To the editor:
The FMC7 antibody, originally described in 1981,1 detects an antigen expressed on mature human B cells and is used in immunophenotypic analysis and differential diagnosis of lymphomas and leukemias. The identity of the FMC7 antigen was unclear for many years. An early unconfirmed report indicated that it might be a 105 kDa protein, based on a Western blot using FMC7 and a radio-iodinated secondary antibody.2 This finding may have deflected attention from the possibility that FMC7 was related to the 33 kDa B-cell antigen, CD20, even though the expression of FMC7 generally correlates well with that of CD20.3,4 Serke et al provided the first evidence that FMC7 is an epitope on the CD20 molecule itself.5 Their study showed that transient transfection of CD20 in the human K562 erythroleukemia cell line caused expression of FMC7 in approximately half of CD20-expressing cells. The data were not definitive, as weak expression and the use of a human hematopoeitic cell line allowed for the alternate interpretation that ectopically expressed CD20 may have upregulated another protein reactive with the FMC7 antibody. In 2003 we confirmed and extended their finding, demonstrating that FMC7 was very strongly and uniformly expressed on 100% of CD20-transfected non–B cells, even of nonhuman, nonhematopoietic origin.6 Furthermore, point mutations in the extracellular region of CD20 destroyed the FMC7 epitope along with all other extracellular CD20 epitopes.6,7 In unpublished data, we have found that deletions in the intracellular regions of CD20 also profoundly reduce FMC7 expression, presumably by affecting CD20 conformation.
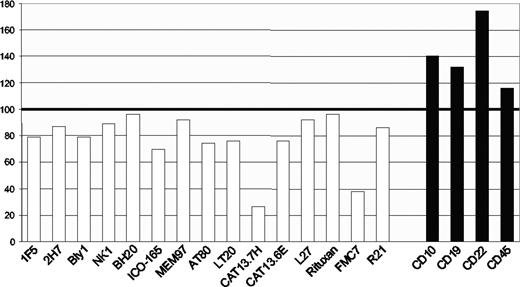
Importantly, the epitope is unusually sensitive to levels of membrane cholesterol.6 FMC7 expression will be different in cells expressing the same amount of CD20 if the cholesterol level varies, as it does among cell lines and during B-cell maturation.8 This provides an explanation for why the correlation between FMC7 and endogenous CD20 expression, while strong, is not strictly held. Cholesterol sensitivity is a common but variable feature of antibodies that detect extracellular CD20 epitopes (Figure 1). Among the 15 antibodies that we tested, FMC7 and one other antibody, CAT13.7H, fall at the extreme end of sensitivity, while rituximab and several other CD20 antibodies are far less affected.
Cholesterol sensitivity of CD20 epitopes. Ramos B cells were treated with methyl-β-cyclodextrin to deplete membrane cholesterol before performing indirect staining with a panel of 15 CD20 antibodies (□), or antibodies against CD10, CD19, CD22, or CD45 (■) for comparison. The bars show the mean fluorescence intensity (MFI) of treated cells as a percentage of MFI values from untreated cells. Data are representative of at least 2 tests of each antibody.
Cholesterol sensitivity of CD20 epitopes. Ramos B cells were treated with methyl-β-cyclodextrin to deplete membrane cholesterol before performing indirect staining with a panel of 15 CD20 antibodies (□), or antibodies against CD10, CD19, CD22, or CD45 (■) for comparison. The bars show the mean fluorescence intensity (MFI) of treated cells as a percentage of MFI values from untreated cells. Data are representative of at least 2 tests of each antibody.
Regrettably, the description by vendors and others of FMC7 as an unidentified 105 kDa antigen persists.9 We hope with this letter to clarify for a wider audience of hematologists and pathologists that FMC7 is, in fact, an epitope of CD20.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie P. Deans, Department of Biochemistry and Molecular Biology, Immunology Research Group, Insititute of Infection, Immunity and Inflammation, University of Calgary, Calgary, Alberta, Canada; e-mail: jdeans@ucalgary.ca

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal