Cryopyrin-associated periodic syndrome (CAPS) is a spectrum of systemic autoinflammatory disorders in which the majority of patients have mutations in the cold-induced autoinflammatory syndrome (CIAS)1 gene. Despite having indistinguishable clinical features, some patients lack CIAS1 mutations by conventional nucleotide sequencing. We recently reported a CAPS patient with mosaicism of mutant CIAS1, and raised the possibility that CIAS1 mutations were overlooked in “mutation-negative” patients, due to a low frequency of mosaicism. To determine whether there were latent mutant cells in “mutation-negative” patients, we sought to identify mutation-associated biologic phenotypes of patients' monocytes. We found that lipopolysaccharide selectively induced necrosis-like cell death in monocytes bearing CIAS1 mutations. Monocyte death correlated with CIAS1 up-regulation, was dependent on cathepsin B, and was independent of caspase-1. Cell death was intrinsic to CIAS1-mutated monocytes, was not mediated by the inflammatory milieu, and was independent of disease severity or anti–IL-1 therapy. By collecting dying monocytes after lipopolysaccharide treatment, we succeeded in enriching CIAS1-mutant monocytes and identifying low-level CIAS1-mosaicism in 3 of 4 “mutation-negative” CAPS patients. Our findings reveal a novel effect of CIAS1 mutations in promoting necrosis-like cell death, and demonstrate that CIAS1 mosaicism plays an important role in mutation-negative CAPS patients.
Introduction
Cryopyrin-associated periodic syndrome (CAPS) is a spectrum of hereditary periodic fever disorders, and is associated with mutations in the cold-induced autoinflammatory syndrome (CIAS)1 gene and its encoded protein, cryopyrin.1 CAPS consists of 3 phenotypically overlapping but relatively distinct syndromes: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and chronic infantile neurologic cutaneous and articular (CINCA) syndrome, also known as neonatal-onset multi-inflammatory disease. These syndromes are diagnosed mainly based upon clinical manifestations, among which an urticaria-like rash beginning in the neonatal or early infantile period is common. While FCAS and MWS are characterized by periodic attacks of urticarial rash, fever, and arthralgia, patients with CINCA syndrome, the most severe condition of CAPS, exhibit continuous disease activity, with fever, urticarial rash, arthropathy, chronic meningitis, papilledema, growth and mental retardation, and hearing loss.1,2
Recent genetic studies revealed that CAPS patients usually carry heterozygous mutations in the CIAS1 coding region (mutation-positive patients3,,,,–8 ). Although they exhibit no recognizable differences in clinical symptoms or in their response to treatment, approximately half of CINCA syndrome patients lack detectable mutations in CIAS1, as assessed by conventional genomic sequencing (mutation-negative patients,3,–5,9,–11 ), indicating the existence of genetic heterogeneity among CAPS patients. Recently, we reported a patient with CINCA syndrome exhibiting mosaicism of a disease-associated mutation of CIAS112 . This case suggested that some mutation-negative CAPS patients might have mosaicism of the CIAS1 mutation; however, the contribution of CIAS1 mosaicism to disease is controversial. Aksentijevich et al claimed that CIAS1 mosaicism is a rare event in mutation-negative patients, based on their analysis of 14 patients in which CIAS1 mosaicism was not identified, even with careful bidirectional sequencing.11,13,14
Somatic mosaicism has been reported in a number of autosomal dominant monogenic disorders.15,–17 Diagnosis of mosaicism by conventional genomic sequencing using the dideoxy termination method is often difficult, because the overlapping chromatogram of the mutant is easily missed when the frequency of a mutant allele is less than 20% to 30%.18 Heteroduplex-based methods15,19 or subcloning-based analysis of mutant alleles enable one to detect such low-level mosaicism; however, these methods are resource-intensive, and cannot distinguish whether the detected mutation is disease-causing or simply a nonfunctional single nucleotide polymorphism (SNP). An alternative approach involves the isolation of mutant cells using functional analyses based on their characteristic biologic features, and then determining the DNA sequence of the isolated cells. In the current study, we set out to identify specific biologic features of CIAS1-mutant cells compared with nonmutated cells, in an effort to specifically isolate CIAS1-mutated cells from mutation-negative patients.
CIAS1 is expressed in peripheral blood polymorphonuclear cells (PBMCs), activated T cells, chondrocytes, and most prominently in monocytes.4 The encoded protein, cryopyrin, belongs to a family of intracellular pattern recognition receptors, which are crucial in the control of immune responses, NF-κB activation, and cell death (reviewed in Ting and Davis20 and Inohara et al21 ). Cryopyrin associates with caspase-1 and an adaptor protein called apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD: ASC), forming a large protein complex called the inflammasome.22,–24 The inflammasome converts biologically inactive prointerleukin (proIL)-1β to active IL-1β, a potent proinflammatory cytokine, thereby causing an inflammatory response. It is speculated that mutant cryopyrin in CAPS patients activates the inflammasome constitutively in a ligand-independent manner. Indeed, enhanced production of IL-1β has been described in CAPS patients,3,10,12,25 and is regarded as a primary cause of their inflammatory symptoms. Disease-associated CIAS1 mutations induce ASC-dependent NF-κB activation in some systems,12,22,26 and we recently reported that they also induce necrotic cell death in the human monocytic cell line THP-1, which is a novel function of CIAS1.27
In this study, we explored whether CIAS1-mutant cells have specific biologic features, using monocytes from mutation-positive patients, and found that CIAS1-mutant monocytes rapidly underwent necrosis-like cell death after treatment with lipopolysaccharide (LPS) to induce CIAS1 expression. This unique phenotype of CIAS1 mutant cells enabled us to differentiate CIAS1-mutated cells and nonmutated cells in 3 of 4 mutation-negative CAPS patients, and we were able to successfully demonstrate that these 3 patients had mutations of CIAS1 as latent mosaicism.
Methods
Patients
We recruited 11 Japanese CAPS patients, among whom 10 patients had clinically diagnosed CINCA syndrome, and 1 was diagnosed with MWS. All of the CINCA patients met previously described diagnostic criteria.5,10 The MWS patient had recurrent episodes of inflammation that were not triggered by cold, progressive deafness, and amyloidosis. Written informed consents were obtained from the patients and their families, according to the protocol of the institutional review board of Kyoto University Hospital and in accordance with the Declaration of Helsinki. Details of patients are described in Table 1.
Clinical profiles and CIAS1 mutations identified in 11 patients with cryopyrin-associated periodic syndrome
| Patient number . | Diagnosis . | Age, y* . | Sex . | Initial classification . | Site of mutation . | Biologics therapy . | Central nervous system . | Skin . | Articular . | Reference number . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental retardation . | Meningitis . | Urticarial rash† . | Arthritis . | Contracture . | ||||||||
| 1 | CINCA | 2 | Female | Mutation-positive | N477K (1431C>A) | None | − | + | + | − | − | (28) |
| 2 | CINCA | 3 | Female | Mutation-positive | G755R (2263G>C) | Anakinra | + | + | + | + | − | (29) |
| 3 | CINCA | 12 | Male | Mutation-positive | M662T (1985T>C) | None | − | + | + | + | − | — |
| 4‡ | CINCA | 12 | Male | Mutation-positive | R260W (778C>T) | None | − | + | + | + | − | — |
| 5 | CINCA | 13 | Male | Mutation-positive | D303N (907G>A) | None | − | + | + | + | − | — |
| 6 | CINCA | 14 | Male | Mutation-positive | Y441H (1321C>T) | Tocilizumab | + | + | + | + | − | — |
| 7 | CINCA | 15 | Male | Mosaic | Y570C (1709A>G) | Anakinra§ | − | + | + | + | + | (12) |
| 8 | CINCA | 18 | Female | Mutation-negative | L264F (790C>T) | None | − | + | + | − | − | (30) |
| 9 | CINCA | 11 | Male | Mutation-negative | G307S (919G>A) | None | − | − | + | + | + | — |
| 10 | MWS | 27 | Female | Mutation-negative | E567K (1699G>A) | None | − | Not assessed | + | + | − | (31) |
| 11 | CINCA | 11 | Male | Mutation-negative | Unknown | None | + | + | + | + | − | (32) |
| Patient number . | Diagnosis . | Age, y* . | Sex . | Initial classification . | Site of mutation . | Biologics therapy . | Central nervous system . | Skin . | Articular . | Reference number . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental retardation . | Meningitis . | Urticarial rash† . | Arthritis . | Contracture . | ||||||||
| 1 | CINCA | 2 | Female | Mutation-positive | N477K (1431C>A) | None | − | + | + | − | − | (28) |
| 2 | CINCA | 3 | Female | Mutation-positive | G755R (2263G>C) | Anakinra | + | + | + | + | − | (29) |
| 3 | CINCA | 12 | Male | Mutation-positive | M662T (1985T>C) | None | − | + | + | + | − | — |
| 4‡ | CINCA | 12 | Male | Mutation-positive | R260W (778C>T) | None | − | + | + | + | − | — |
| 5 | CINCA | 13 | Male | Mutation-positive | D303N (907G>A) | None | − | + | + | + | − | — |
| 6 | CINCA | 14 | Male | Mutation-positive | Y441H (1321C>T) | Tocilizumab | + | + | + | + | − | — |
| 7 | CINCA | 15 | Male | Mosaic | Y570C (1709A>G) | Anakinra§ | − | + | + | + | + | (12) |
| 8 | CINCA | 18 | Female | Mutation-negative | L264F (790C>T) | None | − | + | + | − | − | (30) |
| 9 | CINCA | 11 | Male | Mutation-negative | G307S (919G>A) | None | − | − | + | + | + | — |
| 10 | MWS | 27 | Female | Mutation-negative | E567K (1699G>A) | None | − | Not assessed | + | + | − | (31) |
| 11 | CINCA | 11 | Male | Mutation-negative | Unknown | None | + | + | + | + | − | (32) |
+ indicates present; −, not present; and —, not available
Age at examination.
Observed since neonatal period.
Father has the same heterozygous mutation of CIAS1.
Administration of anakinra began during the study period.
Reagents
Crude LPS (cLPS) from Escherichia coli O127:B8, muramyl dipeptide (MDP) and actinomycin D were purchased from Sigma-Aldrich (St Louis, MO). Pure LPS (pLPS), Pam3CSK, Poly I:C, recombinant flagellin, single-strand RNA (ss-RNA) and CpG DNA (type C, ODN M362) were from InvivoGen (San Diego, CA). Z-Tyr-Val-Ala-Asp(OMe)-CH2F (YVAD-fmk), [L-3-trans-(propylcarbamoyl) oxirane-2-carbonyl]-L-isoleucyl-L-proline methyl ester (CA074-Me), and MG-132 were obtained from EMD (Darmstadt, Germany).
Culture of primary human cells
PBMCs were obtained from CAPS patients and healthy volunteers. CD14+ monocytes and CD14− cells were purified by AutoMACS (Miltenyi Biotec, Gladbach, Germany), or sorted using a FACSVantage System (BD, Franklin Lakes, NJ). The purity of CD14+ monocytes purified by AutoMACS and FACSVantage was more than 75% and 95%, respectively. PBMCs were cultured in RPMI1640 containing 10% fetal calf serum at a density of 106/mL with cLPS, MDP, or Toll-like receptor (TLR) ligands. In some experiments, PBMCs were preincubated for 30 minutes with YVAD-fmk (50 μM), CA074-Me (50 μM), or MG-132 (5 μM) before cLPS treatment.
Genetic analysis
Genomic DNA from PBMCs or whole blood was obtained as previously described.12 To analyze the frequency of the CIAS1-mutant allele, exon 3 or the entire coding region of CIAS1 was amplified using high fidelity DNA polymerase KOD plus (Toyobo, Osaka, Japan) and subcloned using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA).12 Genomic DNA and subclones were sequenced with an ABI 3100 sequencer (Applied Biosystems, Foster City, CA).
Real-time quantitative PCR
Total RNA was extracted from 1 to 2 × 105 monocytes using an RNeasy Mini kit (Qiagen, Venlo, Netherlands). After DNase I digestion (Invitrogen) and first-strand cDNA synthesis with Sensiscript RT (Qiagen), the products were subjected to real-time quantitative polymerase chain reaction (PCR) analysis of CIAS1 and 18S rRNA (as an internal control) using an ABI PRISM 7900HT (Applied Biosystems). The following primers (200 nM) and probes (100 nM) were used: CIAS1, forward 5′-GAGCCTCAACAAACGCTACACA-3′, reverse 5′-CTTGCCGATGGCC-AGAAG-3′, probe 5′-FAM-CTGCGTCTCATCAAGGAGCACCGG-TAM-RA-3′; 18s rRNA, forward 5′-AGTCCCTGCCCTTTGTACACA-3′, reverse 5′-GATCCGAGGGCCTCACTAAAC-3′, probe 5′-FAM-CGCCCGTCGCTA-CTACCGATTGG-TAMRA-3′. Expression of CIAS1 was normalized to 18S rRNA; expression is shown relative to CIAS1 expression in the absence of LPS stimulation, which was set equal to one.
Allele-specific PCR
The PCR settings are described in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) and were optimized to detect at least 0.6% of mutant alleles for each mutation. To make dilution series, patient DNA was diluted with DNA from an individual who was proved not to have latent mosaicism of CIAS1 (confirmed by subcloning).
Flow cytometry
PBMCs were incubated with a FITC-conjugated anti–human CD14 mAb (BD), then sorted and analyzed using a FACScalibur System (BD). For intracellular IL-1β staining, cells were fixed, permeabilized, washed with Permfix and Cytoperm (BD), and incubated with a PE-conjugated anti–human IL-1β mAb (BD), at a concentration of 0.5 μg/mL. Cells were also incubated with propidium iodide (PI) or 7-aminoactinomycin D (7-AAD, purchased from BD) to identify nonviable cells. The expression of phosphatidylserine in the external layer of the plasma membrane was evaluated using PE-conjugated annexin-V (BD). In mutation-negative patients (patients 7–11), LPS-stimulated CD14+/PI+ cells and CD14+/PI− cells were sorted using the FACSVantage System.
Cell viability assay
After incubation with or without cLPS, purified monocytes were centrifuged onto glass slides and were subjected to Giemsa staining. Cell viability was determined using a trypan blue exclusion assay.
Plasmids and cell lines
Expression plasmids for CIAS1 and ASC in the pEF-BOS vector background have been described previously.12 CIAS1 mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).12 The ability of each construct to induce NF-κB activation was assessed using a dual luciferase reporter assay in HEK293 cells as previously described.12 cDNAs encoding carboxy-terminal green fluorescent protein (GFP)-tagged CIAS1 and GFP-tagged mutants of CIAS1 were subcloned into pcDNA/5TO (Invitrogen), before being introduced into human monocytic THP-1 cells using the Cell Line Nucleofector Kit V (Amaxa Biosystems, Cologne, Germany). Phorbol myristate acetate (10 ng/mL) was added to improve gene expression and reduce spontaneous cell death.27 Four hours after the introduction of plasmids, cell death in GFP-positive THP-1 cells was measured by flow cytometry.
Statistical analysis
Data are represented as means plus or minus SD. Statistical analysis was performed using the Students t test. P less than .05 was considered to be statistically significant.
Results
Patient profile
To explore whether mutation-negative CAPS patients were misdiagnosed due to CIAS1 mosaicism and the low frequency of the mutant allele, we recruited 11 Japanese CAPS patients. Clinical and genetic information for these patients is described in Table 1. Among them, 6 CINCA syndrome patients (patients 1–6) were confirmed as heterozygous for CIAS1 mutation by ordinary genomic sequencing, and classified as mutation-positive patients, showing a frequency of the mutant allele of approximately 50% (data not shown). Patient 7 was previously diagnosed as a mosaic, in whom the frequency of the CIAS1 mutant allele in whole blood was approximately 12%, reflecting that approximately 24% of blood cells carried the CIAS1 mutation.12 Three CINCA syndrome patients (patients 8–10) and 1 MWS patient (patient 11) had no evidence of overlapping peaks in their sequencing histograms, indicating that there were no point mutations in CIAS1 that would correlate with amino acid substitutions, based on repeated bidirectional genomic sequencing. These patients were categorized as mutation-negative patients.
Spontaneous IL-1β production in monocytes at single-cell level
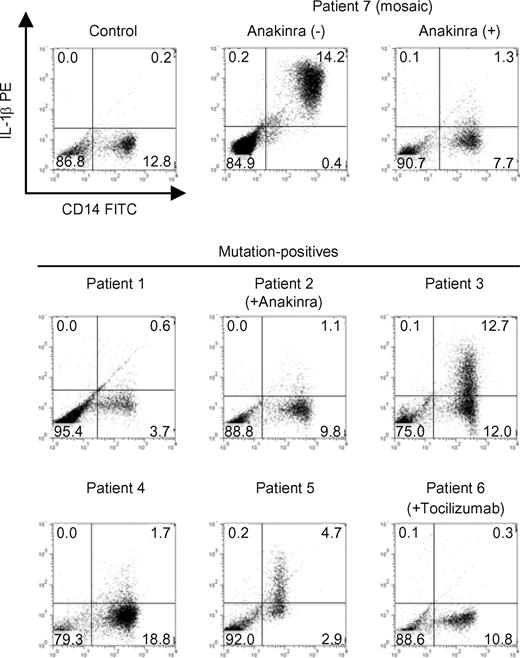
To find possible latent mosaicism in mutation-negative patients, we sought a method which enables mutant and normal cells from the reported mosaic patient to be distinguished at the single cell level. We investigated intracellular IL-1β staining as a candidate technique, because it is generally believed that enhanced production of IL-1β is the underlying molecular cause of CAPS.10,33,–35 To verify this, we first explored in vitro intracellular IL-1β production from CD14-positive peripheral monocytes by flow cytometry. Constitutive IL-1β up-regulation in monocytes was not detected in healthy controls (n = 10), but was observed in the confirmed mosaic patient (patient 7) before starting anakinra treatment (Figure 1). However, we were unable to resolve distinct high-producing (IL-1βhigh) and low-producing (IL-1βlow) subpopulations of CD14-positive monocytes. In addition, while we sorted out relatively higher- and lower-intensity intracellular IL-1β fluorescence-staining populations, the frequency of the CIAS1 mutant allele in each population was almost comparable (data not shown). Furthermore, when patient 7 was treated with anakinra, spontaneous IL-1β up-regulation was canceled; therefore, the status of IL-1β production did not distinguish between monocytes with CIAS1 mutations and those without. Two mutation-positive patients (patient 3 and 5) showed spontaneous up-regulation of intracellular IL-1β in monocytes upon culture of their PBMCs in vitro (Figure 1); this correlated with elevated IL-1β levels in culture supernatant (data not shown). The remaining mutation-positive patients, particularly those who received therapeutic biologics such as anakinra or the anti–IL-6R antibody tocilizumab (patients 2 and 6, respectively), did not show evidence of up-regulation of intracellular IL-1β. The status of intracellular IL-1β therefore seemed to be affected by various factors, such as the treatment applied. Two of the 4 mutation-negative patients (patients 10 and 11, data not shown) also exhibited an up-regulation of intracellular IL-1β. These results show that alterations in intracellular IL-1β levels were not helpful in finding latent mosaicism in mutation-negative patients.
Intracellular IL-1β levels in PBMCs from CAPS patients. PBMCs from healthy controls (n = 10), mutation-positive patients (n = 6), or a mosaic patient (patient 7) were isolated and cultured in vitro for 24 hours. Flow cytometric analysis of CD14 expression and intracellular IL-1β levels was performed. The ratio of CD14+IL-1βhigh monocytes (cells in the right upper quadrant) in healthy controls was 0.26% (± 0.43%; mean ± SD). Data from each of the indicated patients and a representative healthy control are shown. Numbers in each quadrant are the percentages of total cells.
Intracellular IL-1β levels in PBMCs from CAPS patients. PBMCs from healthy controls (n = 10), mutation-positive patients (n = 6), or a mosaic patient (patient 7) were isolated and cultured in vitro for 24 hours. Flow cytometric analysis of CD14 expression and intracellular IL-1β levels was performed. The ratio of CD14+IL-1βhigh monocytes (cells in the right upper quadrant) in healthy controls was 0.26% (± 0.43%; mean ± SD). Data from each of the indicated patients and a representative healthy control are shown. Numbers in each quadrant are the percentages of total cells.
Monocytes from CAPS patients rapidly undergo necrotic cell death when treated with LPS
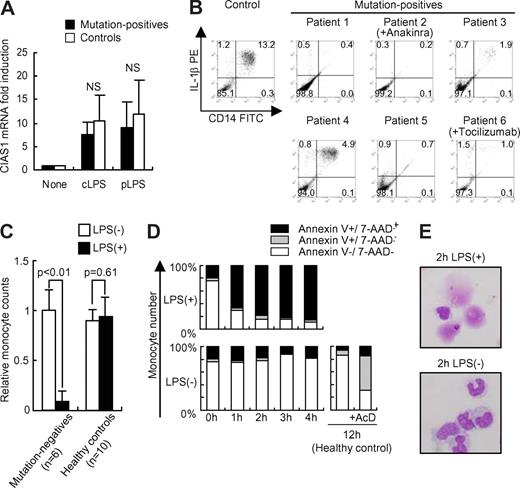
We recently reported that overexpression of disease-associated mutants of CIAS1 caused rapid necrosis-like cell death in a human monocytic cell line, THP-1.27 As CIAS1 is regulated by NF-κB, which is up-regulated by treatment with LPS,36 we next verified whether LPS-induced up-regulation of CIAS1 could induce cell death in CIAS1-mutated monocytes. Quantitative mRNA analysis of CIAS1 in mutation-positive patients and in healthy controls revealed that both cLPS and pLPS markedly enhanced CIAS1 mRNA expression (Figure 2A). When stimulated with cLPS, the number of monocytes from all of the CAPS patients carrying a heterozygous CIAS1 mutation (patients 1–6) decreased significantly, while in healthy controls, it did not (n = 10; Figure 2B,C). Although the magnitude of the decrease in monocyte number varied for each patient, this phenomenon was observed consistently, regardless of the disease severity, the level of spontaneous IL-1β production, or the therapeutic treatment regimen. Monocyte cell number was reduced to equal levels before and after anakinra treatment of patient 4, even though the clinical response to anakinra was excellent (data not shown). These results suggested that monocytes with CIAS1 mutations undergo cell death when CIAS1 expression is induced by LPS. To confirm these results, flow cytometric analysis with PI or 7-AAD staining was performed. As shown in Figure 2D, CD14-positive monocytes from all of the heterozygous CAPS patients rapidly underwent cell death when treated with cLPS, while CD14-negative PBMCs did not (data not shown). When annexin-V staining was also examined, we found no evidence of a population of early apoptotic cells, defined as being annexin-V+/7-AAD− (lower right graph in Figure 2D); rather, cells became annexin-V/7-AAD double-positive directly, implying that they underwent necrosis, not typical apoptosis (Figure 2D). This result was comparable with previous observations using THP-1 cells.27 Giemsa staining of cLPS-stimulated purified monocytes showed evidence of cellular debris from disrupted cells, but failed to detect typical apoptotic morphologic features, such as nuclear condensation or apoptotic bodies, even at early time points (1 or 2 hours) after cLPS stimulation (Figure 2E). Both cLPS and pLPS were equally effective in inducing monocyte death, excluding the possibility that unknown materials in cLPS were responsible for the effect (see Figure 3A). Thus, monocytes isolated from CAPS patients with CIAS1 mutations rapidly underwent a type of cell death consistent with necrosis, rather than apoptosis, when treated with LPS.
Monocytes from mutation-positive patients rapidly undergo cell death when treated with LPS. (A) Real-time quantitative reverse transcription (RT)-PCR analysis of CIAS1 mRNA. Purified monocytes were incubated with cLPS (10 ng/mL) or pLPS (10 ng/mL) for 1 hour, and were then subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression, and represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. NS: statistically not significant. (B) Intracellular IL-1β staining of PBMCs from CAPS patients. PBMCs from mutation-positive patients (n = 6) or healthy controls (n = 10) were incubated with cLPS (10 ng/mL) for 24 hours. Flow cytometric analysis was performed as described for Figure 1. Representative results of each patient, or the control healthy patients, are shown. Numbers in each quadrant are the percentages of total cells. (C) Monocyte cell number decreases upon LPS stimulation. PBMCs were incubated with or without cLPS (10 ng/mL) for 24 hours, as described in Figure 1. Data represent the number of CD14-positive cells in 10 000 PBMCs, compared with the preincubation state, and represent the means (± SD) of the indicated number of patients. (D) PBMCs from mutation-positive patients were stained with FITC-conjugated anti-CD14, PE-conjugated anti–annexin V, and 7-AAD. Samples were analyzed by flow cytometry, gated to select CD14-positive cells, and then analyzed for cell viability. Data from normal PBMCs that were treated with actinomycin D (AcD, 1 μg/mL) for 12 hours were used as a positive control for apoptotis. Representative data from 5 mutation-positive patients are shown. (E) Giemsa staining of monocytes from mutation-positive patients incubated for 2 hours with or without cLPS. Cells were observed by inverted microscopy using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a 40×/0.85 NA objective lens, an Olympus DP70 camera, and DP-controller version 1.1 software (Olympus). Data are representative of 3 mutation-positive patients.
Monocytes from mutation-positive patients rapidly undergo cell death when treated with LPS. (A) Real-time quantitative reverse transcription (RT)-PCR analysis of CIAS1 mRNA. Purified monocytes were incubated with cLPS (10 ng/mL) or pLPS (10 ng/mL) for 1 hour, and were then subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression, and represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. NS: statistically not significant. (B) Intracellular IL-1β staining of PBMCs from CAPS patients. PBMCs from mutation-positive patients (n = 6) or healthy controls (n = 10) were incubated with cLPS (10 ng/mL) for 24 hours. Flow cytometric analysis was performed as described for Figure 1. Representative results of each patient, or the control healthy patients, are shown. Numbers in each quadrant are the percentages of total cells. (C) Monocyte cell number decreases upon LPS stimulation. PBMCs were incubated with or without cLPS (10 ng/mL) for 24 hours, as described in Figure 1. Data represent the number of CD14-positive cells in 10 000 PBMCs, compared with the preincubation state, and represent the means (± SD) of the indicated number of patients. (D) PBMCs from mutation-positive patients were stained with FITC-conjugated anti-CD14, PE-conjugated anti–annexin V, and 7-AAD. Samples were analyzed by flow cytometry, gated to select CD14-positive cells, and then analyzed for cell viability. Data from normal PBMCs that were treated with actinomycin D (AcD, 1 μg/mL) for 12 hours were used as a positive control for apoptotis. Representative data from 5 mutation-positive patients are shown. (E) Giemsa staining of monocytes from mutation-positive patients incubated for 2 hours with or without cLPS. Cells were observed by inverted microscopy using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a 40×/0.85 NA objective lens, an Olympus DP70 camera, and DP-controller version 1.1 software (Olympus). Data are representative of 3 mutation-positive patients.
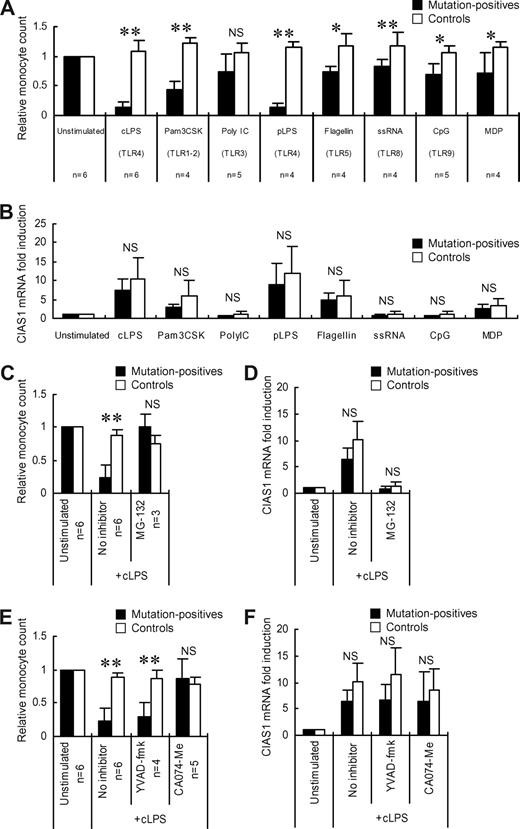
Effects of TLR ligands and various inhibitors on monocyte cell death. (A) PBMCs from mutation-positive patients or healthy controls were incubated for 24 hours with cLPS (10 ng/mL), MDP (5 μg/mL), Pam3CSK (10 μg/mL), Poly I:C (25 μg/mL), pLPS (10 ng/mL), recombinant flagellin (100 ng/mL), single-stranded RNA (ssRNA, 2 μg/mL), or CpG DNA (1 μM). The number of CD14+ cells in 10 000 PBMCs was determined; the ratio of CD14+ cells in stimulated PBMCs to those in unstimulated PBMCs is presented. Data represent the means (± SD) of the indicated number of patients or healthy controls (n = 5). (B) Real-time quantitative RT-PCR analysis of CIAS1 mRNA. Purified monocytes were stimulated with TLR ligands or MDP, as described for (A), for 1 hour, and were then subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression, and represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. (C, E) PBMCs from the indicated number of mutation-positive patients or healthy controls (n = 4) were incubated with or without MG-132 (5 μM), YVAD-fmk (50 μM), or CA074-Me (50 μM) for 30 minutes before the addition of cLPS. The cells were then incubated with or without cLPS (10 ng/mL) for 4 hours. The ratio of CD14+ and 7-AAD− cells in 10 000 PBMCs relative to untreated cells is shown; data represent the means (± SD) (D, F) Real-time quantitative RT-PCR analysis of CIAS1 mRNA. Purified monocytes were preincubated with the indicated inhibitors for 30 minutes, as described for (C and E), and were then stimulated with cLPS (10 ng/mL) for 1 hour. Thereafter, cells were collected and subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression. Values represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. *P less than .05, **P less than .01, NS: not significant, compared with healthy controls by Student t test.
Effects of TLR ligands and various inhibitors on monocyte cell death. (A) PBMCs from mutation-positive patients or healthy controls were incubated for 24 hours with cLPS (10 ng/mL), MDP (5 μg/mL), Pam3CSK (10 μg/mL), Poly I:C (25 μg/mL), pLPS (10 ng/mL), recombinant flagellin (100 ng/mL), single-stranded RNA (ssRNA, 2 μg/mL), or CpG DNA (1 μM). The number of CD14+ cells in 10 000 PBMCs was determined; the ratio of CD14+ cells in stimulated PBMCs to those in unstimulated PBMCs is presented. Data represent the means (± SD) of the indicated number of patients or healthy controls (n = 5). (B) Real-time quantitative RT-PCR analysis of CIAS1 mRNA. Purified monocytes were stimulated with TLR ligands or MDP, as described for (A), for 1 hour, and were then subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression, and represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. (C, E) PBMCs from the indicated number of mutation-positive patients or healthy controls (n = 4) were incubated with or without MG-132 (5 μM), YVAD-fmk (50 μM), or CA074-Me (50 μM) for 30 minutes before the addition of cLPS. The cells were then incubated with or without cLPS (10 ng/mL) for 4 hours. The ratio of CD14+ and 7-AAD− cells in 10 000 PBMCs relative to untreated cells is shown; data represent the means (± SD) (D, F) Real-time quantitative RT-PCR analysis of CIAS1 mRNA. Purified monocytes were preincubated with the indicated inhibitors for 30 minutes, as described for (C and E), and were then stimulated with cLPS (10 ng/mL) for 1 hour. Thereafter, cells were collected and subjected to quantitative real-time RT-PCR analysis. Data were normalized to 18S rRNA expression. Values represent the means (± SD) of 4 mutation-positive patients or 7 healthy controls. *P less than .05, **P less than .01, NS: not significant, compared with healthy controls by Student t test.
Effect of TLR ligands on CIAS1 mutant monocytes
LPS is a ligand of TLR4,37,–39 which is constitutively expressed on monocytes.40 Because monocytes express several types of TLR,40 we investigated whether other TLR ligands were also able to induce cell death in CIAS1-mutant monocytes. As shown in Figure 3A, while several TLR ligands mildly or moderately decreased the number of monocytes from mutation-positive patients, none of them were as potent as LPS. MDP, a bacterial compound that was previously reported to be an activator of the cryopyrin inflammasome,41 also mildly decreased monocyte number. Because some TLRs, such as TLR3 (receptor for poly I:C) and TLR9 (receptor for CpG DNA) are not typically expressed on monocytes,40 modification of the cytokine milieu by other peripheral blood cells may have partly affected the fate of the mutant monocytes. Quantitative mRNA analysis of CIAS1 in mutation-positive patients and in the healthy controls revealed that the effects of Pam3CSK and flagellin on CIAS1 expression were relatively mild, and the effects of poly I:C, single-stranded RNA, and CpG DNA were negligible (Figure 3B), consistent with the finding that LPS is the strongest inducer of cell death of CIAS1 mutant monocytes. Furthermore, to inhibit the LPS-induced up-regulation of CIAS1 we pretreated monocytes with MG-132, a broadly effective proteasome inhibitor also known as a NF-κB inhibitor. The effect of LPS on monocyte death (Figure 3C) and CIAS1 mRNA induction (Figure 3D) was completely abrogated. Although the possibility that MG-132 directly inhibits monocyte death cannot be excluded, these results suggested that the effect of LPS and other TLR ligands in inducing monocyte cell death is associated with their ability to enhance CIAS1 expression.
LPS-induced death of monocytes with CIAS1 mutations is cathepsin B–dependent
Caspase-1 is a key downstream molecule of cryopyrin in IL-1β processing, and forms part of the inflammasome, along with cryopyrin and ASC.23,42 We recently reported that the rapid necrosis of THP-1 cells induced by CIAS1 disease-associated mutants was not mediated by a caspase-1-dependent pathway, but occurred in a lysosomal cathepsin B–dependent manner.27 To explore the contribution of caspase-1 or cathepsin B in the LPS-induced cell death of CIAS1-mutant human primary monocytes, we pretreated PBMCs from mutation-positive patients with a caspase-1 inhibitor, YVAD-fmk, or a cathepsin B-specific inhibitor, CA074-Me (Figure 3E). YVAD-fmk was not effective in preventing monocyte cell death, even at a concentration which substantially suppressed LPS-induced IL-1β production (Figure S1). In contrast, CA074-Me effectively protected mutation-positive monocytes from LPS-induced cell death. Neither YVAD-fmk nor CA074-Me affected the up-regulation of CIAS1 mRNA by LPS (Figure 3F), indicating that the effect of CA074-Me was not at the level of CIAS1 expression, but that it inhibited a posttranscriptional pathway mediated by the mutant form of cryopyrin. Taken together, our findings implied a novel function of mutated CIAS1 in inducing monocyte death in a cathepsin B-dependent, caspase-1–independent manner. The results also suggested that LPS-induced death of monocytes carrying disease-associated mutations of CIAS1 is mediated by a similar mechanism to that reported for the cell death of human monocytic THP-1 cells induced by overexpression of mutated CIAS1.
Selective induction of cell death of CIAS1-mutated monocytes
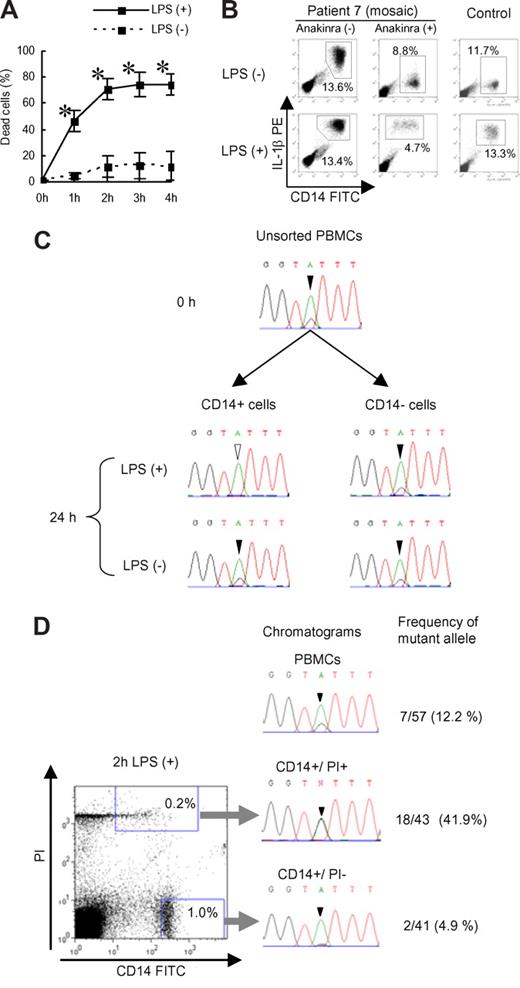
To confirm that the cell death of CIAS1 mutant monocytes was due to an intrinsic mechanism, rather than the result of modifications of the inflammatory milieu, we purified monocytes using magnetic sorting, stimulated them with LPS, and performed a trypan blue exclusion assay. We observed rapid cell death of purified monocytes upon cLPS stimulation, indicating that the monocytic death observed in the previous experiments was not mediated by other cells (Figure 4A). Giemsa staining of the purified monocytes also revealed features of necrosis-like cell death, as seen previously (Figure 2E).
Selective elimination of mutated monocytes from mutation-positive and mosaic patients. (A) Trypan blue exclusion assay of purified monocytes. Purified monocytes were incubated with or without cLPS (10 ng/mL) and dead cells were counted. Values represent the means (± SD) of 3 mutation-positive patients. *P less than .01 compared with LPS (-) counterparts. (B) Representative intracellular IL-1β staining of PBMCs from patient 7 or healthy controls after incubation with or without cLPS (10 ng/mL) for 24 hours. Numbers in each rectangle are the percentages of total cells. (C) PBMCs from patient 7 were cultured with or without cLPS (10 ng/mL) for 24 hours. CD14-positive and -negative cells were sorted, and DNA was extracted and sequenced for analysis of CIAS1. Chromatograms of the CIAS1 gene at nucleotide position 1709 (black or white arrowhead) from each population of cells are shown. Note that the overlapping “G” peak (black arrowhead) disappeared from LPS-treated CD14-positive cells. The data are representative of 3 independent experiments. (D) CIAS1-mutated monocytes from a mosaic patient were enriched in the dying cell population. Left panel: flow cytometry data of PBMCs from patient 7 stimulated with cLPS for 2 hours. Right panel: chromatograms of the CIAS1 gene at position 1709 (arrowhead), and frequency of the mutant allele as determined by subcloning from each population. The data are representative of 3 independent experiments.
Selective elimination of mutated monocytes from mutation-positive and mosaic patients. (A) Trypan blue exclusion assay of purified monocytes. Purified monocytes were incubated with or without cLPS (10 ng/mL) and dead cells were counted. Values represent the means (± SD) of 3 mutation-positive patients. *P less than .01 compared with LPS (-) counterparts. (B) Representative intracellular IL-1β staining of PBMCs from patient 7 or healthy controls after incubation with or without cLPS (10 ng/mL) for 24 hours. Numbers in each rectangle are the percentages of total cells. (C) PBMCs from patient 7 were cultured with or without cLPS (10 ng/mL) for 24 hours. CD14-positive and -negative cells were sorted, and DNA was extracted and sequenced for analysis of CIAS1. Chromatograms of the CIAS1 gene at nucleotide position 1709 (black or white arrowhead) from each population of cells are shown. Note that the overlapping “G” peak (black arrowhead) disappeared from LPS-treated CD14-positive cells. The data are representative of 3 independent experiments. (D) CIAS1-mutated monocytes from a mosaic patient were enriched in the dying cell population. Left panel: flow cytometry data of PBMCs from patient 7 stimulated with cLPS for 2 hours. Right panel: chromatograms of the CIAS1 gene at position 1709 (arrowhead), and frequency of the mutant allele as determined by subcloning from each population. The data are representative of 3 independent experiments.
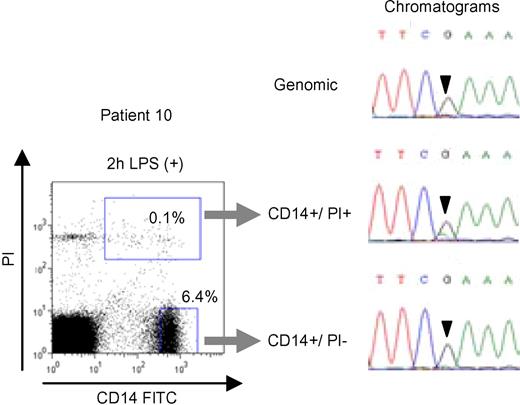
Because patient 7 had mutant and nonmutant cells in the same milieu due to confirmed CIAS1 mosaicism, we were interested in whether LPS-induced cell death was selective for monocytes carrying the CIAS1 mutation. The percentage of CD14-positive monocytes from patient 7 tended to decrease slightly upon cLPS stimulation, but the cell survival profile was more similar to normal controls (Figure 4B). When the DNA of the monocytes from patient 7 was extracted and sequenced, there was an overlapping, small peak on the sequencing chromatogram in unstimulated PBMCs, indicating a mutation of the guanine (G) nucleotide at position 1709 (Figure 4C). In monocytes stimulated with cLPS, the histogram peak corresponding to the mutated G disappeared, while in unstimulated monocytes, or cLPS-stimulated, CD14-negative PBMCs, it did not (Figure 4C). These results suggested that cell death was induced selectively in CIAS1-mutated monocytes. When we sorted CD14-positive/PI-negative cells and CD14-positive/PI-positive cells from cLPS-stimulated PBMCs from patient 7 (Figure 4D) and performed genomic DNA sequencing of each cell population, we found that CIAS1-mutated cells were enriched in the PI-positive population, and eliminated from the PI-negative population. Subcloning-based frequency analysis revealed successful enrichment of the mutant G allele, from a frequency of 12.2% in PBMCs to 41.9% in the PI-positive population of dying monocytes, correlating with an enrichment of mutated cells from approximately 25% to more than 80%. The frequency of the mutant allele in PI-negative monocytes that were not undergoing cell death significantly decreased, to 4.9%. These results suggested that cell death was induced exclusively in CIAS1-mutated monocytes, and not in normal cells, even though both cell types were exposed to the same extracellular milieu.
Identification of mosaicism in 3 of 4 CIAS1 mutation-negative CINCA patients
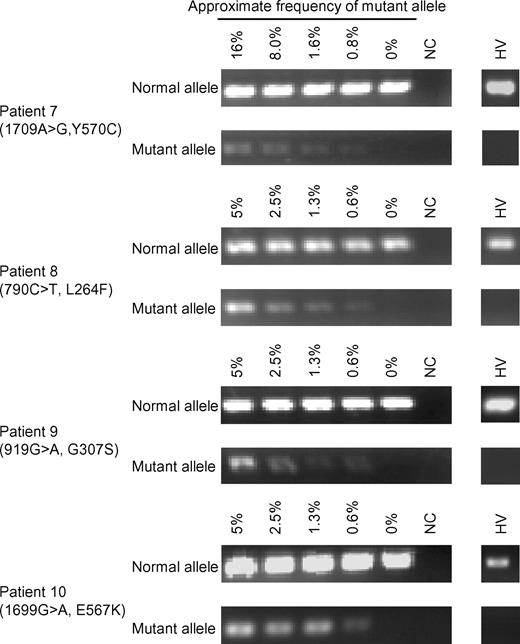
Based on the results from patient 7, we set out to identify CIAS1 mosaicism in the remaining mutation-negative patients by enriching for PI+, dying monocytes after cLPS stimulation. We stimulated PBMCs from patients 8–11 with cLPS, and while the decrease in monocyte cell number was comparable with normal controls (data not shown), we were able to sort dying (PI+) from viable (PI−) monocytes. Subsequent sequencing of the population of monocytes undergoing cell death revealed an overlapping peak on the sequencing chromatogram, indicating mosaicism, in 3 of 4 patients. The nucleotide substitutions were as follows (parentheses indicate the corresponding amino acid change): 790C > T (L264F) in patient 8; 919G > A (G307S) in patient 9; and 1699G > A (E567K) in patient 10 (Figure 5 and Figure S2). Overlapping peaks were not obvious in either chromatogram from unstimulated PBMCs or cLPS-stimulated, PI-negative monocytes. Subcloning analysis of genomic DNA from whole blood revealed that the mutant allele was enriched from 4.3% (2/47) to 19.4% (7/36) in patient 8, 4.3% (2/47) to 11.1% (3/27) in patient 9, and 6.5% (3/46) to 15.2% (7/46) in patient 10 (Table 2). The mutations and corresponding amino acid changes in patients 8, 9, and 10 have not been previously reported as either mutations or SNPs, and were not observed among 100 healthy Japanese donors (data not shown). We confirmed the existence of latent mosaicism in these patients by allele-specific PCR, which can detect mutant alleles at a frequency of 0.6% (Figure 6). We also analyzed 100 healthy controls to exclude the possibility of latent mosaicism among this population, and found no evidence of mutant CIAS1 alleles (Figure 6 and data not shown). Thus, by selectively inducing cell death with LPS, we successfully diagnosed 3 CAPS patients who had been designated mutation-negative by conventional sequencing. Patient 11 had a phenotype that was marked by severe mental and developmental retardation. When we stimulated PBMCs from patient 11 with cLPS, there was very little cell death of monocytes, and we could not resolve overlapping peaks on the sequencing chromatogram of the population of dying monocytes (data not shown). We generated at least 54 subclones of the entire CIAS1 coding region from patient 11, and confirmed that this individual did not carry any mutations, even as a mosaicism of CIAS1.
Enrichment of CIAS1-mutated monocytes from a CIAS1 mutation-negative patient. Flow cytometry analysis of PBMCs from patient 10 stimulated with cLPS for 2 hours (left panel), and chromatograms of the CIAS1 gene at position 1699 from each of the populations of cells (right panel). Numbers in each rectangle are the percentages of total cells.
Enrichment of CIAS1-mutated monocytes from a CIAS1 mutation-negative patient. Flow cytometry analysis of PBMCs from patient 10 stimulated with cLPS for 2 hours (left panel), and chromatograms of the CIAS1 gene at position 1699 from each of the populations of cells (right panel). Numbers in each rectangle are the percentages of total cells.
Frequency of mutant alleles detected in mutation-negative patients
| . | Patient 8 . | Patient 9 . | Patient 10 . |
|---|---|---|---|
| Site of mutation | 790C>T (L264F) | 919G>A (G307S) | 1699G>A (E567K) |
| Frequency of mutant allele | |||
| Whole blood | 2/47 (4.3%) | 2/47 (4.3%) | 3/46 (6.5%) |
| CD14+/PI+ | 7/36 (19.4%) | 3/27 (11.1%) | 7/46 (15.2%) |
| CD14+/PI− | 2/46 (4.3%) | 1/38 (2.6%) | 3/48 (6.3%) |
| . | Patient 8 . | Patient 9 . | Patient 10 . |
|---|---|---|---|
| Site of mutation | 790C>T (L264F) | 919G>A (G307S) | 1699G>A (E567K) |
| Frequency of mutant allele | |||
| Whole blood | 2/47 (4.3%) | 2/47 (4.3%) | 3/46 (6.5%) |
| CD14+/PI+ | 7/36 (19.4%) | 3/27 (11.1%) | 7/46 (15.2%) |
| CD14+/PI− | 2/46 (4.3%) | 1/38 (2.6%) | 3/48 (6.3%) |
Allele-specific PCR for mutant alleles detected in mosaic patients. PCR was performed with mutant or normal allele-specific primers and the corresponding reverse primer (see Table S1). Dilution series were made by mixing patients' DNA and DNA from an individual who was proved not to have latent mosaicism of CIAS1. Representative results of mosaic patients and 100 healthy volunteers (HV) are shown. NC indicates negative control.
Allele-specific PCR for mutant alleles detected in mosaic patients. PCR was performed with mutant or normal allele-specific primers and the corresponding reverse primer (see Table S1). Dilution series were made by mixing patients' DNA and DNA from an individual who was proved not to have latent mosaicism of CIAS1. Representative results of mosaic patients and 100 healthy volunteers (HV) are shown. NC indicates negative control.
Disease-associated mutant CIAS1 induces cell death on THP-1 and spontaneous NF-κB activation
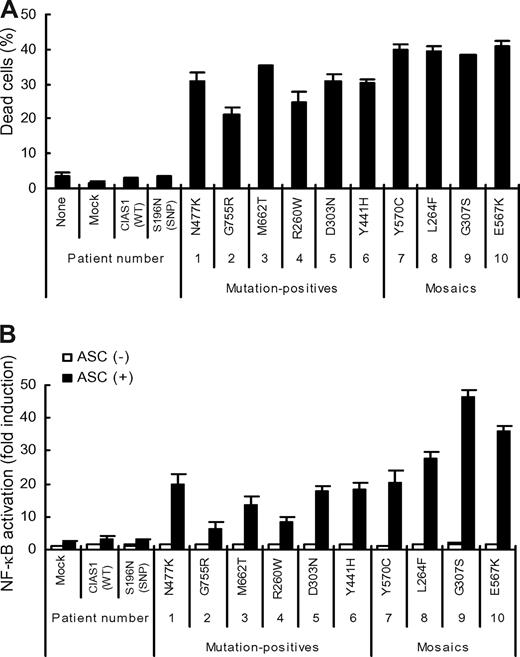
To evaluate whether the newly identified CIAS1 mutations of this study were relevant to disease manifestation, we examined their ability to rapidly induce necrotic cell death when transiently expressed in human monocytic THP-1 cells (Figure 7A). The mutants identified in the mosaics (patients 7–10, Y570C, L264F, G307S, and E567K) had more potent effects than those of the mutation-positive individuals. Transient coexpression of disease-associated CIAS1 mutants and ASC in HEK293FT cells also caused enhanced NF-κB reporter activity, compared with the expression of wild-type CIAS1, or CIAS1-SNP S196N, a disease-unrelated CIAS1 variant (Figure 7B).12 Note that NF-κB activation induced by the mutants identified in mosaic patients was higher than that induced by the mutants identified in mutation-positive patients (patient 1–6; Figure 7B). These findings provided additional evidence that mutations of CIAS1 that are found in both mutation-positive and mosaic patients are functional and related to the development of disease-related symptoms in CAPS patients.
Effect of CIAS1 mutation on the induction of cell death and ASC-dependent NF-κB activation. (A) 106 THP-1 cells were transfected with 0.5 μg of an expression vector for GFP-tagged CIAS1 wild-type (WT), CIAS1 SNP S196N, or one of the disease-associated mutants of CIAS1 (R260W, L264F, D303N, G307S, Y441H, N477K, E567K, Y570C, M662T, and G755R), and incubated with PMA (10 ng/mL) for 4 hours. The percentage of dead cells (7-AAD-positive) among the population of GFP-positive cells is shown. Data represent the means (± SD) of triplicate determinations, and are representative of 3 independent experiments. (B) HEK293FT cells were transfected with 16 ng of an expression vector for CIAS1, or one of its mutants, in the presence or absence of 16 ng of an expression vector for ASC. The induction of NF-κB is shown as fold-change compared with cells that were transfected with a control vector without ASC (set equal to one). Values are the means (± SD) of triplicate determinations, and data are representative of 2 independent experiments.
Effect of CIAS1 mutation on the induction of cell death and ASC-dependent NF-κB activation. (A) 106 THP-1 cells were transfected with 0.5 μg of an expression vector for GFP-tagged CIAS1 wild-type (WT), CIAS1 SNP S196N, or one of the disease-associated mutants of CIAS1 (R260W, L264F, D303N, G307S, Y441H, N477K, E567K, Y570C, M662T, and G755R), and incubated with PMA (10 ng/mL) for 4 hours. The percentage of dead cells (7-AAD-positive) among the population of GFP-positive cells is shown. Data represent the means (± SD) of triplicate determinations, and are representative of 3 independent experiments. (B) HEK293FT cells were transfected with 16 ng of an expression vector for CIAS1, or one of its mutants, in the presence or absence of 16 ng of an expression vector for ASC. The induction of NF-κB is shown as fold-change compared with cells that were transfected with a control vector without ASC (set equal to one). Values are the means (± SD) of triplicate determinations, and data are representative of 2 independent experiments.
Discussion
In the current study, we demonstrated that monocytes carrying disease-associated CIAS1 mutations rapidly undergo cell death upon induction of CIAS1 expression by LPS treatment. Selective induction of monocyte cell death was independent of the disease severity, level of IL-1β production, or therapeutic regimen. Subcloning and analysis of transient expression in cell lines indicated that the ability to induce cell death was specific to disease-associated mutations in CIAS1. Consequently, we were able to use this distinct biologic characteristic to enrich for monocytes carrying CIAS1 mutations. We performed genetic analysis on 4 mutation-negative CAPS patients and found CIAS1 mosaicism in 3 patients, while in the fourth we were able to exclude the presence of a mutation in the CIAS1 coding region. These findings suggest that a majority of CIAS1 mutation-negative patients have disease-associated mutations of CIAS1 as a latent, low-level mosaicism.
Our results indicated that a small number of monocytes carrying CIAS1 mutations are sufficient to evoke systemic inflammation in CIAS1 mosaic patients. The question then arises of how such a small number of mutant cells cause the severe inflammatory responses observed in CAPS patients. As shown in Figure 1, we could not distinguish between CIAS1-mutated and nonmutated cells by intracellular IL-1β staining of PBMCs in patient 7 (mosaic patient); both mutated and nonmutated monocytes appeared to have similar levels of IL-1β. In patients who were treated with the IL-1 receptor antagonist anakinra, not only was IL-1β signaling blocked, but IL-1β production in peripheral blood monocytes was also dramatically reduced (Figure 1). This finding indicates that modification of the cytokine milieu due to the production of IL-1β by CIAS1-mutated cells, which possess constitutive IL-1β producing activity, may cause up-regulation of IL-1β in nonmutated monocytes, thereby leading to a systemic inflammatory condition. In support of this hypothesis, the addition of a neutralizing anti–IL-1β antibody to cultures of PBMCs from patient 7 reduced the levels of intracellular IL-1β, and addition of exogenous IL-1β to control, nonmutated monocytes induced an up-regulation in intracellular IL-1β (data not shown). In addition, we used transient transfection experiments to demonstrate that all of the CIAS1 mosaic mutants (Y570C, L264F, G307S, and E567K) have the potential to induce higher NF-κB activity compared with wild-type CIAS1 (Figure 7); thus, in patients in vivo, these mutations could be highly active, and sufficient to evoke severe systemic inflammation, even when present as a low-level mosaicism.
The clinical symptoms of mosaic patients appear to be milder than those of heterozygous patients. Patient 7 carried a CIAS1-Y570C mutation, which is one of the most common CIAS1 mutations, and is associated with a very severe phenotype including mental retardation and epilepsy.3,5,43 Patient 7 appeared to have milder symptoms than other reported heterozygous patients carrying the same mutation of CIAS1, showing neither mental retardation nor epilepsy, even at 15 years of age. Similarly, patient 8, who had the CIAS1-L264F mutation as mosaicism, exhibited a milder phenotype than patients with the heterozygous L264F mutation.11 Although the CIAS1-G307S mutation in patient 9 has not yet been reported, the symptoms of the patient also seemed to be milder than that of a patient reported to have the G307V mutation.44 The relatively mild phenotypes in mosaic patients, despite the relatively potent effects of their mutations on cellular activity (Figure 7), may be attributable to the lower dose of active mutation. Further study with more CAPS patients with CIAS1 mosaicism and more accurate measurements of mosaicism frequency by real-time PCR could provide clearer view of the correlation between the frequency of mutant allele and disease severity.
The mechanism of LPS-induced monocyte death that we observed remains to be elucidated. When monocytes are treated with LPS, CIAS1 mRNA is induced immediately, and its encoded protein cryopyrin can be detected within 30 to 60 minutes of treatment.36 One simple possibility is that the accumulation of LPS-induced mutant cryopyrin in the cytosol mediates necrosis. This is supported by our recent observation that overexpression of a disease-associated mutant of CIAS1 in THP-1 cells resulted in rapid necrosis-like cell death in a cathepsin B–dependent manner.27 The caspase-1 inhibitor YVAD-fmk failed to inhibit LPS-induced monocyte cell death, while it effectively suppressed LPS-induced IL-1β production. Nigericin, a potassium ionophore, induces not only caspase-1–dependent IL-1β/IL-18 release but also rapid necrosis in LPS-primed THP-1 cells.45 Interestingly, as we observed, the cathepsin B inhibitor CA074-Me inhibited the nigericin-induced necrosis while the caspase-1 inhibitor YVAD-cmk did not.45 This indicates that a common pathway inducing cathepsin B-dependent necrosis in monocytes exists. Cross-talk between the LPS-TLR4 signaling pathway and the cryopyrin inflammasome to cause monocyte cell death is another possibility.
It was recently reported that the cytoplasmic receptor Ipaf recognizes bacterial flagellin, and induces rapid necrosis of Salmonella-infected macrophages.46,47 It has been proposed that cryopyrin functions as a pattern-recognition receptor48,–50 that mediates inflammation; thus, it is possible that cryopyrin-induced rapid necrotic cell death and subsequent release of various cellular components facilitates local inflammation and prevents intracellular bacterial proliferation. Additional experiments are needed to clarify the mechanism of monocyte cell death observed in CIAS1-mutant cells in response to LPS.
While LPS-induced monocyte cell death seemed to be a specific property of CIAS1 disease-associated mutant cells, the clinical and physiologic relevance of this biologic activity is unknown. The primary etiology of CAPS is considered to be excessive IL-1β production by constitutively activated inflammasomes. Although this hypothesis is supported by the fact that the autoinflammatory symptoms of the syndrome are successfully treated with IL-1β–targeted therapy,10,29,33,–35 it remains unclear whether the unique articular and cartilage manifestations of CAPS can also be attributed to IL-1β overproduction. Histologic analysis of the growth cartilage of CINCA syndrome patients revealed necrosis and disorganized proliferation of chondrocytes, and focal calcification, while infiltration of inflammatory cells was not described.51 Feldmann et al speculated that the characteristic growth cartilage burst and epiphyseal overgrowth observed among CINCA patients might be due to dysregulated apoptosis of chondrocytes, which express a high amount of CIAS1.4 One possibility is that certain stimuli, probably other than LPS, cause destructive necrosis of chondrocytes, rather than apoptosis, resulting in a loss of regularity of growth cartilage and subsequent bizarre joint destruction. We observed that LPS induces monocyte cell death independently of anti-IL-1β therapy status; thus, careful observation of anakinra-treated patients will provide a more precise understanding of the involvement of mutant CIAS1-mediated cell death in CAPS symptoms.
While the strategy we used in the current study was good at detecting single nucleotide substitutions, we cannot exclude the possibility that there were other types of genetic abnormalities present that were not detected, such as mis-splicing and noncoding region mutations. However, it is also possible that mosaicism, with an unequal distribution of mutant cells, is prominent in nonhematopoietic cells or tissues, such as the skin or central nervous system. An analysis of nonhematopoietic tissues may therefore be necessary before concluding that the CIAS1 mutation is not responsible for the disease symptoms. Importantly, because diagnosis of CAPS is primarily based on clinical symptoms, a reassessment of the patients' histories and a physical re-evaluation is necessary before reestablishing the disease entity of CIAS1-unrelated patients.
In summary, we found that monocytes bearing mutations in CIAS1 rapidly undergo necrosis-like cell death when treated with LPS, enabling us to diagnose CIAS1 mutation-negative patients as CIAS1 mosaic patients. Our investigation revealed that for a majority of CAPS patients without detectable CIAS1 mutations by ordinary genomic sequencing, disease development may be attributable to low-level mosaicism. Not all CIAS1 mutation-negative patients have CIAS1 mosaicism, presenting the opportunity to uncover genes other than CIAS1 as causative genes for CAPS. Our findings also raise the possibility that low-level mosaicism in other hereditary autoinflammatory syndromes may play a role in disease development, in the absence of detectable gene mutations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank our CAPS patients and their parents for their participation. We thank Dr W. Strober (National Institutes of Health, Bethesda, MD) for critical reading of the manuscript and suggestions, and Dr S. Teramukai (Kyoto University, Kyoto, Japan) for advice on statistical analysis.
This study was supported in part by the Morinaga Hoshi-Kai; the Sapporo Bioscience Foundation; Ministry of Education, Science, Sports, and Culture; and the Ministry of Health, Labor, and Welfare, Japan.
National Institutes of Health
Authorship
Contribution: M.S. performed research and wrote the paper. R.N. and N.K. designed the research, wrote the paper, and analyzed data. A.F. and H. Tanizaki performed research. K.T., T. Imagawa, T. Iehara, H. Takada, T.M., H. Tanaka, H.K., K.K., and S.K. treated the patients and analyzed data. I.O. and T.Y. performed research and discussed results. S.A. wrote the paper and discussed results. T.H., Y.M., and T.N designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryuta Nishikomori, MD, PhD, Department of Pediatrics, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: rnishiko@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal