Abstract
Transcription factors known as CCAAT enhancer binding proteins (C/EBPs) are involved in hematopoietic differentiation, including myelopoiesis and granulopoiesis. C/EBPβ-deficient mice develop normally; however, they exhibit defective macrophage function, resulting in increased susceptibility to infection. Little is known about the role of C/EBPβ in granulopoiesis; therefore, we examined granulopoiesis in C/EBPβ-deficient mice. Morphology, the number of peripheral blood and bone marrow cells, and the expression of genes specific for the myeloid lineage were normal in C/EBPβ-deficient mice. Interestingly, the hematopoietic progenitor cells of C/EBPβ-deficient mice did not respond normally to granulocyte/macrophage-colony stimulating factor and granulocyte colony stimulating factor. In addition, C/EBPβ-deficient neutrophils displayed enhanced apoptosis compared with wild-type neutrophils. Our present results indicate that C/EBPβ helps regulate survival of neutrophils, downstream of the granulocyte colony stimulating factor receptor.
Introduction
A large family of transcription factors known as the CCAAT enhancer binding proteins (C/EBPs) currently includes C/EBPα, C/EBPβ (NF-IL6, LAP), C/EBPγ, C/EBPδ (NF-IL6β, CRP3), C/EBPϵ, and C/EBPζ (CHOP, GADD153).1 These transcription factors regulate the expression of their target genes by homo- or hetero-dimerizing via their C-terminal leucine zipper domains, and are implicated in proliferation, differentiation, and apoptosis in a variety of cell types.2-4
They are involved in hematopoietic cell growth and differentiation, including myelopoiesis and granulopoiesis.3,4 C/EBPα-deficient mice lack neutrophils and eosinophils, and the common myeloid progenitor (CMP) does not effectively dif-ferentiate to granulocyte-monocyte progenitors (GMP).5-7 Approximately 10% to 15% of acute myeloid leukemias have inactivating mutations of C/EBPα.8,9 C/EBPϵ-deficient mice develop normally but fail to generate functional neutrophils and eosinophils.10
C/EBPβ-deficient mice develop normally, but they are highly susceptible to infection by Listeria, Candida albicans, and Brucella abortus.11-13 The macrophages of C/EBPβ-deficient mice have a defect in their ability to kill bacteria and tumor cells.11,12 Long-term culture of B lymphocytes derived from bone marrow of C/EBPβ-deficient mice have impaired expansion as well as decreased number of B lymphocytes in the bone marrow.14 With aging, C/EBPβ-deficient mice develop splenomegaly and lymph node enlargement with an expansion of B cells in these compartments,13 suggesting that C/EBPβ has a critical function in B-cell development. Males of the mutant animals are fertile; however, adult females are sterile because of the deficiency of mature corpora lutea in ovaries, showing that C/EBPβ has an essential role for female reproduction.15
Numerous cytokine-encoding genes, which are important for inflammation and immunity, including interleukin-6 (IL-6), IL-10, IL-12, tumor necrosis factor-α (TNFα), and granulocyte colony-stimulating factor (G-CSF), are downstream targets of C/EBPβ.11,16-21 For example, IL-6, TNFα, and G-CSF were abundantly produced in wild-type macrophages stimulated with IFNγ and lipopolysaccharide (LPS), but their expression was diminished in C/EBPβ-deficient macrophages.11,18
Compared with analysis of B-lymphocytes and macrophages, little is known about the function of neutrophils in C/EBPβ-deficient mice. We can arbitrarily divide the development of neutrophils into 3 stages: immature stage when azurophilic (primary) granules are produced and the cells proliferate (myeloblast and promyelocytes), intermediate stage when secondary granule proteins are made and proliferation ceases (myelocytes and metamyelocytes), and mature nondividing fully functional stage (band cells and segmented neutrophils).22,23 Expression analysis showed that levels of C/EBPα are prevalent at the early myeloid stages and markedly decrease in the mature neutrophils, whereas levels of C/EBPϵ mRNA increase in the myelocytes and metamyelocytes.23 C/EBPβ mRNA is abundant in band cells and segmented neutrophils but is very low in the immature and intermediate mature granulocytic precursor cells.23-25 Interestingly, forced expression of C/EBPβ induces granulocytic differentiation in the erythroleukemia cell line, K562.26 Taken together, these findings suggest that C/EBPβ is involved in neutrophil development. We hypothesized that C/EBPβ is important for granulocyte development or function. To test this, we examined granulopoiesis in C/EBPβ-deficient mice.
Methods
The use of animals in this study was approved by the IACUC at Cedars-Sinai Medical Center.
Cytokines, antibodies, and other reagents
Murine granulocyte-macrophage-colony stimulating factor (GM-CSF), G-CSF, stem-cell factor (SCF), and interleukin-3 (IL-3) as well as human IL-6, IL-11, and thrombopoietin (TPO) were purchased from Calbiochem (San Diego, CA). Human erythropoietin was from Amgen (Thousand Oaks, CA). MethoCult media M3234 and MegaCult-C media were from StemCell Technologies (Vancouver, BC). Isocove modified Dulbecco medium, RPMI 1640, and phosphate-buffered saline (PBS) were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was from Omega Scientific (Tarzana, CA). Fluorescein isothiocyanate (FITC)-conjugated rat antimouse CD14 and peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated rat antimouse Gr-1 antibodies were from BD Bioscience PharMingen (San Jose, CA). Phycoerythrin (PE)-conjugated rat antimouse CD11b/Mac1 antibody was from Serotec (Raleigh, NC). Antiphospho-STAT3 (Tyr705) and anti-STAT3 were obtained from Upstate Cell Signaling (Lake Placid, NY) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Wild-type and C/EBPβ-deficient mice and cell cultures
C/EBPβ-deficient mice were genetically modified as described previously.11,12 The mice were bred under pathogen-free conditions and were killed by CO2 inhalation followed by cervical dislocation. Bone marrow cells were harvested from wild-type and C/EBPβ-deficient mice at 6 to 8 weeks of age. Neutrophils and macrophages were collected by peritoneal lavage with cold PBS at either 18 hours or 3 days after intraperitoneal injection with 1.5 mL of 4% sterile thioglycollate (Sigma-Aldrich, St Louis, MO), respectively. After centrifugation, peritoneal cells were resuspended in Isocove modified Dulbecco medium supplemented with 10% FBS. To isolate nonadherent neutrophils, the lavage cells harvested at 18 hours were cultured for 3 hours and nonadherent cells were collected. To collect macrophages, the lavage cells harvested at day 3 after thioglycollate injection were cultured for 3 hours, nonadherent cells were washed from the plate, and the adherent macrophages were retained. Cytospins were prepared and stained with Diff-Quick (Dade Behring, Dudingen, Switzerland), and the percentage of neutrophils and macrophages was determined by differential counts.
Analysis of peripheral blood and bone marrow cells
Peripheral blood was used directly for blood smears. Cytospins of bone marrow samples were stained with Wright-Giemsa, Sudan Black B as well as staining for myeloperoxidase (MPO) and lipase. For differential counts of bone marrow cytospins, more than 500 cells were counted for each sample. Peripheral blood samples were obtained from the ocular sinus and counted using a HEMAVET 850 (Drew Scientific, Dallas, TX).
RNA extraction, cDNA synthesis, and real-time quantitative RT-PCR
Total RNA was isolated from neutrophils and macrophages either with or without LPS (100 ng/mL, Sigma-Aldrich) treatment for 4 hours, using Trizol reagent (Invitrogen). Total RNA (1 μg) was converted into cDNA by reverse transcription(RT) with Superscript III (Invitrogen). Gene expression was quantified with real-time quantitative polymerase chain reaction (PCR; iCycler, Bio-Rad, Hercules, CA) using either a TaqMan probe or Sybr Green as described previously.27 Sequences of the primer/probe sets will be provided on request.
Colony-forming assay and Western blot analysis
Colony-forming assay was performed as described previously.28 Colony formation was stimulated by multiple combinations of purified recombinant growth factors at the following final concentrations: murine GM-CSF at 20 ng/mL, murine G-CSF at 60 ng/mL, murine IL-3 at 10 ng/mL, murine SCF at 20 ng/mL, and human erythropoietin (EPO) at 3 U/mL. For CFU-E, 2 × 105 bone marrow cells were plated with human EPO (3 U/mL). For BFU-E (7 days culture), bone marrow cells were plated with murine SCF (20 ng/mL) and human EPO (3 U/mL), and colonies were stained with benzidine. For the megakaryocyte colony-forming unit (CFU-Meg) assay, bone marrow cells (105) were cultured with MegaCult-C media in the presence of murine IL-3 (10 ng/mL), human IL-6 (20 ng/mL), human IL-11 (10 ng/mL), and human TPO (50 ng/mL). After 7 days, colonies were stained with acetylcholine esterase. The CFU-Meg colonies were defined as at least 3 megakaryocytes in a cluster.
For detection of phospho-STAT3, bone marrow cells were cultured either with or without 100 ng/mL of G-CSF for 30 minutes. These samples were subjected to SDS-PAGE followed by an electro-transfer to polyvinylidene difluoride membrane, and then the membrane was probed with antiphospho-STAT3 or anti-STAT3 antibodies. The signals were developed with Supersignal West Pico-Chemiluminescent (Pierce Biotechnology, Rockford, IL).
Flow cytometric analysis and apoptosis assay
Mononuclear cells from bone marrow (106) were incubated with the antibodies described above in PBS with 1% BSA for 1 hour at 4°C. These cells were washed with PBS, fixed with 2% paraformaldehyde, and analyzed by flow cytometry.
Apoptosis of neutrophils was detected by the TUNEL assay using the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN) or the annexin V-FITC apoptosis detection kit (BD Bioscience PharMingen). The neutrophils were cultured in either the presence or the absence of GM-CSF (10 ng/mL) or LPS (100 ng/mL) in RPMI 1640 supplemented with 10% FBS and harvested after 2, 6, and 18 hours. Cells were fixed with 4% formaldehyde and permeabilized with 0.2% Triton X-100. Apoptotic neutrophils were counted under the fluorescent microscope (total of 600 cells per slide) or determined by annexin V-FITC staining using flow cytometry either with or without concomitant staining for anti-Gr-1 (neutrophil marker).
5-fluorouracil injection
5-fluorouracil (5-FU; Sigma-Aldrich) was dissolved in saline and given intraperitoneally in a single dose of 5-FU at 150 mg/kg (n = 4 wild-type mice; n = 4 C/EBPβ-deficient mice). Blood samples were taken from the ocular sinus and counted with HEMAVET 850. To confirm the neutrophil count, cytospin preparations were made, stained with Diff-Quick, and analyzed by light microscopy (a total of 500 cells were counted).
Results
Morphologic and cell surface antigen characterization of cells of the peripheral blood and bone marrow
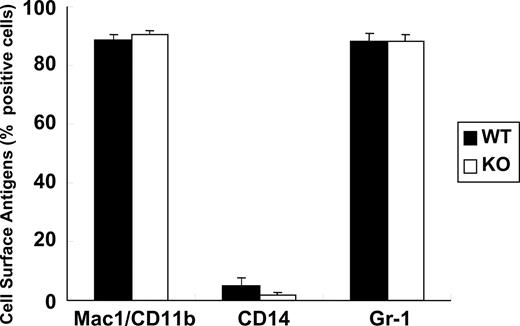
First, we analyzed peripheral blood components in the C/EBPβ-deficient versus wild-type mice. Peripheral blood cells, including red blood cells, white blood cells, neutrophils, platelets, and lymphocytes were almost the same in the C/EBPβ-deficient and wild-type mice (Table 1). We also determined the components of bone marrow cells in the C/EBPβ-deficient and wild-type mice. Morphologic observation revealed that the relative ratios of each bone marrow cell subtype (myeloblasts/promyelocytes, myelocytes/metamyelocytes, neutrophils, monocytes/macrophages, lymphocytes, erythroid cells, and megakaryocytes) were similar in the C/EBPβ-deficient and wild-type mice (Table 2). Bone marrow cells were stained for MPO and Sudan Black B to detect granulocytic cells, and lipase to detect monocytic cells (Table 2). No significant differences were noted between the C/EBPβ-deficient and wild-type mice. We compared the cell-surface makers specific for the myeloid lineage, including Mac1/CD11b, CD14, and Gr-1 in the C/EBPβ-deficient and wild-type bone marrow cells by flow cytometry analysis. Results were similar in both types of mice (Figure 1). Taken together, these results showed that peripheral blood and bone marrow cells from the C/EBPβ-deficient and wild-type mice were morphologically and phenotypically similar.
Blood cell counts in wild-type and C/EBPβ-deficient mice
| Cell type . | Genotypes . | P . | |
|---|---|---|---|
| Wild-type, n = 5 . | C/EBPβ-deficient, n = 4 . | ||
| RBCs, × 1012 cells/L | 7.2 ± 1.3 | 6.8 ± 0.8 | .58 |
| WBCs, × 109 cells/L | 9.3 ± 4.3 | 10.3 ± 4.6 | .73 |
| Neutrophils, × 109 cells/L | 1.0 ± 0.9 | 0.5 ± 0.4 | .25 |
| Lymphocytes, × 109 cells/L | 7.5 ± 3.0 | 9.2 ± 3.7 | .47 |
| Platelets, × 109 cells/L | 833 ± 378 | 1376 ± 643 | .2 |
| Others, × 109 cells/L | 1.4 ± 0.8 | 1.3 ± 0.2 | .92 |
| Cell type . | Genotypes . | P . | |
|---|---|---|---|
| Wild-type, n = 5 . | C/EBPβ-deficient, n = 4 . | ||
| RBCs, × 1012 cells/L | 7.2 ± 1.3 | 6.8 ± 0.8 | .58 |
| WBCs, × 109 cells/L | 9.3 ± 4.3 | 10.3 ± 4.6 | .73 |
| Neutrophils, × 109 cells/L | 1.0 ± 0.9 | 0.5 ± 0.4 | .25 |
| Lymphocytes, × 109 cells/L | 7.5 ± 3.0 | 9.2 ± 3.7 | .47 |
| Platelets, × 109 cells/L | 833 ± 378 | 1376 ± 643 | .2 |
| Others, × 109 cells/L | 1.4 ± 0.8 | 1.3 ± 0.2 | .92 |
Blood samples from mice of each genotype were analyzed. Values represent the mean plus or minus the SD.
RBCs indicates red blood cells; WBCs, white blood cells; and others, eosinophils and basophils.
Differential counts of bone marrow cells in wild-type and C/EBPβ-deficient mice
| Cell type . | Genotypes . | P . | |
|---|---|---|---|
| Wild-type, n = 4 . | C/EBPβ-deficient, n = 4 . | ||
| Myeloblasts/promyelocytes | 3 ± 1 | 4 ± 1 | .26 |
| Myelocytes/metamyelocytes | 29 ± 5 | 31 ± 6 | .61 |
| Neutrophils | 27 ± 2 | 25 ± 4 | .76 |
| Monocytes/macrophages | 4 ± 1 | 2 ± 1 | .14 |
| Lymphocytes | 14 ± 0 | 11 ± 1 | .22 |
| Erythroid cells | 27 ± 5 | 27 ± 4 | .99 |
| Megakaryocytes | 6 ± 1 | 6 ± 2 | .82 |
| MPO-positive cells | 60 ± 2 | 64 ± 1 | .62 |
| Sudan Black B–positive cells | 61 ± 5 | 71 ± 5 | .15 |
| Lipase positive cells | 4 ± 1 | 2 ± 1 | .75 |
| Cell type . | Genotypes . | P . | |
|---|---|---|---|
| Wild-type, n = 4 . | C/EBPβ-deficient, n = 4 . | ||
| Myeloblasts/promyelocytes | 3 ± 1 | 4 ± 1 | .26 |
| Myelocytes/metamyelocytes | 29 ± 5 | 31 ± 6 | .61 |
| Neutrophils | 27 ± 2 | 25 ± 4 | .76 |
| Monocytes/macrophages | 4 ± 1 | 2 ± 1 | .14 |
| Lymphocytes | 14 ± 0 | 11 ± 1 | .22 |
| Erythroid cells | 27 ± 5 | 27 ± 4 | .99 |
| Megakaryocytes | 6 ± 1 | 6 ± 2 | .82 |
| MPO-positive cells | 60 ± 2 | 64 ± 1 | .62 |
| Sudan Black B–positive cells | 61 ± 5 | 71 ± 5 | .15 |
| Lipase positive cells | 4 ± 1 | 2 ± 1 | .75 |
Bone marrow samples of 4 mice from each genotype were analyzed. Cytopreps of cells were stained with Wright-Giemsa, MPO, Sudan Black B, and lipase. Megakaryocytes were counted in 20 fields using ×400 magnification. Values represent the mean cell percentages plus or minus the SD.
Myeloid lineage analysis of bone marrow cells. Bone marrow cells were isolated from wild-type (WT; n = 4) and C/EBPβ-deficient (KO; n = 4) adult mice, stained with fluorescent-labeled antibodies against myeloid lineage-specific antigens (Mac1/CD11b, CD14, and Gr-1), and analyzed by flow cytometry. Results represent the mean plus or minus SD of 4 mice in each cohort.
Myeloid lineage analysis of bone marrow cells. Bone marrow cells were isolated from wild-type (WT; n = 4) and C/EBPβ-deficient (KO; n = 4) adult mice, stained with fluorescent-labeled antibodies against myeloid lineage-specific antigens (Mac1/CD11b, CD14, and Gr-1), and analyzed by flow cytometry. Results represent the mean plus or minus SD of 4 mice in each cohort.
Molecular characterization of bone marrow cells
Next, we investigated mRNA levels of myeloid-specific genes in the bone marrow cells of the C/EBPβ-deficient and wild-type mice. As summarized in Table 3, the expression levels of primary (azurophilic)-granule (MPO), and secondary and tertiary neutrophil granule protein genes (lactoferrin, neutrophil collagenase, neutrophil gelatinase, neutrophil gelatinase-associated lipocalin, murine cathelin-like protein, and cathelin B9) as well as eosinophil granule protein genes (major basic protein and eosinophil peroxidase) were comparable between both C/EBPβ-deficient and wild-type bone marrow cells.
Relative expression of primary, secondary, and tertiary granule- and eosinophil-specific mRNAs in bone marrow cells
| Genes . | Genotypes . | |
|---|---|---|
| Wild-type, n = 4 . | C/EBPβ-deficient, n = 4 . | |
| MPO | 1.00 ± 0.23 | 1.21 ± 0.21 |
| LF | 1.00 ± 0.34 | 0.83 ± 0.22 |
| NC | 1.00 ± 0.23 | 1.21 ± 0.12 |
| NG | 1.00 ± 0.18 | 0.92 ± 0.08 |
| NGAL | 1.00 ± 0.32 | 1.00 ± 0.18 |
| MCLP | 1.00 ± 0.24 | 1.00 ± 0.22 |
| B9/NGP | 1.00 ± 0.23 | 1.13 ± 0.28 |
| MBP | 1.00 ± 0.18 | 1.07 ± 0.26 |
| EPX | 1.00 ± 0.14 | 1.00 ± 0.18 |
| Genes . | Genotypes . | |
|---|---|---|
| Wild-type, n = 4 . | C/EBPβ-deficient, n = 4 . | |
| MPO | 1.00 ± 0.23 | 1.21 ± 0.21 |
| LF | 1.00 ± 0.34 | 0.83 ± 0.22 |
| NC | 1.00 ± 0.23 | 1.21 ± 0.12 |
| NG | 1.00 ± 0.18 | 0.92 ± 0.08 |
| NGAL | 1.00 ± 0.32 | 1.00 ± 0.18 |
| MCLP | 1.00 ± 0.24 | 1.00 ± 0.22 |
| B9/NGP | 1.00 ± 0.23 | 1.13 ± 0.28 |
| MBP | 1.00 ± 0.18 | 1.07 ± 0.26 |
| EPX | 1.00 ± 0.14 | 1.00 ± 0.18 |
Expression of each gene was determined by RT-PCR and normalized to the expression level of 18S RNA. Values are means plus or minus SD.
MPO indicates myeloperoxidase; LF, lactoferrin; NC, neutrophil collagenase; NG, neutrophil gelatinase; NGAL, neutrophil gelatinase-associated lipocalin; MCLP, murine cathelin-like protein; B9/NGP, cathelin B9; MBP, major basic protein; and EPX, eosinophil peroxidase.
Analysis of hematopoietic progenitor cells
Clonogenic assay in methylcellulose revealed that the number of myeloid clonogenic cells was similar between wild-type and C/EBPβ-deficient bone marrow cells cultured simultaneously with IL-3, IL-6, EPO, and SCF (Figure 2A). On the other hand, the number of colonies stimulated in the presence of either GM-CSF, SCF/G-CSF, or G-CSF was significantly decreased from bone marrow cells of C/EBPβ-deficient mice compared with wild-type mice. In contrast, the number of erythroid- and megakaryocytic-lineage committed bone marrow clonogenic stem cells cultured with EPO (CFU-E), EPO/SCF (BFU-E), or TPO/IL-3/IL-6/IL-11 (CFU-mk) were comparable in C/EBPβ-deficient and wild-type mice (Figure 2B).
Analysis of bone marrow cells. Bone marrow cells were isolated from wild-type (n = 4) and C/EBPβ-deficient (n = 4) adult mice. (A) Bone marrow cells (2 × 104) were cultured in 1% methylcellulose supplemented with IL-3 (10 ng/mL), IL-6 (10 ng/mL), SCF (20 ng/mL), and EPO (3 U/mL);or GM-CSF (20 ng/mL); or G-CSF (60 ng/mL) and SCF (20 ng/mL); or G-CSF (60 ng/mL) alone. Colonies were scored if they contained more than 50 cells at day 7. Results in panels A and B represent the mean plus or minus SD of 4 mice in each cohort (*P < .001; **P < .005). (B) Bone marrow cells (2 × 105) were cultured in either 1% methylcellulose or MegaCult-C media (CFU-Meg), supplemented with either EPO (3 U/mL); or EPO (3 U/mL) and SCF (20 ng/mL); or TPO (50 ng/mL), IL-3 (10 ng/mL), IL-6 (20 ng/mL), and IL-11 (10 ng/mL). BFU-E (EPO and SCF) and CFU-Meg (TPO, IL-3, IL-6, and IL-11) were counted on day 7 of culture; CFU-E (EPO alone) was enumerated on day 2. Black and white bars show wild-type and C/EBPβ-deficient mice, respectively. (C) Detection of phospho-STAT3 in bone marrow cells. Bone marrow cells were isolated from C/EBPβ heterozygous mutant and C/EBPβ-deficient adult mice, and the cells were stimulated either with or without G-CSF (100 ng/mL) for 30 minutes. Signals of phospho-STAT3 (pSTAT3) and STAT3 were detected by Western blot analysis.
Analysis of bone marrow cells. Bone marrow cells were isolated from wild-type (n = 4) and C/EBPβ-deficient (n = 4) adult mice. (A) Bone marrow cells (2 × 104) were cultured in 1% methylcellulose supplemented with IL-3 (10 ng/mL), IL-6 (10 ng/mL), SCF (20 ng/mL), and EPO (3 U/mL);or GM-CSF (20 ng/mL); or G-CSF (60 ng/mL) and SCF (20 ng/mL); or G-CSF (60 ng/mL) alone. Colonies were scored if they contained more than 50 cells at day 7. Results in panels A and B represent the mean plus or minus SD of 4 mice in each cohort (*P < .001; **P < .005). (B) Bone marrow cells (2 × 105) were cultured in either 1% methylcellulose or MegaCult-C media (CFU-Meg), supplemented with either EPO (3 U/mL); or EPO (3 U/mL) and SCF (20 ng/mL); or TPO (50 ng/mL), IL-3 (10 ng/mL), IL-6 (20 ng/mL), and IL-11 (10 ng/mL). BFU-E (EPO and SCF) and CFU-Meg (TPO, IL-3, IL-6, and IL-11) were counted on day 7 of culture; CFU-E (EPO alone) was enumerated on day 2. Black and white bars show wild-type and C/EBPβ-deficient mice, respectively. (C) Detection of phospho-STAT3 in bone marrow cells. Bone marrow cells were isolated from C/EBPβ heterozygous mutant and C/EBPβ-deficient adult mice, and the cells were stimulated either with or without G-CSF (100 ng/mL) for 30 minutes. Signals of phospho-STAT3 (pSTAT3) and STAT3 were detected by Western blot analysis.
STAT3 is a secondary signal molecule activated by G-CSF; therefore, next we examined phosphorylation status of STAT3 in bone marrow cells. As shown in Figure 2C, phospho-STAT3 was detected in the G-CSF-stimulated bone marrow cells, and expression levels of phospho-STAT3 were comparable in C/EBPβ heterozygous mutant and C/EBPβ-deficient mice. This finding suggests that STAT3 pathway is intact in C/EBPβ-deficient bone marrow cells.
Gene expression of cytokines in neutrophils
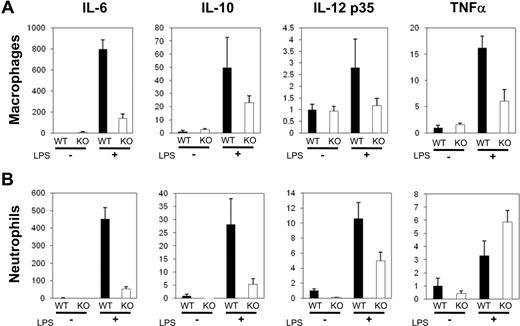
Several genes encoding cytokines, including IL-6, IL-10, IL-12, and TNFα, which are important for inflammation and immunity, are downstream targets of C/EBPβ.11,16-19 Neutrophils and macrophages play an essential role for the innate immune response. To analyze the mRNA levels of these cytokine genes, neutrophils or macrophages were collected by peritoneal lavage with cold PBS at either 18 hours or 3 days after intraperitoneal injection with 1.5 mL of 4% thioglycollate. These cells were cultured either with or without LPS (100 ng/mL) for 4 hours, and expression of cytokine genes was quantified by real-time PCR; data were normalized to 18S RNA levels. Expression of IL-6, IL-10, IL-12 p35, and TNFα mRNAs was increased by LPS in wild-type macrophages (Figure 3A, black bars). Under the same culture conditions, C/EBPβ-deficient macrophages exposed to LPS had lower levels of each of these cytokines (Figure 3A white bars). LPS also stimulated expression of these cytokines in LPS-stimulated wild-type neutrophils (Figure 3B). Induction of IL-6, IL-10, and IL-12 p35 gene expression was impaired in neutrophils of C/EBPβ-deficient mice (Figure 3B). In contrast, levels of TNFα mRNA were higher in the LPS-treated C/EBPβ-deficient neutrophils compared with LPS-treated wild-type neutrophils (Figure 3B).
Differential cytokine gene expression in primary macrophages and neutrophils. Macrophages (A) and neutrophils (B) from wild-type and C/EBPβ-deficient mice were harvested from peritoneal cavity and cultured either with (+) or without (−) LPS (100 ng/mL, 4 hours). Total RNAs were isolated, and expression of cytokine genes was quantified by real-time RT-PCR as a ratio of these transcripts/18S transcripts, and wild-type LPS-untreated samples are calculated as 1. ■ and □ show wild-type and C/EBPβ-deficient mice, respectively. Results represent the mean plus or minus SD.
Differential cytokine gene expression in primary macrophages and neutrophils. Macrophages (A) and neutrophils (B) from wild-type and C/EBPβ-deficient mice were harvested from peritoneal cavity and cultured either with (+) or without (−) LPS (100 ng/mL, 4 hours). Total RNAs were isolated, and expression of cytokine genes was quantified by real-time RT-PCR as a ratio of these transcripts/18S transcripts, and wild-type LPS-untreated samples are calculated as 1. ■ and □ show wild-type and C/EBPβ-deficient mice, respectively. Results represent the mean plus or minus SD.
Apoptosis assay of neutrophils
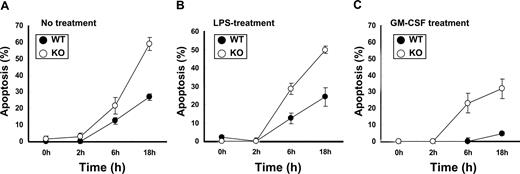
Neutrophils constitutively undergo apoptosis; next, we examined the affect of C/EBPβ on apoptosis of neutrophils. Neutrophils of C/EBPβ-deficient and wild-type mice were harvested from the peritoneal cavity and cultured for 0, 2, 6, and 18 hours with either diluent, LPS or GM-CSF, and apoptosis was measured by TUNEL assay. After 18 hours, wild-type neutrophils had 27%, 24%, and 5% of apoptotic cells in the presence of diluent, LPS, or GM-CSF, respectively (Figure 4). In contrast, C/EBPβ-deficient neutrophils had an approximately 2-fold increase in apoptosis in the untreated and LPS-treated conditions at 18 hours (Figure 4A,B). Inflammatory stimuli, such as GM-CSF and G-CSF, are known to enhance survival of neutrophils.29,30 We found decreased apoptosis in both wild-type and C/EBPβ-deficient neutrophils treated with GM-CSF (Figure 4C); but still, C/EBPβ-deficient neutrophils had an approximately 6-fold increased apoptosis compared with the wild-type neutrophils. Flow cytometry analysis also showed that the Gr-1+, annexin V+, and PI− population (apoptotic neutrophils) was increased in C/EBPβ-deficient neutrophils compared with the wild-type mice (data not shown). These results show that the survival of the C/EBPβ-deficient neutrophils is markedly decreased in the untreated as well as the LPS- and GM-CSF-treated cultures compared with the wild-type neutrophils under similar culture conditions.
Apoptosis assay of neutrophils. Neutrophils of wild-type and C/EBPβ-deficient mice were harvested from peritoneal cavity and cultured for 0, 2, 6, and 18 hours with RPMI 1640 and 10% FBS alone (A) or with LPS (100 ng/mL) (B) or GM-CSF (10 ng/mL) (C). Apoptosis was measured by TUNEL assay. Results represent the mean plus or minus SD of 4 mice in each cohort.
Apoptosis assay of neutrophils. Neutrophils of wild-type and C/EBPβ-deficient mice were harvested from peritoneal cavity and cultured for 0, 2, 6, and 18 hours with RPMI 1640 and 10% FBS alone (A) or with LPS (100 ng/mL) (B) or GM-CSF (10 ng/mL) (C). Apoptosis was measured by TUNEL assay. Results represent the mean plus or minus SD of 4 mice in each cohort.
Neutrophil counts in 5-FU-treated mice
Because our in vitro experiments suggested that committed myeloid stem cells have decreased response to GM-CSF and G-CSF, and C/EBPβ enhanced survival of neutrophils in vitro, we examined peripheral blood neutrophil counts in wild-type and C/EBPβ-deficient mice at 0 to 14 days after injecting 5-FU. 5-FU kills cycling hematopoietic progenitor cells (Figure 5). Neutrophil counts rapidly decreased from day 1 and reached a nadir at day 4. The number of neutrophils was significantly decreased in the C/EBPβ-deficient mice compared with the wild-type mice on day 4: 0.42 × 109 cells/L (SD = 0.03 × 109 cells/L) in wild-type mice and 0.04 × 109 cells/L (SD = 0.03 × 109 cells/L) in C/EBPβ-deficient mice (P < .01). The difference at day 7 was not statistically significant. By day 10, peripheral blood neutrophil counts had returned to normal in both C/EBPβ-deficient and the wild-type mice.
Neutrophil counts in mice after 5-FU treatment. 5-FU (150 mg/kg) was given intraperitoneally to the wild-type and C/EBPβ-deficient mice. Blood samples were taken from the ocular sinus at 0, 1, 4, 7, 10, and 14 days, and neutrophils were counted. Black and broken lines show their mean plus or minus SD results from wild-type and C/EBPβ-deficient mice, respectively.
Neutrophil counts in mice after 5-FU treatment. 5-FU (150 mg/kg) was given intraperitoneally to the wild-type and C/EBPβ-deficient mice. Blood samples were taken from the ocular sinus at 0, 1, 4, 7, 10, and 14 days, and neutrophils were counted. Black and broken lines show their mean plus or minus SD results from wild-type and C/EBPβ-deficient mice, respectively.
Discussion
The present study showed that peripheral blood and bone marrow morphology and differential counts were the same in wild-type and C/EBPβ-deficient mice. Furthermore, levels of expression of genes specific for the myeloid lineage did not differ between the 2 types of mice. These findings indicated a variety of functions as well as differentiation were normal in the myeloid lineage in the C/EBPβ-deficient mice. However, production of cytokines (IL-6, IL-10, and IL-12 p35) was markedly diminished in C/EBPβ-deficient neutrophils as well as macrophages; the latter has been noted.11
Interestingly, the committed myeloid clonogenic cells stimulated with GM-CSF, G-CSF/SCF, or G-CSF were diminished in the C/EBPβ-deficient mice compared with wild-type mice. These results extend those of Hirai et al,31 who recently reported a crucial function for C/EBPβ in emergency granulopoiesis. These investigators showed that G-CSF increased peripheral granulocyte counts in wild-type mice, but not in C/EBPβ-deficient mice. Furthermore, peripheral blood granulocyte counts in C/EBPβ-deficient mice did not increase even in the presence of fungal infection.31 Notably, C/EBPβ-deficient bone marrow cells showed normal expression of G-CSF receptor mRNA (data not shown),31 suggesting that impaired response to G-CSF is not because of loss of expression of the receptor, but an aberrant molecular pathway(s) downstream of the receptor in these cells. STAT3 is a secondary signal of activated G-CSF receptor. Conditional STAT3 knockout mice showed impaired response to G-CSF with failure to increased their peripheral neutrophil counts.32 Our results revealed that levels of phospho-STAT3 in bone marrow cells stimulated with G-CSF are comparable in C/EBPβ heterozygous mutant and C/EBPβ-deficient mice, suggesting that other pathways might be aberrant.
A transcription factor, lipopolysaccharide-induced TNFα factor, regulates the expression of TNFα gene, and lipopolysaccharide-induced TNFα factor–deficient macrophages stimulated with LPS have a decrease expression of TNFα gene compared with wild-type macrophages.33 C/EBPβ-deficient macrophages also showed a similar phenotype; therefore, these molecules might play a key role in the innate immune system in macrophages. Interestingly, C/EBPβ-deficient neutrophils demonstrated an increase expression of TNFα mRNA in the presence of LPS stimulation. The difference of the signal transduction pathway(s) between macrophages and neutrophils remains unclear.
Our current data showed that C/EBPβ-deficient neutrophils have increased apoptosis in vitro compared with wild-type mice. This is the first time that C/EBPβ has been reported to be involved in suppressing apoptosis in neutrophils. This transcription factor has shown a similar ability to suppress apoptosis in several other cell types. C/EBPβ-deficient keratinocytes displayed increased apoptosis instead of tumor development in the response to the carcinogen dimethylbenzenthracene,34 and C/EBPβ-deleted hepatic stellate cells also had increased apoptosis.35 Immortalized macrophages infected with Myc/Raf retrovirus showed growth factor–independent survival; in contrast, C/EBPβ-deficient immortalized macrophages were completely dependent on growth factor.36 Here, we found enhanced survival of neutrophils from wild-type mice compared with C/EBPβ-deficient mice, consistent with C/EBPβ promoting survival of neutrophils by inhibiting apoptosis.
Certain features of the C/EBPβ-deficient mice are reminiscent of myelodysplastic syndrome. Both have increased apoptosis of myeloid cells and increased production of TNFα. Both can have decreased clonogenic growth of committed myeloid progenitor cells. Furthermore, blood cell response to various hematopoietic stresses is often blunted in both. We previously examined the C/EBPβ gene for mutations in myelodysplastic syndrome but detected none.37
In conclusion, we found that C/EBPβ-deficient mice had impaired response of their committed myeloid progenitor cells to G-CSF and GM-CSF, deficiency of cytokine production (IL-6, IL-10, and IL-12 p35) by their neutrophils, enhanced apoptosis of their granulocytes, and a decreased ability to withstand a cytotoxic stress (5-FU) in the C/EBPβ-deficient mice compared with the wild-type mice. Further studies are needed to elucidate the downstream targets of C/EBPβ that mediate these effects.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work is dedicated to the memory of David Golde, a mentor and friend. The authors thank members of our laboratory for helpful discussions.
This work was supported by the National Institutes of Health (grant 5R01CA026038-30) and the Parker Hughes Fund. H.P.K. is the holder of the Mark Goodson endowed Chair in Oncology Research and is a member of the Jonsson Cancer Center and the Molecular Biology Institute, UCLA.
National Institutes of Health
Authorship
Contribution: T.A. and T.S. performed the research, analyzed the data, and wrote the paper; J.O. assisted with the colony assay; S.A. provided C/EBPβ-deficient mice; A.F.G. designed and performed the research; H.P.K. directed the overall study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tadayuki Akagi, Division of Hematology and Oncology, Cedars-Sinai Medical Center, UCLA School of Medicine, 8700 Beverly Blvd, Los Angeles, CA 90048; e-mail: akagit@cshs.org.
References
Author notes
T.A. and T.S. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal