B-non-Hodgkin lymphomas (B-NHLs) use a raft-associated signalosome made of the constitutively active Lyn kinase, the tyrosine phosphorylated Cbp/PAG adaptor, and tyrosine phosphorylated STAT3 transcription factor. No such “signalosome” is found in rafts of ALK+ T lymphoma and Hodgkin-derived cell lines, despite similar Cbp/PAG, Lyn, and STAT3 expression and similar amounts of raft sphingolipids. Stable association of the signalosome with B-NHL rafts requires (1) a Lyn kinase (auto)phosphorylated in its regulatory and active site tyrosines, (2) a Cbp/PAG adaptor phosphorylated at tyrosine 317 and bound to Lyn SH2 via phosphotyrosine 299 and neighboring residues, and (3) a tyrosine phosphorylated STAT3 linked via SH2 to the regulatory, C-terminal tyrosine of Lyn. No Csk appears to be part of this B-NHL signalosome. An oncogenic role for Lyn was shown after exposure of B-NHL lines to Lyn inhibitors that prevented Lyn and Cbp/PAG phosphorylation, dissociated the signalosome from rafts, and eventually induced death. Cell death followed decreases in Lyn or Cbp/PAG expression levels in one mantle cell lymphoma line, but not in a Hodgkin-derived one. The Lyn-Cbp/PAG signalosome appears to control proliferation and survival in most B-NHLs and constitutes a therapeutic target in B-NHL cells that exhibit oncogenic “addiction” to the Lyn kinase.
Introduction
Human lymphomas develop mainly from B lymphocytes after chromosomal translocations involving the IgH promoter and an oncogene,1,2 but the type-specific molecular basis for lymphoma cell proliferation remains largely unknown.1 It was recently suggested that, in B-cell lymphomas, CD40 and its ligand CD154 were coexpressed and associated in a raft-based signalosome leading to constitutive expression of NF-κB.3 Considering that the Lyn Src-family kinase and the Cbp/PAG adaptor are the major phosphotyrosylated proteins of lymphoma rafts,4,5 and therefore components of a proximal signaling platform,6 we set out to investigate their membrane organization and oncogenic potential in a panel of human B/T non-Hodgkin and Hodgkin lymphoma cell lines and tissues.
In normal lymphocytes, the Csk-binding protein (Cbp)/phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) adaptor7,–9 is a major phosphotyrosylated protein involved in the negative regulation of signaling in murine T lymphocytes10,,–13 and mast cells.14 Cbp/PAG (hereafter PAG), phosphorylated at tyrosine 317, was shown to bind Csk15 and allow phosphorylation of the Src-kinase C-terminal regulatory tyrosine and inactivation of the Src kinase.16,17 PAG is tyrosine phosphorylated in resting T lymphocytes and rapidly dephosphorylated upon activation.10,11,16,17 Likewise, tyrosine phosphorylated PAG associated with Csk was shown to inhibit bovine B lymphocyte proliferation.18 In contrast, several human B–non-Hodgkin lymphoma (B-NHL) cell lines proliferate with a tyrosine phosphorylated PAG adaptor,4 suggesting that, in lymphoma cells, this form of PAG rather promotes proliferation.
PAG is a 432-amino acid protein localized in sphingolipid-enriched membrane microdomains.19 The cytoplasmic portion of the PAG single transmembrane protein contains 10 phosphorylatable tyrosines and 2 proline-rich domains (residues 131–138 and 257–263), to potentially interact with SH2 and SH3 domains, respectively. Palmitoylation facilitates PAG association with rafts8 and contributes to the formation of a “lipid shell” around PAG in sphingolipid and cholesterol-rich domains.20
We investigated how PAG, Lyn, and Csk interact within membrane microdomains in B-NHLs (Burkitt, follicular, mantle cell, and diffuse large B-cell lymphomas of both germinal center– and activated B cell–type), anaplastic large cell T lymphomas (ALCLs) expressing the nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) fusion protein (ALK+ lymphomas) and Hodgkin lymphoma-derived cell lines and tissues. The PAG protein was expressed in all those cells, but only in B-NHL lines did the phosphorylated PAG protein associate with sphingolipid-enriched membrane domains (or rafts) and strongly interact with Lyn. The phosphorylated signal transducer and activator of transcription 3 (STAT3) was also part of the Lyn/PAG raft complex in B-NHL lines and tissues and may modulate gene expression under the control of the Lyn/PAG signalosome.
Methods
This study was approved by the review boards of each participating institution.
Cell lines
The Raji, SupM2, SR786, Karpas 299, L428, and L591 cell lines were from the European Cell Culture Collection, the DoHH2 cell line from Dr A. Kluin-Nelemans, Groningen, The Netherlands, SUDHL6 and OCI-Ly3 lines from Dr A. Rosenwald, Würzburg, Germany, and Granta-519, Jeko-1 and VAL from Dr F. Bertoni, Bellinzona, Switzerland. Human cell lines were cultured in RPMI 1640, supplemented with 10% fetal calf serum (FCS), except ALK+ lymphomas SupM2, SR786, Karpas 299, and VAL, which grow in 20% FCS, and Granta-519, in DMEM 10% FCS, 10 mM HEPES, penicillin (50 units/mL), streptomycin (50 μg/mL), and 2 mM l-glutamine, at 37°C in 5% CO2 humidified atmosphere. Normal human B lymphocytes from peripheral blood were isolated on immunomagnetic beads coated with anti-CD19 antibodies (Miltenyi Biotec, Auburn, CA). Table 1 summarizes the properties of the cell lines used.
Properties of the cell lines used
| Cell lines . | Lymphoma type . | Signalosome assembly . | Activation receptors . | Src kinases . | Oncogenic proteins . | EBV expression . |
|---|---|---|---|---|---|---|
| Raji | B-NHL, Burkitt-derived | Yes | CD40, BCR | Lyn | C-Myc | Yes |
| DoHH2 | B-NHL, follicular immunoblastic | Yes | CD40, BCR | Lyn | Bcl-2 | No |
| SUDHL6 | B-NHL DLBCL GC-type | Yes | BCR | Lyn | Bcl-2 | No |
| SupM2 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| SR786 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| Karpas 299 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| L428 | Hodgkin | No | CD30 | Lyn | ? | No |
| L591 | Hodgkin | No | CD30 | Lyn | ? | Yes |
| Jeko-1 | B-NHL, MCL | Yes | ? | Lyn | Cyclin D1 | No |
| Granta-519 | B-NHL, MCL | Yes | ? | Lyn | Cyclin D1 | Yes |
| OCI-LY3 | B-NHL, DLBCL, ABC-type | Yes | ? | Lyn | ? | No |
| VAL | B-NHL, DLBCL, ABC-type | Yes | ? | Lyn | Bcl-2 | Yes |
| Cell lines . | Lymphoma type . | Signalosome assembly . | Activation receptors . | Src kinases . | Oncogenic proteins . | EBV expression . |
|---|---|---|---|---|---|---|
| Raji | B-NHL, Burkitt-derived | Yes | CD40, BCR | Lyn | C-Myc | Yes |
| DoHH2 | B-NHL, follicular immunoblastic | Yes | CD40, BCR | Lyn | Bcl-2 | No |
| SUDHL6 | B-NHL DLBCL GC-type | Yes | BCR | Lyn | Bcl-2 | No |
| SupM2 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| SR786 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| Karpas 299 | T lymphoma ALCL, ALK+ | No | CD30 | Lyn, Lck, Fyn | NPM-ALK | No |
| L428 | Hodgkin | No | CD30 | Lyn | ? | No |
| L591 | Hodgkin | No | CD30 | Lyn | ? | Yes |
| Jeko-1 | B-NHL, MCL | Yes | ? | Lyn | Cyclin D1 | No |
| Granta-519 | B-NHL, MCL | Yes | ? | Lyn | Cyclin D1 | Yes |
| OCI-LY3 | B-NHL, DLBCL, ABC-type | Yes | ? | Lyn | ? | No |
| VAL | B-NHL, DLBCL, ABC-type | Yes | ? | Lyn | Bcl-2 | Yes |
EBV indicates Epstein-Barr virus; B-NHL, B-non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; GC, germinal center; ALCL, anaplastic large cell T lymphoma; ALK, anaplastic lymphoma kinase; MCL, mantle cell lymphoma; ABC, activated B-cell; and ?, unknown.
Antibodies and reagents
Rabbit antibodies to Lyn, Csk, and pSTAT3 were from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine mAb 4G10 was from Upstate Biotechnology. Rabbit antibodies anti-Src 418 (catalytic site) was from BioSource International (Camarillo, CA) and anti-Lck 505 (regulatory site) from Cell Signaling Technology (Danvers, MA). Anti-PAG mouse mAb MEM-255 (for WB) and PAG C-1 (for immunoprecipitation [IP]) were from Exbio (Prague, Czech Republic). The rabbit anti-PAG pY317 antibody was raised against the peptide KEISAMpYSS. Cholera toxin B subunit (peroxidase-labeled) was from Sigma-Aldrich (St Louis, MO); 4-amino-5-[4-chlorophenyl]-7-t-butyl]pyrazolo[3,4-d]pyrimidine (PP2) was from Calbiochem (San Diego, CA) and 7-{4-[2-(2methoxy-ethylamino-ethoxy]-phenyl}-5-(3-methoxy-phenyl)-7H–pyrrolo[2,3-d]pyrimidine-4-ylamine (CGP76030) from Novartis (Basel, Switzerland).
Subcellular fractionation
To isolate sphingolipid-enriched rafts, 5 × 107 cells were lysed in TKM buffer (50 mM Tris-HCl (pH 7.4), 25 mM KCl, 5 mM MgCl2, and 1 mM EGTA) containing 1% TX-100, plus protease inhibitors (10 μM leupeptin, 25 μM aprotinin, and 2 mM Pefabloc) and 0.1 mM Na3VO4 as phosphatase inhibitor, and gradient separation carried out as previously described.4 The sedimentability of the proteins was tested by ultracentrifuging a 100-fold diluted aliquot of each fraction at 100 000g for 60 minutes. The pelleted material was solubilized in SDS sample buffer and probed by WB.
Immunoprecipitation
PAG IP was carried out with the PAG C-1 mAb covalently linked to cyanogen bromide-activated Sepharose 4B beads (GE Healthcare, Little Chalfont, United Kingdom). Raft and nonraft gradient fractions (containing protease and phosphatase inhibitors) were first incubated with 50 mM octyl-glucoside (OTG) to dissociate sphingolipid aggregates, and then mixed with the PAG C-1 beads. Binding of rabbit antibodies (anti-Lyn, anti-pY418 Src and anti-pY505 Lck) to protein A/G agarose beads (Santa Cruz Biotechnology) was carried out according to the manufacturer's instructions. After overnight incubation at 4°C with raft or nonraft material from gradient fractions, the Sepharose and agarose beads were washed with phosphate-buffered saline and extracted with SDS sample buffer. After SDS-PAGE separation and semi-dry transfer to nitrocellulose, the IP and coIP proteins were identified by WB. Alternatively, cells were extracted on ice for 15 minutes in 50 mM OTG, 1% NP-40/1% saponin, or 1% TX-100-TKM, centrifuged at 5000g, and the resulting supernatant subjected to IP with anti-PAG C-1. The IP proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-Lyn or anti-Src pY418.
PAG and Lyn siRNA constructs and silencing procedure
5 siRNAs targeting Lyn were identified using the siRNA software design program. Four siRNAs for PAG were identified in the same manner, and the PAG-targeting sequence identified by Smida et al13 was used to silence PAG. The Lyn and PAG siRNAs used for silencing and control are defined in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
The selected sequences were annealed and cloned into the ClaI-MluI sites of the lentiviral vector pLVTHM.21 Recombinant lentiviruses containing the Lyn or PAG siRNA were produced by cotransfection of 293 T cells with the packaging plasmid psPAX2 and the vesicular stomatitis virus-G envelope-protein expression plasmid pMDG2g. Details of the lentiviral vectors and protocols are given at the Tronolab website.
Jeko-1 and L428 cell lines were transduced twice and cultured under standard conditions for 48 and 72 hours, before assessment of transduction level, viability, and extraction in SDS-PAGE sample buffer for measurement of PAG and Lyn by WB.
Cell culture with the tyrosine kinase inhibitors CGP76030 and PP2 and cell death measurement
Cells were cultured at 5 × 105/mL in medium containing 10% FCS, with increasing concentrations of DMSO-solubilized CGP7603022 or PP2 (3-25 μM) for 24, 48, or 72 hours, harvested and incubated in ethidium bromide (20 μg/mL) for 5 minutes. The cell viability was measured using a FACScan (BD Biosciences, San Jose, CA) apparatus. Proliferation of cells after CGP76030 exposure was evaluated after loading with 1.25 μM carboxy fluorescein succinimidyl ester (CFSE) by measuring the fluorescence at 48, 72, and 96 hours using a FACScan (BD Biosciences) apparatus.
Preparation of human lymphoma extracts
Lymphoma tissues were obtained from patients with their informed consent, in accordance with the Helsinki protocol. The deep-frozen (−80°C) lymphoma tissues in Tissue-Tek were thin-sectioned (10 μm) in a cryostat, and 50 to 100 sections were immersed in 0.5 mL of TKM containing protease inhibitors (10 μM leupeptin, 25 μM aprotinin, and 2 mM Pefabloc) and phosphatase inhibitor (0.1 mM Na3VO4) to remove the excess Tissue-Tek. Tissues were then extracted in TKM-TX-100 1% with protease and phosphatase inhibitors for 1 hour at 4°C, homogenized and centrifuged at 1000g, and then 10 000g. The last supernatant was made 50 mM OTG and mixed with Sepharose 4B-anti-PAG C-1 antibodies for IP.
In silico modeling of the PAG/Lyn complex
Sequences of human Lyn and PAG were downloaded from Swissprot (P07948 LYN and Q9NWQ8 PAG1). The PAG structure was modeled using the Robetta full-chain protein structure prediction server,23 as no suitable template was available from the RCSB Protein Data Bank (PDB). The Lyn SH2 domain was modeled using the 1lck entry (PDB) as a template, with the same server. Twelve structures were obtained for PAG and 5 for the Lyn SH2 domain. Root mean square deviation was measured for all structures, using the MOLMOL program24 and average structures were selected for both PAG and Lyn SH2.
Ten phosphoprotein sequences were selected at random from PDB, with details for the three-dimensional coordinates of p-Tyr. From these 10 phosphoproteins, representative 3-dimensional coordinates data for 15 pY were selected, and averages for each atomic bond length (Cα backbone and side chain), bond angles, and temperature factors calculated. Root mean square deviations for both atoms and dihydrals were calculated using the Monte Carlo, dihedral probability grid method to build first the backbone conformation and then the side chain ring of Y and phosphate coordinates. These average coordinates of pY then served to rebuild the atomic coordinates at each tyrosine (105, 163, 181, 227, 299, 317, 341, 359, 387, and 417) in the PAG protein, individually or in combination. For further analysis, structures obtained for PAG phosphorylated at tyrosines 299 and 317 or unphosphorylated PAG were used for docking with Lyn SH2, using ClusPro25 to generate a binding model. All models were evaluated for their binding contacts using the CMA server of the SPACE suite of tools.26 The selected models were then analyzed with the CMA server and residue per residue contact maps generated.
Results
Cbp/PAG and Lyn codistribute in detergent-resistant raft membranes of B-NHLs
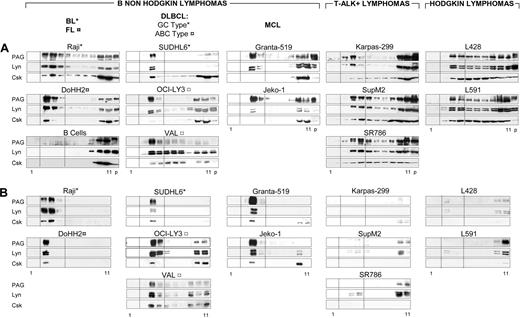
Density distributions of PAG, Lyn, and Csk were compared (Figure 1A) for all lymphoma lines (Table 1) and normal B lymphocytes. In the B-NHL cell lines Raji, DoHH2, SUDHL6, OCI-Ly3, VAL, Granta-519, and Jeko-1, 90% of PAG, 50% to 90% of Lyn and 10% to 30% of Csk were recovered in the raft fractions. In all B-NHL gradients, except Granta-519, the amounts of PAG and Lyn recovered in the gradient pellets (Figure 1A, “p”) were minimal, suggesting little or no association with cytoskeleton or nuclei. By contrast, only 30% to 50% of PAG and Lyn and negligible amounts of Csk were recovered in rafts of ALK+ lymphomas (Karpas 299, SupM2, and SR786) and HL cell lines (L428 and L591). Lyn and PAG therefore exhibited cell-specific interactions with membrane components leading to preferential association with rafts in B-NHLs (Figure 1A). In nonactivated human peripheral blood B lymphocytes, neither PAG nor Lyn nor Csk was recovered in the raft fractions (Figure 1A bottom left column), although GM1-containing rafts were clearly identifiable in normal B lymphocytes (not shown).
PAG and Lyn codistribute in detergent-resistant raft membranes of B-NHLs. (A) Subcellular fractionation of TX-100 lysates. TX-100 lysates (50 × 106 cells) of the indicated cell line loaded on sucrose gradient, ultracentrifuged to equilibrium, and 10 μL of each fraction was probed by WB with anti-PAG (MEM-255), anti-Lyn and anti-Csk. Fractions 1 to 11, from top to bottom. p: pellet of the equilibrium gradient containing nuclei and cytoskeleton. Dotted lines indicate the raft-containing fractions 3-5. (B) Sedimentability of the TX-100-resistant material. An aliquot (10 μL) of each gradient fraction was diluted 100-fold and ultracentrifuged (100 000g for 1 hour). The sedimented material probed by WB and raft fractions between dotted lines, as in panel A. Gradient fractionation was carried out at least twice for all cell lines and more than 5 times for Raji, DoHH2, SUDHL6, ALK+, and L428 lines.
PAG and Lyn codistribute in detergent-resistant raft membranes of B-NHLs. (A) Subcellular fractionation of TX-100 lysates. TX-100 lysates (50 × 106 cells) of the indicated cell line loaded on sucrose gradient, ultracentrifuged to equilibrium, and 10 μL of each fraction was probed by WB with anti-PAG (MEM-255), anti-Lyn and anti-Csk. Fractions 1 to 11, from top to bottom. p: pellet of the equilibrium gradient containing nuclei and cytoskeleton. Dotted lines indicate the raft-containing fractions 3-5. (B) Sedimentability of the TX-100-resistant material. An aliquot (10 μL) of each gradient fraction was diluted 100-fold and ultracentrifuged (100 000g for 1 hour). The sedimented material probed by WB and raft fractions between dotted lines, as in panel A. Gradient fractionation was carried out at least twice for all cell lines and more than 5 times for Raji, DoHH2, SUDHL6, ALK+, and L428 lines.
After ultracentrifugation of sucrose-free gradient fractions, PAG, Lyn, and Csk cosedimented exclusively with raft membranes from the B-NHL lines Raji, DoHH2, SUDHL6, OCI-Ly3, VAL, Granta-519, and Jeko-1 (Figure 1B). By contrast, the PAG, Lyn, and Csk detected in the raft fractions of the ALK+ lines SupM2, SR786, and Karpas, and Hodgkin lymphoma (HL) lines L428 and L591 did not cosediment with detergent-resistant membranes, reflecting weaker association with such microdomains (Figure 1B). As expected, no PAG, Lyn, or Csk sedimented with normal B lymphocyte rafts (not shown).
Human B-NHL cell rafts contain a predominant tyrosine phosphorylated component of 80 kDa and doubly phosphorylated Lyn
In gradients of Burkitt-, follicular-, and diffuse large B-cell lymphomas (DLBCL) of GC type, the antiphosphotyrosine antibody (Figure 2A) identified a single component of 80 kDa (open arrowhead) comigrating with PAG in raft fractions, and in activated B-cell type (ABC-type) DLBCLs (OCI-Ly3 and VAL) and mantle cell lymphomas (MCL) (Granta-519 and Jeko-1), additional phosphotyrosylated bands in the Src kinase molecular weight (MW) range (50–60 kDa) were also detected. In ALK+ T lymphomas (SupM2, SR786, and Karpas 299), phosphotyrosylated bands of all MW were detected (including Src kinase bands), without selective accumulation in raft fractions. In the HL lines L428 and L591, the tyrosine phosphorylated 80-kDa component accumulated in nonraft fractions.
B-NHL rafts contain a predominant tyrosine phosphorylated component of 80 kDa. (A) Subcellular fractionation of TX-100 lysates as in Figure 1; 10 μL of each gradient fraction (as in Figure 1A) was probed with the antiphosphotyrosine 4G10 mAb. (B) Tyrosine phosphorylated PAG is part of the 80-kDa raft component. Raft and nonraft gradient fractions from the indicated cell lines containing each equivalent amounts of anti-PAG-reactive protein. R indicates fraction 3-5 (raft) proteins; NR, fraction 10-11 (nonraft) proteins. First row, WB with antiphosphotyrosine 4G10 antibody; second row, WB with antibody against peptide KEISAMpYSS, recognizing pY317 in PAG; third row, WB with anti-PAG antibody MEM-255. (C) Doubly tyrosine phosphorylated Lyn is part of the 80-kDa component in selected B-NHL rafts. Raft (R) and nonraft (NR) gradient fractions from the indicated cell lines containing each equivalent amounts of Lyn. First row, WB with anti-pY418 Src antibody; second row, WB with anti-pY505 Lck antibody; third row, WB with anti-Lyn antibody. Open arrowheads indicate the 80-kDa, antiphospho-Lyn-reactive component. Gradients and IPs were carried out 3 times for all cell lines, and representative experiments are shown.
B-NHL rafts contain a predominant tyrosine phosphorylated component of 80 kDa. (A) Subcellular fractionation of TX-100 lysates as in Figure 1; 10 μL of each gradient fraction (as in Figure 1A) was probed with the antiphosphotyrosine 4G10 mAb. (B) Tyrosine phosphorylated PAG is part of the 80-kDa raft component. Raft and nonraft gradient fractions from the indicated cell lines containing each equivalent amounts of anti-PAG-reactive protein. R indicates fraction 3-5 (raft) proteins; NR, fraction 10-11 (nonraft) proteins. First row, WB with antiphosphotyrosine 4G10 antibody; second row, WB with antibody against peptide KEISAMpYSS, recognizing pY317 in PAG; third row, WB with anti-PAG antibody MEM-255. (C) Doubly tyrosine phosphorylated Lyn is part of the 80-kDa component in selected B-NHL rafts. Raft (R) and nonraft (NR) gradient fractions from the indicated cell lines containing each equivalent amounts of Lyn. First row, WB with anti-pY418 Src antibody; second row, WB with anti-pY505 Lck antibody; third row, WB with anti-Lyn antibody. Open arrowheads indicate the 80-kDa, antiphospho-Lyn-reactive component. Gradients and IPs were carried out 3 times for all cell lines, and representative experiments are shown.
To characterize this 80-kDa component, aliquots of pooled raft and nonraft fractions containing equivalent amounts of PAG were probed with antiphosphotyrosine antibody (4G10), anti-pY317 of PAG, and anti-PAG (Figure 2B). As expected, the 80-kDa component was strongly 4G10-positive (Figure 2B first row) and similarly, positive for pY317 PAG in rafts (Figure 2B second row), whereas the PAG protein was detected equally well in rafts and nonrafts (Figure 2B third row). Hyperphosphorylated raft PAG was of consistently higher MW than the nonraft PAG. In contrast, in HL and ALK+ cell lines, the anti-PAG pY317- and 4G10-positive bands were predominant in nonrafts. These results show that, in B-NHL lines, PAG phosphorylated at Y317 was preferentially found in rafts as part of the 80-kDa component.
The phosphorylation state of Lyn was probed with the anti-Src pY418 to detect pY396 in the Lyn catalytic site, and with the anti-Lck pY505 to detect the regulatory C-terminal Y507 of Lyn (Figure 2C). The double phosphorylation of Lyn was verified by reciprocal IP and WB with the anti-pY418 Src and anti-p505 Lck antibodies (not shown). In the SR786 ALK+ line, raft and nonraft Lyn were phosphorylated at both sites, with a weak 80-kDa band positive with the anti-Lck pY505 antibody in the raft and nonraft fractions. In B-NHL and ALK+ lines, the Lyn kinase in raft and nonraft fractions was phosphorylated in both the catalytic (Figure 2C first row) and the regulatory (Figure 2C second row) sites. Only in the HL line L428 was the Lyn kinase detectable with neither of the 2 anti-pY antibodies. In the raft fractions of Raji, DoHH2, SUDHL6, and VAL lines, Lyn phosphorylated at Y396 or Y507 was also detected at 80 kDa (open arrowhead) in addition to the lower MW phosphoforms, but not in rafts of OCI-Ly3, Granta-519, or Jeko-1. The same cell lines (Raji, DoHH2, SUDHL6, and VAL) were those in which the phosphotyrosylated 80 kDa predominated in rafts (Figure 2A). Thus, in Raji, DoHH2, SUDHL6, and VAL, part of Lyn phosphotyrosylated at the catalytic and regulatory sites comigrated with the 80-kDa, phosphotyrosylated component of B-NHL rafts.
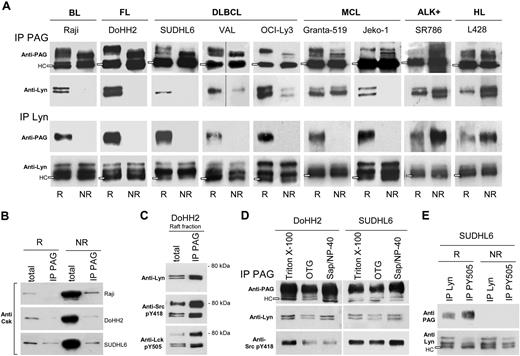
PAG and Lyn associate in B-NHL rafts
Evidence for selective association of Lyn and PAG in rafts was obtained by comparing the extent of reciprocal coprecipitation in raft and nonraft PAG (Figure 3A). In B-NHL cell lines (with the exception of Granta-519), Lyn coprecipitated only with the raft PAG phosphoform (Figure 3A second row) and reciprocally, the raft PAG phosphoform coprecipitated only with raft Lyn (Figure 3A third row). By contrast, in ALK+ and HL lines, Lyn preferentially coIP with nonraft PAG; likewise, PAG with nonraft Lyn, suggesting that in those cell lines, the PAG/Lyn interaction takes place in nonraft fractions. To rule out trapping of PAG or Lyn in IPs carried out with TX-100–resistant membrane material, coIP was evaluated in the presence of 50 mM OTG, a detergent that disperses sphingolipid aggregates.4,27
Lyn and PAG are associated in B-NHL rafts. (A) Raft (R) and nonraft (NR) aliquots (100 mL) from Raji, DoHH2, SUDHL6, Val, OCI-Ly3, Granta-519, Jeko-1, SR786, and L428 cells containing each equivalent amounts of either PAG or Lyn were IP with Sepharose-4B-coupled anti-PAG C-1 antibody (IP PAG, top 2 rows), or protein A/G-bound anti-Lyn antibody (IP Lyn, bottom 2 rows). IP materials were probed with anti-PAG MEM-255 mAb (first and third rows) or anti-Lyn antibody (second and fourth rows). Open rectangles indicate the H chain (HC) of the antibody used for IP. Vertical lines indicate repositioned gel lanes. (B) R and NR material (100 μL) from Raji, DoHH2, and SUDHL6, as in panel A) was IP with anti-PAG C-1 bound to Sepharose, and probed with anti-Csk antibody. Total: R and NR material (10 μL) probed directly with anti-Csk. (C) R material from DoHH2 (100 μL) was IP with Sepharose-bound anti-PAG C-1 (IP PAG) in 3 aliquots and probed by WB with: top, anti-Lyn; middle, anti-pY418 Src; and bottom, anti-pY505 Lck. Total: R material (10 μL) probed directly with the indicated antibodies. (D) PNS of 15 × 106 DoHH2 and SUDHL6 cells extracted with different detergents, subjected to IP with anti-PAG (PAG C-1) and WB with anti-PAG MEM 255, anti-Lyn, and anti-Src pY418. (E) R and NR material (100 μL) from SUDHL6 was IP with protein A/G-bound anti-Lyn, or anti-pY505 Lck antibodies, and probed with anti-PAG MEM-255 (first row) and anti-Lyn (second row). IPs described in this figure were carried out at least 3 times for each cell line.
Lyn and PAG are associated in B-NHL rafts. (A) Raft (R) and nonraft (NR) aliquots (100 mL) from Raji, DoHH2, SUDHL6, Val, OCI-Ly3, Granta-519, Jeko-1, SR786, and L428 cells containing each equivalent amounts of either PAG or Lyn were IP with Sepharose-4B-coupled anti-PAG C-1 antibody (IP PAG, top 2 rows), or protein A/G-bound anti-Lyn antibody (IP Lyn, bottom 2 rows). IP materials were probed with anti-PAG MEM-255 mAb (first and third rows) or anti-Lyn antibody (second and fourth rows). Open rectangles indicate the H chain (HC) of the antibody used for IP. Vertical lines indicate repositioned gel lanes. (B) R and NR material (100 μL) from Raji, DoHH2, and SUDHL6, as in panel A) was IP with anti-PAG C-1 bound to Sepharose, and probed with anti-Csk antibody. Total: R and NR material (10 μL) probed directly with anti-Csk. (C) R material from DoHH2 (100 μL) was IP with Sepharose-bound anti-PAG C-1 (IP PAG) in 3 aliquots and probed by WB with: top, anti-Lyn; middle, anti-pY418 Src; and bottom, anti-pY505 Lck. Total: R material (10 μL) probed directly with the indicated antibodies. (D) PNS of 15 × 106 DoHH2 and SUDHL6 cells extracted with different detergents, subjected to IP with anti-PAG (PAG C-1) and WB with anti-PAG MEM 255, anti-Lyn, and anti-Src pY418. (E) R and NR material (100 μL) from SUDHL6 was IP with protein A/G-bound anti-Lyn, or anti-pY505 Lck antibodies, and probed with anti-PAG MEM-255 (first row) and anti-Lyn (second row). IPs described in this figure were carried out at least 3 times for each cell line.
A total of 10% to 30% of Csk also distributed in raft fractions of B-NHL lines (Figure 1A) and sedimented with PAG and Lyn (Figure 1B), but most of Csk was recovered from nonraft fractions in B-NHL cell lines (Figure 1A) and showed no significant coIP with PAG or Lyn, in rafts, or in nonraft fractions (Figure 3B, IP PAG).
In PAG IPs of DoHH2 raft material, the coIP Lyn kinase was probed with anti-Lyn, anti-catalytic (anti-Src pY418), and anti-regulatory (anti-Lck pY505) site antibodies (Figure 3C). The 53/56 Lyn kinase was detected by all antibodies, but in addition, the 2 antiphosphotyrosine antibodies also detected the 80-kDa component. The selectivity of Lyn coIP with PAG compared with that of Csk was shown with the material from the same DoHH2 cell line, where raft Csk was undetectable in the PAG IP (Figure 3B), whereas significant amounts of Lyn and phosphorylated Lyn coprecipitated with raft PAG (Figure 3C). The anti-pY505 Lck antibody (recognizing the Lyn C-terminal, regulatory pY507) was more efficient at coprecipitating raft PAG than the anti-Lyn antibody (Figure 3E), suggesting that phosphorylated Lyn interacted selectively with raft PAG. Lyn IP with the antibody against the active site pY396 was also more efficient to coIP the PAG protein (not shown).
To avoid the possibility that spurious lipid-protein interactions occurring during Triton X-100 extraction, postnuclear supernatants were generated with NP-40/1%-saponin 1%,28 or OTG, and compared with the same supernantants obtained after extraction with TKM-Triton X-100 1% (Figure 3D). In the DoHH2 and SUDHL6 lines studied, the 53-56 Lyn kinase coprecipitated equally well with PAG in TX-100 and NP-40-saponin. In SUDHL6, the coprecipitated Lyn was equally tyrosine phosphorylated in its active site (anti-Src pY418), and less so in DoHH2 extracted, probably reflecting dephosphorylation.
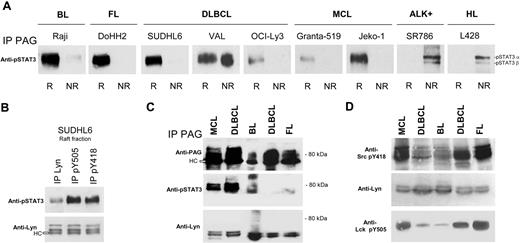
pSTAT3 selectively associates with the raft PAG/Lyn complex in B-NHL lines and lymphomas
To investigate how the PAG/Lyn raft platform could signal further downstream, we found that pSTAT3 selectively coIP with PAG in rafts of all B-NHL lines. The VAL cell line, however, contained as much coIP pSTAT3 in rafts as in nonraft fractions (Figure 4A). Both pSTAT3α (92 kDa) and pSTAT3β (83 kDa) coIP with PAG in nonraft fractions of the L428 HL and SR786 ALK+ lines, suggesting a raft-independent interaction with PAG. Finally pSTAT3, for equivalent amounts of IP Lyn (Figure 4B second row), was more efficiently coIP with antiphosphotyrosine kinase antibodies than with anti-Lyn antibodies in rafts of SUDHL6 (Figure 4B first row), suggesting that pSTAT3 could interact with a pY motif on Lyn in the complex.
pSTAT3 selectively associates with the raft PAG/Lyn complex in B-NHL lines and lymphomas. (A) Raft (R) and nonraft (NR) material (100 μL) of the indicated cell lines was IP with anti-PAG C-1 antibody (same material as in Figure 3A) and probed with anti-pSTAT3 antibody. (B) Raft material from SUDHL6 was IP with protein A/G-bound anti-Lyn, anti-pY505 Lck, or anti-pY418 Src antibodies, and the IP material probed with anti-pSTAT3 (first row) and anti-Lyn (second row). (C) TX-100–resistant raft material isolated from frozen lymphoma tissues was treated with 50 mM OTG and IP with Sepharose-bound anti-PAG C-1 antibody. The IP material was probed with anti-PAG MEM-255 (top), anti-pSTAT3 (middle), and anti-Lyn (bottom). (D) Same material as in panel C: WB with anti-Src pY418, anti-Lyn, and anti-Lck pY505. pSTAT3 association with Lyn/PAG was ascertained at least 3 times for all cell lines, and IPs with patient material carried out twice with lymphomas of each type.
pSTAT3 selectively associates with the raft PAG/Lyn complex in B-NHL lines and lymphomas. (A) Raft (R) and nonraft (NR) material (100 μL) of the indicated cell lines was IP with anti-PAG C-1 antibody (same material as in Figure 3A) and probed with anti-pSTAT3 antibody. (B) Raft material from SUDHL6 was IP with protein A/G-bound anti-Lyn, anti-pY505 Lck, or anti-pY418 Src antibodies, and the IP material probed with anti-pSTAT3 (first row) and anti-Lyn (second row). (C) TX-100–resistant raft material isolated from frozen lymphoma tissues was treated with 50 mM OTG and IP with Sepharose-bound anti-PAG C-1 antibody. The IP material was probed with anti-PAG MEM-255 (top), anti-pSTAT3 (middle), and anti-Lyn (bottom). (D) Same material as in panel C: WB with anti-Src pY418, anti-Lyn, and anti-Lck pY505. pSTAT3 association with Lyn/PAG was ascertained at least 3 times for all cell lines, and IPs with patient material carried out twice with lymphomas of each type.
Snap-frozen samples of one mantle-cell lymphoma, one Burkitt, one follicular lymphoma, and 2 DLBCLs, were extracted with TX-100 and the TX-100-resistant material was further extracted with OTG. We have previously shown that 50 mM OTG did not dissociate the Lyn/PAG complexes, before IP with the insolubilized anti-PAG C-1 antibody. PAG IPs of OTG-solubilized lymphoma rafts were then probed on nitrocellulose with anti-PAG, anti-pSTAT3, and anti-Lyn (Figure 4C). The Lyn kinase coIP with PAG in all 5 tissue samples and pSTAT3 with PAG in one MCL, one DLBCL and one Burkitt lymphoma. Tumor cells amounted to 60% to 90% of the sample cellularity for all cases. We could also show that Y396 and Y505 of Lyn were detectable in all Burkitt lymphoma, follicular lymphoma, and DLBCL tested (Figure 4D).
ALK+ and Raji cell lines exhibit quantitatively similar, but qualitatively different, raft sphingolipid patterns
The raft lipid contents of Raji cells was compared with that of SupM2 and SR786 ALK+ cell lines (Figure S1) by direct visualization of the sphingolipids (Figure S1A) and autoradiography of 14C-serine labeled sphingolipids (Figure S1B). The sphingomyelin bands were a major sphingolipid in both Raji and ALK+ rafts. Moving ahead of sphingomyelin, the CD77 (globotriaosylceramide, a Burkitt lymphoma marker29 ) was detectable by direct staining and autoradiography in Raji raft extracts. Lactosylceramide was less abundant in the Raji than in the SR786 and SupM2 extracts (10% vs 15% and 19%, respectively, Figure S1B). The total amount of unlabeled sphingolipids recovered from raft fractions equivalent in protein contents was highest for SR786 (100%, lane 2), 92% for Raji (lane 1), and 79% for SupM2 (lane 3). Charged glycosphingolipids were minor components not detectable by direct staining (Figure S1A), but a weak 14C-serine-labeled band comigrating with the GM2 marker was detectable in Raji rafts (Figure S1B). GM1 was only detectable after cholera toxin B overlay of the methacrylate-treated TLC plate (Figure S1C, corresponding to the insert in Figure S1A). The 2 ALK+ cells showed substantially stronger GM1 bands than Raji, suggesting that GM1 is at least twice more abundant in ALK+ rafts than in Raji rafts.
Kinase inhibitors trigger cell death of most B-NHL lines, block proliferation, inhibit the Lyn kinase in isolated B-NHL rafts, block tyrosine phosphorylation of both PAG and Lyn in rafts, and dissociate Lyn from PAG and rafts
Incubation of B-NHL cell lines (SUDHL6, OCI-Ly3, VAL, DoHH2, Granta-519, and Jeko-1) with 10 μM CGP76030 killed more than 50% of the cells after 48 hours of incubation, and PP2 was slightly less potent to induce cell death (Figure 5A). Comparatively, Raji remained more than 70% viable up to 25 μM of both inhibitors, as were L428 HL cells. Interestingly, the ALK+ lymphoma SR786 cells were resistant to PP2, but 100% sensitive to CGP76030, suggesting that CGP76030 selectively inhibited the NPM-ALK fusion protein on which ALK+ lymphomas are dependent for proliferation.7 Phosphorylation of NPM-ALK was indeed abrogated by CGP76030 in SR786 cells (not shown). To evaluate the effects of CGP76030 (0.5 and 1 μM) on DoHH2 proliferation, CFSE-loaded cells were analyzed after 48, 72, and 96 hours (Figure 5B). A dose-dependent inhibition of cell-cycle progression was manifest at 72 and 96 hours (decreased numbers of cells in M2 and M3). Moreover, the numbers of viable cells considerably decreased during the observation period (not shown).
Exposure to CGP76030 triggers cell death of SUDHL6, Granta-519, DoHH2, VAL, Jeko-1, OCI-Ly3, and SR786 cell lines, blocks tyrosine phosphorylation of both PAG and Lyn in rafts, and dissociates Lyn from PAG and rafts. (A) Death of Raji, DoHH2, SUDHL6, Val, OCI-Ly3, Granta-519, Jeko-1, SR786, and L428 cells in presence of CGP76030 or PP2 at the indicated concentrations after 48 hours. These results are representative of 3 independent experiments. (B) Measurement of the proliferation rate of DoHH2 cells, loaded with CFSE, after CGP76030 exposure for 48, 72, and 96 hours. M1 corresponds to cells that have least proliferated, and M3 to cells with the highest proliferation rate. The results shown in panel B are representative of 3 independent experiments. (C-E) CGP76030 selectively dephosphorylates Lyn and PAG in rafts. (C) DoHH2 cells were cultured for 7 hours in the presence of 10 μM CGP76030, lysed in SDS sample buffer and WB with anti-P-Tyr antibody (lysate, left panel), or subjected to sucrose gradient fractionation in the presence of TX-100 as in Figure 1A. R and NR material containing equal amounts of Lyn was also probed with anti-P-Tyr antibody (right panel). (D) R and NR material (as in panel C) containing equal amounts of PAG were WB with anti-pY317 PAG, anti-PAG (MEM-255), and anti-pSTAT3 antibodies. (E) R and NR material (as in panel C) containing equal amounts of Lyn, was WB with anti-pY418 Src, anti-pY505 Lck, or anti-Lyn antibodies. Results in panels C through E are representative of 2 independent experiments. (F) CGP76030 redistributes Lyn of the raft fractions but minimally affects PAG localization. Equal volumes of sucrose gradient fractions of CGP76030-treated DoHH2 cells were WB with anti-PAG MEM-255 or anti-Lyn antibodies. Intensities were measured with ImageQuant and expressed as arbitrary units. Identical results were obtained in 2 independent experiments.
Exposure to CGP76030 triggers cell death of SUDHL6, Granta-519, DoHH2, VAL, Jeko-1, OCI-Ly3, and SR786 cell lines, blocks tyrosine phosphorylation of both PAG and Lyn in rafts, and dissociates Lyn from PAG and rafts. (A) Death of Raji, DoHH2, SUDHL6, Val, OCI-Ly3, Granta-519, Jeko-1, SR786, and L428 cells in presence of CGP76030 or PP2 at the indicated concentrations after 48 hours. These results are representative of 3 independent experiments. (B) Measurement of the proliferation rate of DoHH2 cells, loaded with CFSE, after CGP76030 exposure for 48, 72, and 96 hours. M1 corresponds to cells that have least proliferated, and M3 to cells with the highest proliferation rate. The results shown in panel B are representative of 3 independent experiments. (C-E) CGP76030 selectively dephosphorylates Lyn and PAG in rafts. (C) DoHH2 cells were cultured for 7 hours in the presence of 10 μM CGP76030, lysed in SDS sample buffer and WB with anti-P-Tyr antibody (lysate, left panel), or subjected to sucrose gradient fractionation in the presence of TX-100 as in Figure 1A. R and NR material containing equal amounts of Lyn was also probed with anti-P-Tyr antibody (right panel). (D) R and NR material (as in panel C) containing equal amounts of PAG were WB with anti-pY317 PAG, anti-PAG (MEM-255), and anti-pSTAT3 antibodies. (E) R and NR material (as in panel C) containing equal amounts of Lyn, was WB with anti-pY418 Src, anti-pY505 Lck, or anti-Lyn antibodies. Results in panels C through E are representative of 2 independent experiments. (F) CGP76030 redistributes Lyn of the raft fractions but minimally affects PAG localization. Equal volumes of sucrose gradient fractions of CGP76030-treated DoHH2 cells were WB with anti-PAG MEM-255 or anti-Lyn antibodies. Intensities were measured with ImageQuant and expressed as arbitrary units. Identical results were obtained in 2 independent experiments.
To further investigate the difference between the inhibitor-sensitive B-NHL lines and the resistant Raji cells, isolated rafts from Raji, DoHH2, Jeko-1, and VAL cell lines were subjected to in vitro kinase assay (S2). The raft Lyn kinase isolated from each B-NHL cell line was similarly inhibited (70%-90%) by PP2 and CGP76030, leading to decreased Lyn (53-56 kDa bands) and 80-kDa component phosphorylation, suggesting that, in Raji cells, a block further downstream prevents cell death induction by CGP76030.
We next investigated the consequences of Lyn inhibition by CGP76030 on PAG and Lyn tyrosine phosphorylation in the DoHH2 line (Figure 5C), which is 90% killed by 10 μM CGP76030 (Figure 5A). We chose to study DoHH2 cells after 7 hours of CGP76030 exposure, time at which only negligible apoptosis and intracellular proteolysis were detectable. The phosphotyrosylated protein pattern in the whole lysate of CGP76030-treated cells was very comparable with that of untreated cells, emphasizing the selectivity of CGP76030 (Figure 5C left panel). However, in isolated rafts, CGP76030 abolished tyrosine phosphorylation of the 80-kDa (PAG and phosphoLyn) and 53- to 56-kDa Lyn bands, and likewise in nonrafts (Figure 5C right panel). Phosphorylation of raft and nonraft PAG Y317 was also abolished by CGP76030 (Figure 5D first row), with a noticeably decreased MW for the raft PAG (Figure 5D second row) but no change in the MW of nonraft PAG (Figure 5D second row). CGP76030 abolished pSTAT3 detection in rafts, whereas the intensity of pSTAT3 α and β was slightly decreased in nonrafts (Figure 5D third row). The anti-pY418 of Src and anti-p505 of Lck antibodies no longer detected phosphoLyn in the 80-kDa complex (rafts) and the 53- to 56-kDa Lyn (rafts and nonrafts) after CGP76030 treatment (Figure 5E). CGP76030 therefore inhibited phosphorylation of both catalytic and regulatory tyrosines in Lyn. As the IC50 value of CGP76030 is 53 for Lyn and 560 for Csk,22 the loss of Lyn Y507 phosphorylation most likely resulted from CGP76030-mediated inhibition of Lyn autophosphorylation, rather than phosphorylation by Csk. The effect of PAG and Lyn dephosphorylation by CGP76030 on the gradient distribution of the 2 proteins (Figure 5F) was examined in the same setting. The raft-association of PAG was minimally affected (15% decrease in raft PAG with concomitant increase in nonraft PAG), whereas 30% of raft Lyn was shifted from rafts to nonrafts after CGP76030 treatment.
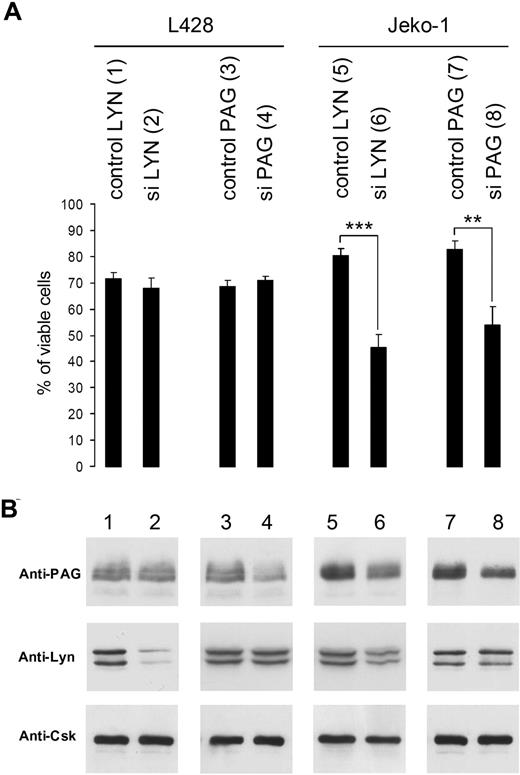
Silencing of Lyn or PAG induces cell death in Jeko-1 (MCL), but not in L428 Hodgkin cells. (A) Viability of L428 and Jeko-1 cells 72 hours after transduction of Lyn or PAG sequences (in Table S1: control Lyn, sequence 3) and siLyn (sequence 2), control PAG (sequence 9), and siPAG (sequence 10), evaluated after ethidium bromide labeling. Statistical analysis was carried out by Student t test (**P < .001; ***P < .0001). (B) Lyn and PAG protein expression was measured by WB with anti-Lyn and anti-PAG MEM255 antibodies. Equivalent loading was verified by anti-Csk WB because the Csk protein is not affected by Lyn or PAG silencing. Intensities of Lyn and PAG bands were measured with ImageQuant. These results are representative of 3 independent silencing experiments.
Silencing of Lyn or PAG induces cell death in Jeko-1 (MCL), but not in L428 Hodgkin cells. (A) Viability of L428 and Jeko-1 cells 72 hours after transduction of Lyn or PAG sequences (in Table S1: control Lyn, sequence 3) and siLyn (sequence 2), control PAG (sequence 9), and siPAG (sequence 10), evaluated after ethidium bromide labeling. Statistical analysis was carried out by Student t test (**P < .001; ***P < .0001). (B) Lyn and PAG protein expression was measured by WB with anti-Lyn and anti-PAG MEM255 antibodies. Equivalent loading was verified by anti-Csk WB because the Csk protein is not affected by Lyn or PAG silencing. Intensities of Lyn and PAG bands were measured with ImageQuant. These results are representative of 3 independent silencing experiments.
The B-NHL line Jeko-1 (MCL) cell line dies after decrease of Lyn or PAG gene expression whereas the L428 Hodgkin cell line remains unaffected
The L428 Hodgkin cell lines remained essentially unaffected (Figure 6A) with 90% of Lyn silencing (Figure 6B lane 2), or 50% of PAG silencing (Figure 6B lane 4). In contrast, the viability of the Jeko-1 MCL was considerably decreased after a 65% Lyn silencing (Figure 6B lane 6) and 50% silencing of PAG (Figure 6B lane 8) expression. Interestingly, the remaining PAG expression was reduced by 50% in Jeko-1 cells silenced for Lyn (Figure 6B lane 6).
In silico modeling of the PAG/Lyn complex
Modeling of PAG with phosphorylated Y317 and Y299 generated a conformation accommodating the Lyn SH2 loop (circled in red, Figure 7A right panel). The Lyn SH2/phosphorylated PAG contact map (Figure 7B) shows that the PAG residues (horizontal axis) involved in binding Lyn SH2 are clustered between Phe288 and Arg302 and include Tyr299, but not Tyr317. This stands in contrast with the binding model of Lyn SH2 with unphosphorylated PAG, where the PAG residues involved in binding to Lyn SH2 are spread between Phe224 and Lys367, not including Tyr 299 and 317, and the Lyn SH2 uses N- and C-terminal residues (Figure 7A left panel). This implies that interaction of the complete Lyn protein with unphosphorylated PAG is not favored because Lyn domains preceding and following SH2 are likely to interfere with the Lyn SH2/unphosphorylated PAG interaction. In contrast, the N- and C-termini of Lyn SH2 (arrowheads) are not involved in the contact of phosphorylated PAG and the part of Lyn SH2 (circled in red) contacting phosphorylated PAG is doing so independently of the non-SH2 portions of the Lyn protein (Figure 7A right panel). Moreover, comparison of values for the binding contacts (estimated by CMA) between residues of phosphorylated and unphosphorylated PAG with Lyn SH2 suggests that the Lyn-PAG complex is much more stable when PAG is phosphorylated on Y299 and Y317 and phosphorylation of PAG tyrosines other than 299 and 317 did not result in binding clefts accommodating Lyn SH2 (not shown).
Modeling of the PAG/Lyn SH2 complexes and Lyn, PAG, STAT3, and Csk interactions in lymphoma cell membranes. (A) Binding models of PAG (red) and Lyn SH2 (blue) and the fitting of Lyn SH2 are shown for both nonphosphorylated and phosphorylated (Y299 and Y317) PAG. The orientation of phosphorylated PAG is different from that of nonphosphorylated PAG. The phosphorylated PAG constitutes a best-fitting model. The N- and C-termini of Lyn SH2 (small arrowheads) are facing the nonphosphorylated PAG structure, and SH2 binding to PAG should be affected when the complete Lyn protein interacts with PAG. In contrast, the N- and C-termini of Lyn SH2 (small arrowheads) are facing away from the contact area with the phosphorylated PAG structure, an orientation that is likely not to be affected when the whole Lyn protein interacts with phosphorylated PAG. (B) The residue per residue contact maps show the residues involved in the contact area for phosphorylated PAG. The Lyn residues involved in contacts are shown in the vertical axis and PAG residues on the horizontal axis. Y299 of phosphorylated PAG is shown to make contacts with Pro 18, Gly 19, Asn 20, Ser 21, Ala 22, Arg 42, and Asp 50 of Lyn SH2. The encircled area of Lyn SH2 (Figure 7A) contains Pro 46, Val 47, and His 48 (in a coiled region between 2 β-sheets) that make contacts with different residues of phosphorylated PAG. (C) According to Cary and Cooper,16 Csk docks onto PAG (pY317), phosphorylates Lyn pY507, causing the Lyn SH2 to interact with Lyn pY507 and to form an intramolecular loop, resulting in a Lyn kinase of low catalytic activity. (D) In the raft-based “signalosome” of B-NHLs, the acylated Lyn kinase and the transmembrane PAG are both inserted in rafts. Modular interactions through Lyn SH2 and SH3 further stabilize the Lyn/PAG signalosome. The SH2 of Lyn is not interacting with its regulatory, C-terminal pY507 (intramolecular interaction), but instead with a cleft in PAG containing pY299. The Lyn C-terminal pYQQQ could also be recognized by STAT3 SH2, an interaction that further limits the possibility of intramolecular binding to Lyn SH2. PAG is phosphotyrosylated by Lyn at least at Y299 and Y317, and Lyn autophosphorylates at Y396 in the catalytic domain (SH1) and at the regulatory site Y507.
Modeling of the PAG/Lyn SH2 complexes and Lyn, PAG, STAT3, and Csk interactions in lymphoma cell membranes. (A) Binding models of PAG (red) and Lyn SH2 (blue) and the fitting of Lyn SH2 are shown for both nonphosphorylated and phosphorylated (Y299 and Y317) PAG. The orientation of phosphorylated PAG is different from that of nonphosphorylated PAG. The phosphorylated PAG constitutes a best-fitting model. The N- and C-termini of Lyn SH2 (small arrowheads) are facing the nonphosphorylated PAG structure, and SH2 binding to PAG should be affected when the complete Lyn protein interacts with PAG. In contrast, the N- and C-termini of Lyn SH2 (small arrowheads) are facing away from the contact area with the phosphorylated PAG structure, an orientation that is likely not to be affected when the whole Lyn protein interacts with phosphorylated PAG. (B) The residue per residue contact maps show the residues involved in the contact area for phosphorylated PAG. The Lyn residues involved in contacts are shown in the vertical axis and PAG residues on the horizontal axis. Y299 of phosphorylated PAG is shown to make contacts with Pro 18, Gly 19, Asn 20, Ser 21, Ala 22, Arg 42, and Asp 50 of Lyn SH2. The encircled area of Lyn SH2 (Figure 7A) contains Pro 46, Val 47, and His 48 (in a coiled region between 2 β-sheets) that make contacts with different residues of phosphorylated PAG. (C) According to Cary and Cooper,16 Csk docks onto PAG (pY317), phosphorylates Lyn pY507, causing the Lyn SH2 to interact with Lyn pY507 and to form an intramolecular loop, resulting in a Lyn kinase of low catalytic activity. (D) In the raft-based “signalosome” of B-NHLs, the acylated Lyn kinase and the transmembrane PAG are both inserted in rafts. Modular interactions through Lyn SH2 and SH3 further stabilize the Lyn/PAG signalosome. The SH2 of Lyn is not interacting with its regulatory, C-terminal pY507 (intramolecular interaction), but instead with a cleft in PAG containing pY299. The Lyn C-terminal pYQQQ could also be recognized by STAT3 SH2, an interaction that further limits the possibility of intramolecular binding to Lyn SH2. PAG is phosphotyrosylated by Lyn at least at Y299 and Y317, and Lyn autophosphorylates at Y396 in the catalytic domain (SH1) and at the regulatory site Y507.
Discussion
To define the proximal control of constitutive proliferation and apoptosis resistance in human lymphomas, we have investigated the membrane organization of the Lyn tyrosine kinase and the PAG adaptor, a negative regulator of Src-kinase activity in murine and human T lymphocytes.10,11,13 We show that, in B-NHL rafts, PAG is functionally associated with Lyn and pSTAT3 and could function as part of a constitutively active “signalosome.”
Lyn, PAG, and pSTAT3 form such a signalosome in all B-NHLs and corresponding lymphoma tissues, whereas in ALK+ and HL cell lines expressing the same signaling proteins and similar amounts of raft sphingolipids, no such raft-associated signalosome is detectable, although STAT3 is activated by cytoplasmic or nuclear oncogenic kinases in ALK+30 and HL.31 In contrast to pSTAT3, the Csk cytoplasmic kinase that binds PAG and down-regulates Src-family kinases in normal T lymphocytes10,–12,16 was not associated with the B-NHL signalosome, despite PAG Y317 phosphorylation, the binding motif for Csk SH2. The catalytically active Lyn in B-NHL rafts most likely autophosphorylates its regulatory tyrosine because it was shown to do so in isolated Lyn32 and in chronic lymphocytic leukemia cells.33
This organization of proximal signaling in human lymphoma B cells stands in contrast to what was shown in murine11 and human13 T lymphocytes where Csk associates with PAG after phosphorylation of tyrosine 317 (human) or 314 (murine) by the Fyn kinase. However, whereas formation of a complex between PAG and Fyn was restricted to unstimulated murine T cells,11 formation of a multimolecular complex including PAG and Fyn was also demonstrated in stimulated human T cells.13
In human B-NHLs, the Lyn/PAG signalosome (Figure 7D) appears to result from tyrosine phosphorylation of PAG by doubly autophosphorylated Lyn, which then allows interaction of Lyn SH2 with PAG. The Lyn SH2, as we suggest, could bind PAG in a cleft including Y299, even though Y299 is not part of a consensus motif recognized by Src SH2. Several clusters of coordinated residues can be predicted between Lyn SH2 and the phosphorylated PAG cleft (Figure 7A), including phosphorylated Y299 (Figure 7B) to suggest a strong interaction unperturbed by other domains of Lyn. Moreover, the binding of Lyn SH3 to PAG could also consolidate the Lyn/PAG interaction and prevent Lyn SH3 from binding the linker region of Lyn, a prerequisite for achieving a closed-inactive conformation (Figure 7C). Finally, Lyn pY507 would remain available for binding by STAT3 SH2 (pY507QQQ, similar to the pYXXQ STAT3 SH2 consensus motif34 ).
The apparent MW of tyrosine phosphorylated PAG in rafts (80-90 kDa) is approximately twice the molecular size deducible from the polypeptide chain length (432 amino acids, 46.77 kDa). This higher than expected MW is thought to derive from a high contents in glutamic and aspartic acid (13.9%) that causes anomalous interactions of the polypeptide chain with SDS. In particular, the phosphotyrosylated 80-kDa component detected by antiphosphotyrosine, anti-PAG pY317, and antiphosphoLyn antibodies remains incompletely defined, and further work is needed to characterize the multimers resulting from Lyn-PAG interactions in B-NHLs.
The stable interaction of PAG with rafts,8,9 its functional interaction with Lyn and of Lyn with pSTAT3, define an organization of signaling that makes B-lymphoma cells particularly dependent on the Lyn kinase. This functional organization is destroyed by CGP76030, which inhibits Lyn and PAG phosphorylation and dissociates Lyn from rafts and PAG and by decreasing the expression of Lyn or PAG that results in cell death. This situation is reminiscent of the “oncogene addiction” characteristic of leukemias and solid tumors, where the balance of prosurvival and proapoptotic signals is tipped in favor of cell death induction on acute oncogene inactivation (“oncogenic shock”).35,36 The dependence of B-NHLs on Lyn is probably due to the association of Lyn with PAG and STAT3 in rafts, which puts the Lyn kinase in control of both prosurvival and proapoptotic pathways. Lyn inhibition could therefore result in cell death because of greater attenuation of prosurvival than proapoptotic signals. A similar oncogenic interaction of Lyn was recently described with ITAM motifs in the Kaposi sarcoma-associated herpesvirus K1 protein,37 and an oncogenic role for Lyn was also postulated in chronic myelogenous leukemia cells showing resistance to Imatimib mesylate (Glivec) independently of Bcr/Abl.38 Likewise, in acute lymphoblastic leukemic cells expressing BCR-ABL1 gene, the Lyn kinase was shown to constitute a critical oncogene.39 Finally, in case of SLP-65 (BLNK) linker protein deficiency, a direct oncogenic activity was also demonstrated for the Lyn kinase.40 Despite its oncogenic and regulatory properties, PAG appears redundant in lymphocyte physiology because mice with a disrupted Cbp/PAG gene exhibit normal lymphocyte activation and maturation.41,42 PAG may therefore constitute an adaptor selectively capable of increasing the oncogenic potential of Lyn in B-NHL cells.
The signalosome in B-NHL can be modulated by therapeutic antibodies, such as the anti-CD20 rituximab, which decreases Lyn activity in rafts.4 Defining the structure and mode of assembly of the Lyn-PAG signalosome should be of value to design therapeutic compounds that target rafts and disrupt proximal signaling. The failure of Raji cells to die after Lyn inhibition is not the result of the lack of Lyn inactivation by CGP76030, but rather to a block in cytoplasmic assembly of the apoptosome in Burkitt-derived lymphoma cells.43 B-NHLs (with the exception of Burkitt-derived lymphomas) are therefore best suited for targeting the Lyn-PAG signalosome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank I. Dunand, E. Leimgruber, and W. Reith for their kind and efficient help with the silencing experiments and B. Huard for assessing the proliferation experiments.
This work was supported by grants from the Swiss National Science Foundation (3100AO-102158), Oncosuisse (OCS 01408-08-2003), the Ligue genevoise contre le cancer, and Bernische Krebsliga. This work was supported in part by the Pakistan Academy of Sciences (N.-u.-D.).
Authorship
Contribution: S.T, H.D., and K.K. performed the experiments, prepared the illustrations, and revised the manuscript; D.B. carried out the experiments; I.A. and N.-u.-D. designed and performed the bioinformatics analysis; G.v.E.-D. performed and analyzed the lipid study; J.A.L. and B.S. provided important reagents; B.B. and D.C.H. designed the study; and D.C.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Hoessli, Department of Pathology and Immunology, Centre médical universitaire, 1 rue Michel-Servet, CH-1211 Genève 4, Switzerland; e-mail: Daniel.Hoessli@medecine.unige.ch.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal