For acute myeloid leukemia (AML), the major form that occurs in children with Down syndrome (DS) is acute megakaryoblastic leukemia (AMKL). In 14% of these children, there is a history of transient myeloproliferative disorder (TMD) at birth.1 Somatic mutations of GATA1 gene are found in both children with DS-AMKL and TMD.2 Because acquired mutations of JAK2 in patients with non–DS-AMKL3 and mutations of JAK3 in patients with AMKL with or without DS4,–6 have been described, Norton et al investigated the frequency of JAK2 and JAK3 mutations in 19 and 16 children with TMD or DS-AMKL, respectively.7 However, they failed to detect any mutations for either of the genes in any of the patients. Therefore, they concluded that there is a low incidence of JAK3 mutation and that there is no evidence yet that JAK2 mutations contribute to DS malignancies. Here, we report the first case of a DS patient who had mutations of JAK2, JAK3 and GATA1 in her AMKL cells.
At age 29 months, a female child with DS presented with right exophthalmos. A Head CT scan showed a tumor in her right orbit. An immunohistochemical examination of the tumor revealed that the blast cells were positive for LCA, MT-1 and CD68, and negative for UCHL-1, MB-1, L-26 and Ki-1. Five months later she developed overt leukemia. Bone marrow aspiration revealed a predominance of blast cells with basophilic cytoplasm and cytoplasmic blebs, which were negative for myeloperoxidase and nonspecific esterase stain. Immunophenotypically, the blast cells were positive for CD7, CD13, CD33, CD42b, and CD117. Chromosomal analysis revealed 47, XX, del(7)(p15), +8, del(13)(q12q32), −14, der(14;21)(q10;q10)c, del(17)(p11), +21c, +mar. She was diagnosed as AMKL and received intensive chemotherapy, although she did not respond. She died from the progressive disease 10 months after the initial presentation. We analyzed the GATA1, JAK2 and JAK3 mutations in her peripheral blood samples. Written informed consent for banking and molecular analysis of leukemic cells in accordance with the Declaration of Helsinki was obtained from the parents of the patient. High-molecular weight DNA was extracted from the samples using standard methods. We amplified the genomic DNA corresponding to exon2 of GATA1,2 exon12 of JAK2,8 and all 23 exons of the JAK34 gene, respectively and the amplified products were sequenced directly.
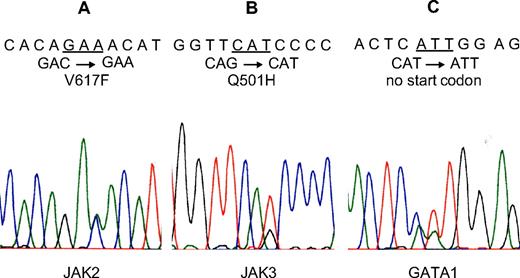
Mutations of JAK2 V617F, JAK3 Q501H, and GATA1 (2-3TG > AT, no start codon), were found in the patient (Figure 1). Until now, 4 active mutations (A572V,4 A573V,5,6 A593T,5 and V722I4 ) of the JAK3 gene have been found in samples obtained from patients with DS-AMKL. All of these were uniformly located in the JH2 pseudokinase domain. A novel mutation (Q501H) that was found in this patient occurred in the JH3 SH2 domain. The function of this domain is not completely understood and the binding partners also have not yet been identified. Park et al reported that there were no mutations of the JAK2 gene found in any of the 23 samples from the patients with TMD or DS-AMKL.9 JAK2 and JAK3 mutation may have contributed to the resistance to the intensive chemotherapy in our patient.
Mutations of JAK2, JAK3, and GATA1 in a child with DS-AMKL. Mutations of JAK2 V617F (A), JAK3 Q501H (B), and GATA1 (2-3TG > AT, no start codon; C) were found in a child with DS-AMKL.
Mutations of JAK2, JAK3, and GATA1 in a child with DS-AMKL. Mutations of JAK2 V617F (A), JAK3 Q501H (B), and GATA1 (2-3TG > AT, no start codon; C) were found in a child with DS-AMKL.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Kojima, Department of Pediatrics, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, 466-8550, Japan; e-mail: kojimas@med.nagoya-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal