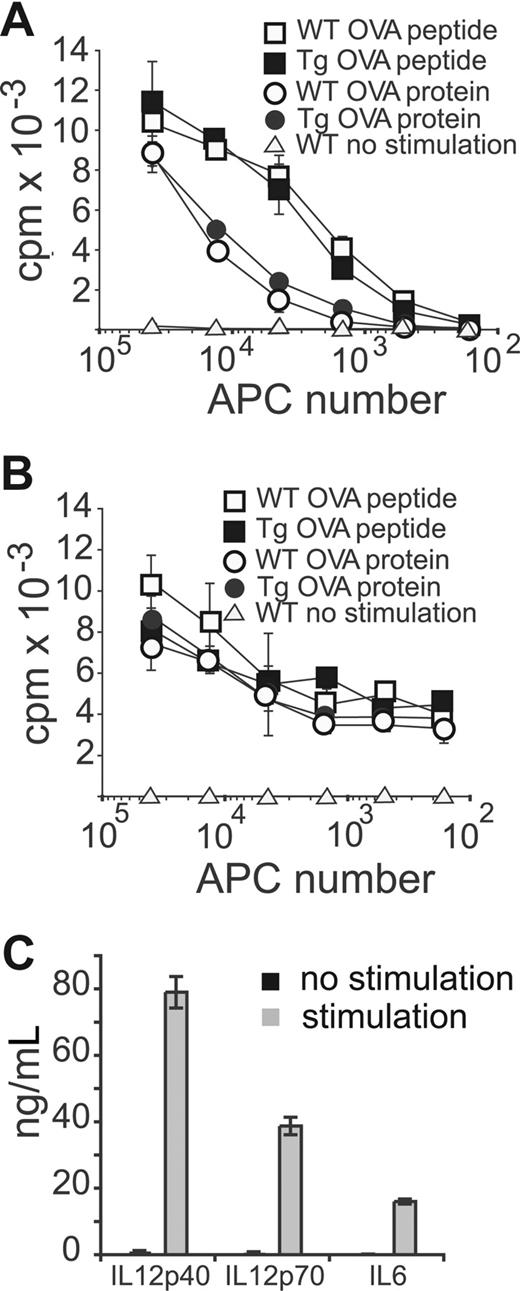
A major function of CD8α+ DCs is the efficient MHC class I and MHC-II restricted antigen-(cross)presentation to naive T cells.42 Despite a described in vivo block in maturation in LCH,18,43,44 LCH cells can fully differentiate into APCs in vitro.45 To test these functions, we titrated different numbers of splenic DCs isolated from diseased Tg or WT mice for antigen presentation to OTI or OTII TCR Tg T cells when incubated with ovalbumin (Ova) protein or peptide. As shown in Figure 4A and B, Tg DCs induced similar T-cell proliferation to control DCs. To specifically address the Ova presentation to naive T cells, we used T cells from OTI mice on a RAG−/− background. Again, Tg DCs behaved like their normal counterparts (not shown).
Sick Tg DCs maintain their antigen presentation and cytokine secretion capacity. CD11c+ spleen DCs from WT or sick Mushi1 Tg mice were magnetically purified. (A) Ova protein or peptide (cross)-presentation after 1-hour pulsing of irradiated DCs to OTI CD8+ T cells. Proliferation of T cells was measured by 3H-thymidine incorporation after 3 days of coincubation. (B) Ova or peptide presentation of irradiated DCs to OTII CD4+ T cells. Peptide or protein was left in the culture during the 3-day incubation period. Proliferation was measured as in panel A. (C) Secretion of indicated cytokines was measured by ELISA in supernatants of DCs from sick Mushi1 Tg mice that were activated with CpG, poly IC, IFN-γ, IL-4, and GM-CSF or not activated for 18 hours. The mean and standard deviation are shown for triplicate measurements.
Sick Tg DCs maintain their antigen presentation and cytokine secretion capacity. CD11c+ spleen DCs from WT or sick Mushi1 Tg mice were magnetically purified. (A) Ova protein or peptide (cross)-presentation after 1-hour pulsing of irradiated DCs to OTI CD8+ T cells. Proliferation of T cells was measured by 3H-thymidine incorporation after 3 days of coincubation. (B) Ova or peptide presentation of irradiated DCs to OTII CD4+ T cells. Peptide or protein was left in the culture during the 3-day incubation period. Proliferation was measured as in panel A. (C) Secretion of indicated cytokines was measured by ELISA in supernatants of DCs from sick Mushi1 Tg mice that were activated with CpG, poly IC, IFN-γ, IL-4, and GM-CSF or not activated for 18 hours. The mean and standard deviation are shown for triplicate measurements.
Freshly isolated splenic DCs do not secrete high amounts of cytokines.46 Activation of their Toll-like receptors (TLRs) results in the secretion of large amounts of IL-12p40 and IL-6. Large quantities of IL-12p70 can be secreted only with the combined signaling of TLR and cytokine receptors.46 To evaluate the inducible cytokine secretion by Tg DCs of diseased mice, DCs were activated with the synergistic TLR ligands CpG and poly IC in the presence of cytokines (IL-4, IFN-γ, and GM-CSF). High-level secretion of IL-6, IL-12p40, and IL-12p70 was detected after activation as described for WT CD8α+ cDCs (Figure 4C). Likewise, they produced CCL3, interferon β, IL-1β, IL-10, and TNFα. In addition, MCP-1—specifically produced by CD8α− DCs—was not detected (not shown). In summary, our data show that transformed CD8α+ cDCs maintain a normal DC differentiation program.
Preferential transformation of differentiated CD8α+ cDCs
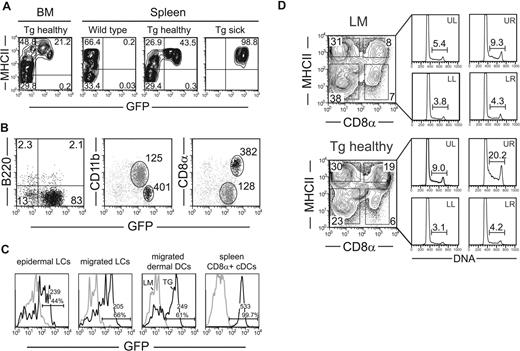
Because tumorigenesis usually affects precursor and dividing cells, GFP expression was evaluated in bone marrow or splenic CD11c−, CD115+ or CD115−, CD117high common DC/macrophage precursors or MHC-II− CD11c+ Tg DC precursors by flow cytometry.9,,,–13 For both types of precursors, no Tg expression was found. In bone marrow from young mice, MHC-II− DC precursors were clearly GFP negative (Figure 5A and data not shown). In splenic DC precursors, GFP expression was practically absent or very low in 4 studied cases. In those with partial expression, the few GFP+ CD11c+ MHC-II− cells had a mature phenotype (eg, CD8α expression, not shown). In diseased mice, practically no MHC-II−CD11c+ precursors were found in spleens. These observations suggest that tumorigenesis occurs in the mature CD8α+ DC subset.
Preferential transformation of CD8α+ DCs is correlated with absence of Tg expression in DC precursors, increased Tg expression in CD8α+, and higher steady-state proliferation in CD8α+ DCs in Mushi1 and in WT mice. (A) Tg expression is barely detectable in bone marrow DC precursors. Bone marrow cells and CD11c+ magnetically purified splenocytes of indicated mice were analyzed for MHC-II and GFP reporter expression by FACS analysis. CD11c+ gated cells are shown. (B) Expression levels of GFP were analyzed in spleen DC subsets of healthy Mushi1 Tg mice as in panel A. For pDCs, the percentage of B220+ and/or GFP+ cells is indicated. For cDCs, the geometric mean of GFP level is shown in indicated regions of cells stained for CD11b or CD8α. (C) Expression levels of GFP were analyzed in epidermal LCs (gate: CD45+MHCIIhigh), migrated LCs (gate: CD11c+CD205brightCD8α−), and dermal DCs (gate: CD11c+CD205intCD8α−) in skin-draining LNs, and spleen CD8α+ cDCs from 3.5-month-old (Tg) and non-Tg (LM) mice. The percentage and the geometric mean (Geo) of GFP+ cells with the indicated gates are shown. (D) Spleen cells of control from Mushi1 healthy 2-month-old (Tg healthy) and non-Tg (LM) mice were purified and gated as in panel A. In the left part, the percentage of cells positive for CD8α and MHC-II expression are shown. In the right part, the gate positions are indicated. Steady-state proliferation of indicated DC subsets was determined by Hoechst staining. The percentage of S and 4N cells is indicated.
Preferential transformation of CD8α+ DCs is correlated with absence of Tg expression in DC precursors, increased Tg expression in CD8α+, and higher steady-state proliferation in CD8α+ DCs in Mushi1 and in WT mice. (A) Tg expression is barely detectable in bone marrow DC precursors. Bone marrow cells and CD11c+ magnetically purified splenocytes of indicated mice were analyzed for MHC-II and GFP reporter expression by FACS analysis. CD11c+ gated cells are shown. (B) Expression levels of GFP were analyzed in spleen DC subsets of healthy Mushi1 Tg mice as in panel A. For pDCs, the percentage of B220+ and/or GFP+ cells is indicated. For cDCs, the geometric mean of GFP level is shown in indicated regions of cells stained for CD11b or CD8α. (C) Expression levels of GFP were analyzed in epidermal LCs (gate: CD45+MHCIIhigh), migrated LCs (gate: CD11c+CD205brightCD8α−), and dermal DCs (gate: CD11c+CD205intCD8α−) in skin-draining LNs, and spleen CD8α+ cDCs from 3.5-month-old (Tg) and non-Tg (LM) mice. The percentage and the geometric mean (Geo) of GFP+ cells with the indicated gates are shown. (D) Spleen cells of control from Mushi1 healthy 2-month-old (Tg healthy) and non-Tg (LM) mice were purified and gated as in panel A. In the left part, the percentage of cells positive for CD8α and MHC-II expression are shown. In the right part, the gate positions are indicated. Steady-state proliferation of indicated DC subsets was determined by Hoechst staining. The percentage of S and 4N cells is indicated.
CD8α+ cDCs express enhanced Tg levels and show higher spontaneous cell proliferation
We searched for a correlation between Tg expression levels and preferential transformation of CD8α+ DCs (Figure 5B). Flow cytometry was performed with splenic DCs from young healthy Tg mice. Most CD11c+B220− cDCs expressed the Tg, while the majority of pDCs did not. Among cDCs, the Tg was expressed at 3-fold higher levels in CD8α+ compared with CD11b+ subsets.
We next examined Tg expression in tissue-derived DCs by flow cytometry. In epidermis, 10% to 40% of LCs were GFP positive, and the expression level was lower than in spleen CD8α+ cDCs (Figure 5C). In skin-draining LNs, migrated LCs and dermal DCs also expressed lower Tg levels. Similar Tg expression was observed in MLN (data not shown). Tg expression was confirmed to be highest in CD8α+ cDCs by quantitative RT-PCR, and the lower expression in CD8α− cDCs, LCs, and B cells perfectly matched the flow cytometry data (Figure S5). In addition, SV40 Ags could not be detected in LCs by immunofluorescence (data not shown). These results suggest that enhanced Tg expression could play a role in the preferential transformation of CD8α+ DCs.
The G0 status of peripheral DCs was very recently called into question by the description of locally dividing DCs in spleen.12,,–15 In our model, a higher proliferation rate of MHC-II+ CD8α+ DCs in the steady state could favor transformation. To address this hypothesis, DNA content was determined in spleen DCs isolated from young healthy Tg and control mice (Figure 5D). Cycling cells were more frequent among MHC-II+ DCs than among their MHC-II− precursors, and mature CD8+ DCs cycled more (9.3%) than their CD8− counterparts (5.4%). B lymphocytes cycled at lower levels (2.6% not shown). In young Tg mice, cycling of DC precursors was similar. In contrast, a preferential increase in cell cycling was observed in MHC-II+ CD8α+ Tg DCs (2.1-fold vs 1.6-fold in CD8α− DCs). These data show that the higher steady-state cycling of MHC-II+CD8α+ DCs in spleen can be further increased by T antigen expression before disease onset.
Discussion
Tg Mushi mice serve as the long sought-after animal model for human multisystem histiocytosis.20 Our Tg approach to express SV40 T oncogenes was designed to target cDCs to cycle without noticeable dedifferentiation.47 Indeed, in Tg Mushi mice, cDCs rather than LCs are transformed with maintained phenotypic and functional features. Remarkably, these mice develop lesions sharing key characteristics with human multisystem LCH: (1) similar organ distribution (except bone and skin where the transgene is expressed at lower levels in LCs), (2) histologic features and immunohistologic markers such as Langerin and S100, and (3) APC functions similar to LCH cells.45 These features allow a formal validation of this animal model for multisystem histiocytosis. The severe and progressive development of disease resembles the historical Letterer-Siwe disease. Interestingly, the combined features of frequent and yet normal mitosis with largely conserved DC differentiation in Mushi mice are reminiscent of the rare cases of LCH with high proliferation rather than of LCS defined by abnormal mitosis. In other words, the transformed cells in our model have characteristics of “low-grade DC tumors.” Our data raise the provocative possibility that multisystem LCH is a neoplastic disease. This would be a deregulation of the recently demonstrated physiological cell proliferation of differentiated DCs.12,,–15
A historical cornerstone in the understanding of LCH was reached when pathological histiocytes were identified as being LCs.48 The classic finding of Birbeck granules, which are composed of the Langerin protein in DCs, defines them as LCs, and the presence of such cells in the biopsy is diagnostic for LCH. Histiocytic disorders are divided into LCH and non-LCH types based on detection of Langerin or Birbeck granules. In rare cases, downmodulation of Langerin in activated LCs makes the classical view less certain that the clinical diagnosis of LCH can be based solely on Langerin expression.49 Recently, the specificity of Langerin expression for LCs was further challenged in mice. Using GFP knockin BALB/c mice in the Langerin locus, Langerin expression was found in LN, spleen, thymus, and liver, whereas similar findings in humans have been reported (a first description of such cells in human spleen is found in Figure 2G).34,50 Importantly, in mice, the major mouse DC subset expressing Langerin besides LC is the CD8α+ cDC population.50
Surprisingly, in diseased Mushi mice, the lesions do not contain CD8α− CD11b+ Langerin+ LCs but rather CD8α+ CD11b− Langerin+ cDCs. Indeed, LCs are unlikely to become transformed for the following reasons: (1) the activity of the CD11c promoter fragment is lower in LCs than in CD8α+ DCs (Figure 4B)26 ; (2) skin and skin-draining LNs that contain LCs are not affected; (3) the proliferating cells are CD11b− and CD8α+, in contrast to resting or activated LCs (Figure 3);50 (4) LCs express IRF-4, CD68, and DC-SIGN, which are only weakly expressed in control and proliferating CD8α+ DCs (Figure S4); and (5) Langerin expression is not restricted to skin LCs. Our Mushi mice thus challenge the current classification of histiocytosis. Based on our findings and the current knowledge on Langerin expression in spleen and liver in both species (Figure 2G),34 the importance of Langerin expression in the understanding of pathogenesis and of clinical entities should be revisited. In particular, it would be of interest to test a cDC origin of pathogenic cells in LCH lesions involving internal organs only or of lesions lacking eosinophil infiltration. It should be stressed that this hypothesis does not underscore the importance of Langerin expression in the diagnosis of histiocytosis and a central role of LCs in skin lesions.34 In future experiments, the role of LCs could be addressed by generating a new Tg model based on Langerin-driven oncogene expression. The comparison of this new LCH model and the model described here will help to tackle the involvement of various DC populations in histiocytosis.
In several Tg models the impact of SV40 antigens on cell differentiation was not detected.47 The subtle differences observed such as the expression profile of CIITA isoforms in sick Tg mice (Figure 3D) could be due to somatic mutations or epigenetic changes. The large increase in Langerin expression in CD8a+ cDCs already taking place in 2-month-old Tg mice (data not shown) might be due to similar mechanisms or to indirect factors such as altered DC homeostasis.
SV40 T oncogenes are able to induce tumors in Tg mice through deregulation of cell cycling and cell death.47 Several factors influence tumorigenesis: besides the continuous expression of high levels of T antigens, both genetic and cell lineage/differentiation factors are important. In contrast to most cell types, neurons seem resistant to transformation by T antigens probably due to their inability to induce cycling in terminally differentiated cells that are in the G0 phase of the cell cycle. In contrast to the traditional view on DCs as being terminally differentiated cells, it was recently shown that peripheral cDCs locally divide in the spleen.12,,–15 Therefore, the DC compartment should be amenable to transformation by T antigens. Several lines of evidence suggest that the cells undergoing transformation are the differentiated splenic CD8α+ cDCs. First, Tg expression is not detectable in early (CD11c− CD115+ or− CD117high) or late (CD11c+ MHC-II−) precursors.10,13 Second, proliferation of MHC-II+CD8α+ DCs is highest followed by MHC-II+CD8α− DCs and lowest in MHC-II− precursors. These differences are increased in healthy Tg mice. Third, in sick Tg mice, all proliferating cells were MHC-II+CD8α+ cells. Fourth, in asymptomatic 3.5-month-old mice, single nodules and increase in Langerin mRNA were specifically found in spleen without abnormality in the gut (not shown).
Our model suggests that deregulated proliferation in peripheral DCs could lead to histiocytosis. Consistent with this hypothesis, the 2 DC subsets with convincing evidence for self-renewal capacity—namely LC and splenic DC populations—are the ones involved in human histiocytosis and our mouse model, respectively. In other words, LCH could be understood as a disease caused by deregulated homeostasis of DC subsets having self-renewal capacity. The physiological relevance of cell cycling on DC function has been proposed to extend the duration of antigen presentation,15 while the functional impact of deregulated cycling on immune function of DCs in pathological conditions is currently unknown. The existence of our animal model opens the way to address such issues.
It can be argued that all transformed DCs conserve their self-renewal capacity in malignant histiocytosis, but in LCH such a simple scenario is unlikely because of lesions with low frequency of cycling histiocytes. However, a neoplastic process cannot be excluded because of infrequent mitoses. Indeed, in humans, neoplastic diseases have been described with low levels of proliferation.51 In these cases, a tumor with 2 compartments has been proposed where a small pool of transformed or pretransformed cells (cancer stem cells or cancer progenitors) further differentiates into terminally differentiated cells. In myeloproliferative disorders, mature cells accumulate and/or proliferate but the oncogenic mutation is already found in precursors. In mixed lineage leukemia, the recent description of acquisition of self-renewal capacity in the transient amplifying pool highlighted the concept of tumor stem cells derived from subverted progenitor cells.52
There is an ongoing debate over the malignant or reactive nature of LCH. The rather low frequencies of proliferating DCs, the activated phenotype of accumulated LCs, lymphocytes, and macrophages in the lesions, as well as local overproduction of cytokines have been taken as evidence for reactive accumulation of cells.18,19 The occasionally observed progression from a disseminated leukemia-like disease to LCH, the response to chemotherapy regimens used in the treatment of other hematopoietic malignancies, increased LC proliferation, and the frequent loss of heterozygosity are more in favor of somatically acquired mutations and transformation.21,,,–25 In our model, intrinsic DC defects in cycling/renewal were demonstrated by the Tg expression being restricted to differentiated cDCs and the ease to transfer the disease to recipient mice (data not shown). The requirement for secondary mutation events leading to transformation has been described for SV40 large T antigens and presents an exciting testable possibility of shared mutations between human and murine multisystem histiocytosis.24,25,53
In conclusion, our model of multisystem histiocytosis shows that transforming mature cDCs can lead to a disease with striking similarities to aggressive systemic LCH with high proliferation index. This model allows for the definition of shared molecular pathways of DC transformation between humans and mice, leading to the discovery of potential targets for diagnosis or treatment of human disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank R. Ceredig and A. Wilson for critical reading of the paper; A. Fischer, W. Held, H. R. MacDonald, W. Reith, and F. Tacchini-Cottier for helpful discussions; and P. Zaech for FACS sorting. We are especially grateful to T. Bianchi, S. Chevrier, B. Hu, K. Lefort, S. Lattmann, and A. Wilson for valuable help.
This work was supported by grants of the Swiss National Science Foundation, the Swiss Cancer League, the American Histiocytosis Society, and an award from the Histiocytosis Research Trust.
Authorship
Contribution: Q.-G.S. designed and performed the experiments, interpreted the data, and assisted with the paper; L.A.O. coordinated the study, designed and performed the experiments, interpreted the data, and wrote the paper; M.J.H. and F.G. contributed additional experiments and assisted with the paper; G.K. contributed additional experiments and intellectual input; E.S., C.L., and F.B. performed experiments; K.L.M. interpreted data, assisted with the paper, and contributed intellectual input; H.A.-O. supervised the project, designed and performed experiments, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luc A. Otten or Hans Acha-Orbea, Dept of Biochemistry, University of Lausanne, 155 chemin des Boveresses 155, CH-1066 Epalinges, Switzerland; e-mail: luc.otten@hotmail.com or hans.acha-orbea@unil.ch.
References
Author notes
Q.-G.S. and L.A.O. contributed equally to this work.