Abstract
Protein S expresses cofactor activity for activated protein C (APC) by enhancing the APC-catalyzed proteolysis at R306 in factor Va. It is generally accepted that only free protein S is active and that complex formation with C4b-binding protein (C4BP) inhibits the APC-cofactor activity of protein S. However, the present study shows that protein S-C4BP expresses APC-cofactor activity and stimulates APC-catalyzed proteolysis at R306 more than 10-fold, but instead inhibits proteolysis at R506 by APC 3- to 4-fold. Free protein S stimulates APC-catalyzed cleavage at R306 approximately 20-fold and has no effect on cleavage at R506. The resulting net effect of protein S-C4BP complex formation on APC-catalyzed factor Va inactivation is a 6- to 8-fold reduction in factor Va inactivation when compared with free protein S, which is not explained by inhibition of APC-cofactor activity of protein S at R306, but by generation of a specific inhibitor for APCcatalyzed proteolysis at R506 of factor Va. These results are of interest for carriers of the factor VLeiden mutation (R506Q), as protein S-C4BP effectively enhances APC-catalyzed factor Va (R306) inactivation in plasma containing factor VLeiden.
Introduction
Protein S is a vitamin K–dependent glycoprotein of 75 kDa that expresses multiple anticoagulant activities.1 Protein S circulates in plasma at a concentration of 350 nM, of which 60% is tightly bound in a noncovalent complex with C4b-binding protein (C4BP).2 C4BP is a high-molecular-weight plasma protein of 570 kDa with a plasma concentration of 200 nM. C4BP is a regulator of the classical pathway of the complement system and consists of 7 α-chains and one β-chain, which are held together by disulfide bonds in a central core.3 The β-chain of C4BP binds with high affinity to the sex hormone–binding globulin homology region of protein S in a 1:1 stoichiometry.4,5
It was reported that only free protein S functions as a nonenzymatic cofactor for activated protein C (APC), which accelerates APC-catalyzed inactivation of factor Va and factor VIIIa.6-8 Protein S also exhibits anticoagulant activity in the absence of APC in that direct interactions between protein S, factor Va, factor Xa, and phospholipids were reported to inhibit prothrombin activation. However, many of these observations were later explained by the presence of in vitro–generated protein S multi-mers in purified protein S preparations.9-13 More recently, it was reported that protein S is a cofactor for tissue factor pathway inhibitor (TFPI) in the down-regulation of tissue factor activity by stimulating the inhibition of factor Xa by full-length TFPI.14
Inactivation of factor Va by APC occurs by limited proteolysis in the heavy chain of factor Va at residues R306, R506, and R679.15 Factor Va inactivation by APC proceeds via biphasic reaction; a rapid proteolysis at R506 that results in the formation of an intermediate with partial cofactor activity, followed by a slow proteolysis at R306, which leads to full inactivation of factor Va.16 Due to its relatively low rate constant, proteolysis at R679 is not considered to be physiologically relevant. Factor VLeiden17 is characterized by an arginine to glutamine mutation at position 506 and as a result, inactivation of factor VaLeiden proceeds exclusively through proteolysis at R306.17,18
Optimal proteolysis of factor Va by APC requires the presence of negatively charged phospholipids and the cofactor protein S.19-21 In the presence of protein S, APC-catalyzed proteolysis at R306 of factor Va and factor VaLeiden is enhanced approximately 20-fold, while the proteolysis at R506 in general is not affected, although a 4- to 5-fold stimulation of the proteolysis at R506 by APC in the presence of protein S can be achieved, depending on the experimental design.22,23 It was reported that the APC-cofactor activity of protein S is inhibited by proteolysis of the thrombin-sensitive region of protein S24 or by complex formation with C4BP.25-27 In addition, it was observed that protein S relocated the active site of phospholipid-bound APC to a position above the membrane surface that favors factor Va inactivation, offering a rationale for APC-cofactor activity expression of protein S.28,29 More recently, it was shown that C4BP directly inhibits APC-catalyzed inactivation of factor Va30 both in the presence and absence of protein S.
In line with the fact that C4BP in complex with protein S acts as cofactor in APC-catalyzed factor VIIIa inactivation,31 we demonstrate in the present study that protein S-C4BP enhances APC-catalyzed factor Va inactivation at R306. It was observed that C4BP did not completely inhibit the APC-cofactor activity of protein S in plasma, whereas polyclonal antibodies against protein S did fully block the APC-cofactor activity of protein S. The absence of inhibition of APC-cofactor activity of protein S by C4BP was most pronounced in plasma containing factor VLeiden. More detailed kinetic analysis demonstrated that protein S-C4BP is a cofactor for APC that stimulates APC-catalyzed proteolysis at R306 of factor Va more than 10-fold and inhibits cleavage at R506 approximately 4-fold.
Methods
Materials
Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), Tris (tris(hydroxymethyl)aminomethane), and ovalbumin were purchased from Sigma-Aldrich (St Louis, MO), bovine serum albumin from MP Biomedicals (Illkirch, France). The chromogenic substrate S2238 was supplied by Chromogenix, Instrumentation Laboratory, Milan, Italy. Fluorogenic substrate I-1140 was from Bachem (Bubendorf, Switzerland). 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-Dioleoyl-sn-glycero-3-phosphoserine (DOPS), and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were obtained from Avanti Polar Lipids (Alabaster, AL). Phospholipid vesicles (20% DOPS, 20% DOPE, 60% DOPC, and 10%DOPS, 90% DOPC) were prepared as described previously.32
Polyclonal antibodies against protein S were purchased from DAKO (Glostrup, Denmark). Monoclonal antibody 9H10 against C4BP was a kind gift of Prof B. N. Bouma (The Scripps Research Institute). Inhibitory antibodies against factor VIII were a kind gift of Baxter (Vienna, Austria). Protein S was purified as described previously.13 Human normal factor V and factor VLeiden32 , human prothrombin,6 bovine factor Xa,33 and human C4BP34 were purified as described. Preparation of recombinant factor V (R506Q/R679Q and R306Q/R679Q) has been described previously.21 Activated protein C was obtained from Enzyme Research Laboratories (ERL, South Bend, IN), and tissue factor (TF) was from Dade Innovin (Behring, Germany). Thrombin calibrator was from Thrombinoscope (Maastricht, The Netherlands). Thrombin was obtained from Kordia Lab Supplies (Leiden, The Netherlands).
Calibrated automated thrombin generation assay
The anticoagulant activity of protein S and protein S-C4BP in plasma (0.32% sodium citrate) was measured using thrombin generation assays. In normal pooled plasma and in plasma from a homozygous carrier of the factor VLeiden mutation, thrombin generation was initiated with 6.8 pM TF, 240 μM phospholipid vesicles (20:60:20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations). Thrombin formation was monitored with the fluorogenic substrate I-1140. Prior to initiation of thrombin generation, normal pooled plasma or factor VLeiden plasma (80 μL) was incubated at 37°C during 15 minutes with or without 10 μL of purified C4BP or antibodies against protein S with or without antibodies against factor VIII. In normal plasma, 1.87 μM antiprotein S antibodies completely inhibited the APC-cofactor activity of protein S. Thrombin generation was measured in the presence or absence of APC (final concentration 4 nM in normal plasma and 35 nM in factor VLeiden plasma, respectively).
Thrombin generation in clotting plasma was monitored by calibrated automated thrombin generation (CAT) assays, where conversion of the low-affinity fluorogenic substrate by thrombin is followed in time in a Fluoroskan Ascent reader (Thermo Labsystems, Helsinki, Finland) with filter sets 390 nm-excitation and 460 nm-emission.35 Thrombin generation curves and the area-under-the-curve (endogenous thrombin potential, ETP) were calculated using the Thrombinoscope software (Synapse, Maastricht, The Netherlands).35
Normal factor Va and factor VaLeiden inactivation in a model system
Purified human factor V (100 μL 12 nM) in Hepes-buffered saline (HBS: 25 mM Hepes, pH 7.7, 175 mM NaCl) containing 5 mM CaCl2 and 0.5% bovine serum albumin (BSA) was activated with thrombin (3 nM) and after 15 minutes Phe-Pro-Arg-chloromethylketone (PPACK; 3.5 nM) was added. Factor Va (at a final concentration of 1.2 nM) was incubated at 37°C in a mixture containing 50 μM phospholipids (20:60:20 DOPS/DOPC/DOPE) and 5 mM CaCl2 with or without 200 nM protein S or 200 nM protein S-C4BP. At time 0, APC was added (final concentration, 125 pM), and at different time points samples were taken from the incubation mixture. The factor Va concentration in these aliquots was determined with a prothrombinase-based factor Va assay: 10 μL of the inactivation mixture was added to a mixture (240 μL) containing 5 nM bovine factor Xa, 1 μM human prothrombin, 40 μM phospholipids (10:90 DOPS/DOPC), and 2.5 mM CaCl2. After 1-minute prothrombin activation was stopped by a 40-fold dilution in an ice-cold buffer containing 50 mM Tris-HCl, pH 7.4, 175 mM NaCl, 20 mM EDTA (ethylenediaminetetraacetic acid) and 0.5% ovalbumin. The amount of thrombin formed was quantified using the chromogenic substrate S2238 as described before.36 Time courses of factor Va inactivation were fitted to a biphasic exponential equation according to Nicolaes et al.16 Protein S-C4BP complex was prepared by incubation of 2 μM protein S with 2.2 μM C4BP in HBS containing 3 mM CaCl2 for 15 minutes. After 10-fold dilution in the reaction mixture, a final concentration of 200 nM protein S-C4BP was reached. Confirmation of complex formation was performed after the inactivation experiments using Superose-6 size-exclusion chromatography.
Size-exclusion chromatography
Fifty microliters normal plasma or normal plasma with a molar excess of C4BP (450 nM) over free protein S or 150 μL of the factor Va inactivation mixtures were applied to a Superose-6 size-exclusion chromatography column at a flow rate of 0.25 mL/min in Hepes-buffered saline (HBS: 25 mM Hepes, pH 7.7, 175 mM NaCl) containing 1 mM CaCl2. Fractions (0.25 mL) were collected and immediately diluted 1:1 in HBS containing 30 mg/mL BSA. Protein S and protein S-C4BP in the eluting fractions were quantified by enzyme-linked immunosorbent assay (ELISA).
Protein S and protein S-C4BP ELISA
Protein S ELISA was performed as described.13 Protein S-C4BP in the elution fractions was quantified by ELISA using the following procedure. Microtiter plate wells were coated with capturing monoclonal antibodies 9H10 against the α-chains of C4BP diluted 1:750 in 0.1 M Na2CO3, pH 9.0 at 4°C overnight. Wells were washed and blocked with blocking buffer (HBS containing 3% BSA and 3 mM CaCl2) for 2 hours. Column elution fractions were applied at several dilutions in HBS containing 0.5% BSA and 3 mM CaCl2 and left incubating for 1 hour. Wells were washed 3 times with washing buffer (HBS containing 3 mM CaCl2 and 0.03% Tween-20), followed by incubation with a 1:1000 dilution of horseradish peroxidase (HRP)–conjugated polyclonal antibodies against protein S for 1 hour. After washing of the wells 5 times with washing buffer, detection of bound HRP-labeled antibodies was performed with a HRP-substrate kit (Pierce, Rockfield, IL).
Inactivation of recombinant factor Va (R506Q/R679Q and R306Q/R679Q)
Recombinant factor V (R506Q/R679Q or R306Q/R679Q) was activated by thrombin (2 nM), and after 15 minutes PPACK was added to a final concentration of 2.5 nM. Recombinant factor Va (R506Q/R679Q or R306Q/R679Q) at a final concentration of 20 pM was incubated at 37°C with phospholipids (50 μM 20:60:20 DOPS/DOPC/DOPE), protein S, protein S-C4BP (200 nM), or C4BP (200 nM) in HBS containing 5 mM CaCl2 and 0.5% BSA. At time zero, APC (100 pM) was added, and samples were taken at different time points. The time course of factor Va inactivation was monitored with the following prothrombinase-based factor Va assay: 115 μL inactivation mixture of recombinant factor Va (R506Q/R679Q and R306Q/R679Q) was added to 10 μL of a prothrombin/bovine factor Xa mixture yielding final concentrations of 5 nM bovine factor Xa and 1 μM prothrombin or 0.1 nM bovine factor Xa and 1 μM prothrombin, respectively. After 1 minute prothrombin activation was stopped by a 40-fold dilution in an ice-cold buffer containing 50 mM Tris-HCl, pH 7.4, 175 mM NaCl, 20 mM EDTA, and 0.5% ovalbumin, and the amount of thrombin formed was determined with the chromogenic substrate S2238.36 Time courses of factor Va inactivation were fitted as described.16
Results
Regulation of APC-cofactor activity of protein S by C4BP
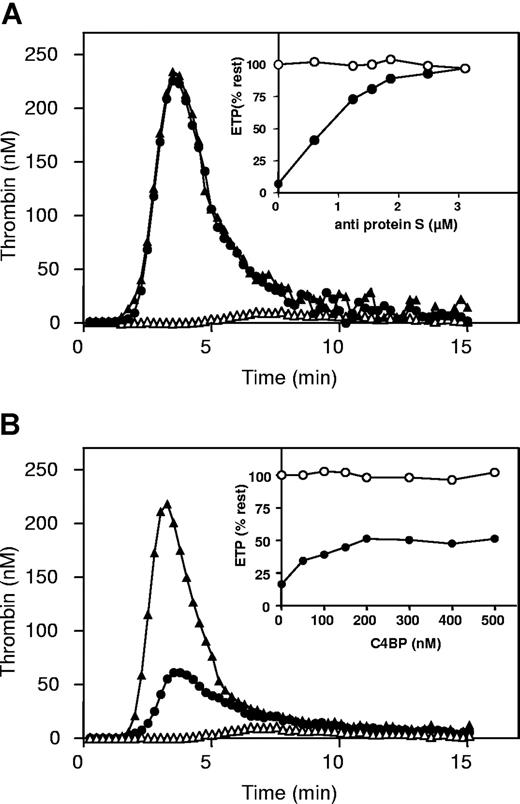
Homozygous factor VLeiden plasma was used to study the APC-cofactor activity of protein S in plasma. Because the possibility of rapid R506-cleavage by APC in factor VLeiden was absent, optimal differences in factor Va-inactivation by APC were detected in the absence compared with the presence of protein S due to the 20-fold enhancement of the slow cleavage at R306 by protein S.19-21 When coagulation in factor VLeiden plasma was initiated with tissue factor, phospholipids, and CaCl2, a typical thrombin generation curve was obtained (Figure 1). After a lag time representing the clotting time of plasma, thrombin generation was observed before reaching a peak, followed by inactivation of thrombin by the natural anticoagulants antithrombin and α2-macroglobulin. The area under the thrombin generation curve, called the endogenous thrombin potential (ETP), was used as a measure for the total amount of thrombin generated during the coagulation process. In factor VLeiden plasma the ETP was determined to be 854 nM IIa.min (Figure 1A). When APC was present during thrombin generation, the ETP was reduced due to the proteolytic inactivation of factors Va and VIIIa by APC and its cofactor protein S (Figure 1A). In these experiments an APC concentration was chosen that resulted in a residual ETP in the presence of APC that was around 10% (104 nM IIa.min) of the ETP determined in the absence of APC (Figure 1).
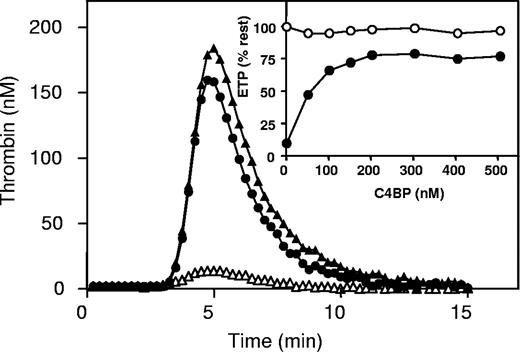
The effect of C4BP and antibodies against protein S on thrombin generation in plasma with factor VLeiden. (A) Thrombin generation curves in factor VLeiden plasma without APC (▲), with 35 nM APC (△), and with 35 nM APC with 3.1 μM antibodies against protein S (●). Insets: percentage of endogenous thrombin potential (ETP) as a function of anti–protein S antibody concentration in factor VLeiden plasma in the absence of APC (○) or in the presence of APC (●). (B) Thrombin generation curves in plasma with factor VLeiden, without APC (▲), with 35 nM APC (△), and with 35 nM APC and 500 nM C4BP (●). Insets: percentage of ETP as a function of C4BP concentration in plasma with factor VLeiden in the absence of APC (○) or in the presence of APC (●).
The effect of C4BP and antibodies against protein S on thrombin generation in plasma with factor VLeiden. (A) Thrombin generation curves in factor VLeiden plasma without APC (▲), with 35 nM APC (△), and with 35 nM APC with 3.1 μM antibodies against protein S (●). Insets: percentage of endogenous thrombin potential (ETP) as a function of anti–protein S antibody concentration in factor VLeiden plasma in the absence of APC (○) or in the presence of APC (●). (B) Thrombin generation curves in plasma with factor VLeiden, without APC (▲), with 35 nM APC (△), and with 35 nM APC and 500 nM C4BP (●). Insets: percentage of ETP as a function of C4BP concentration in plasma with factor VLeiden in the absence of APC (○) or in the presence of APC (●).
Addition of antibodies against protein S in factor VLeiden plasma in the presence of APC resulted in a dose-dependent increase of the ETP to a level that was more than 95% of the ETP determined in the absence of APC (Figure 1A, insert). This experiment confirmed that the anticoagulant effect of APC in plasma was for more than 95% dependent on the presence of its cofactor protein S.37
Next, titration experiments were performed with C4BP in plasma from the same factor VLeiden homozygous individual (Figure 1B). Varying amounts of C4BP were added to factor VLeiden plasma, followed by incubation for 15 minutes at 37°C prior to the initiation of thrombin generation. Titration with C4BP resulted in a dose-dependent increase of the ETP in the presence of APC (Figure 1B; insert). However, at the highest concentration of C4BP added (500 nM), in the absence of free protein S, APC was still able to reduce the ETP by more than 50% in the presence of protein S-C4BP (Figure 1B), which would not have been observed if complex formation with C4BP had inhibited the APC-cofactor activity of protein S. Complex formation of free protein S in plasma with the added C4BP was verified by size-exclusion chromatography (Figure 2). Addition of C4BP to plasma resultedin the complete disappearance of free protein S eluting at approximately 17 mL.
Verification of plasma protein S-C4BP in factor VLeiden plasma by Superose-6 size-exclusion chromatography. (A) Superose-6 size-exclusion chromatography of factor VLeiden plasma: protein S was measured with a protein S ELISA (▲) and with a protein S-C4BP complex (△)ELISA. (B) Superose-6 size-exclusion chromatography of factor VLeiden plasma saturated with C4BP: protein S was measured with a protein S ELISA (▲) and with a protein S-C4BP complex (△)ELISA. Vo: void column volume; Vt: total column volume.
Verification of plasma protein S-C4BP in factor VLeiden plasma by Superose-6 size-exclusion chromatography. (A) Superose-6 size-exclusion chromatography of factor VLeiden plasma: protein S was measured with a protein S ELISA (▲) and with a protein S-C4BP complex (△)ELISA. (B) Superose-6 size-exclusion chromatography of factor VLeiden plasma saturated with C4BP: protein S was measured with a protein S ELISA (▲) and with a protein S-C4BP complex (△)ELISA. Vo: void column volume; Vt: total column volume.
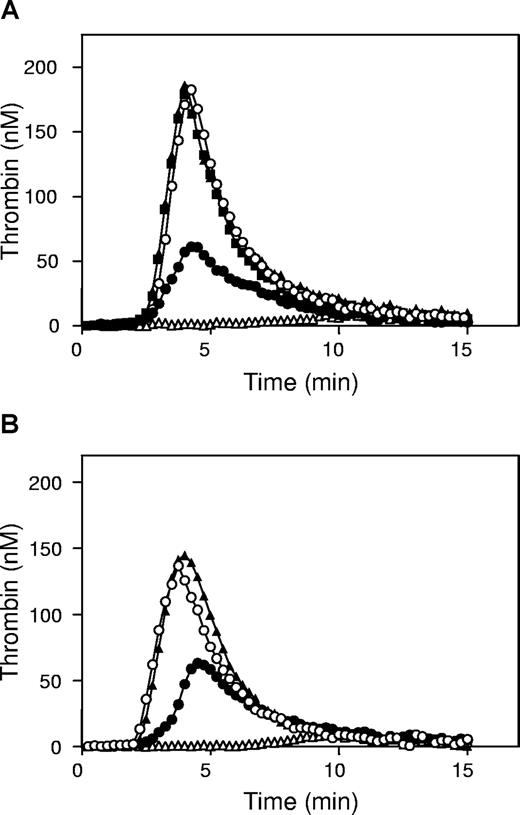
Because antibodies against protein S normalized the ETP of factor VLeiden plasma in the presence of APC for more than 95%, this suggested that antibodies against protein S inhibited the cofactor activity of both free protein S and protein S-C4BP. To demonstrate that the remaining 50% of the ETP in the presence of APC and excess C4BP (Figure 1B) was due to the cofactor activity of protein S-C4BP for APC, antibodies against protein S were added in the presence of C4BP, which caused the ETP to be restored for more than 95% compared with the absence of APC (Figure 3A). This indicated that in factor VLeiden plasma, the 50% reduction of the ETP in presence of APC was caused by the APC-cofactor activity of protein S-C4BP complex in factor VaLeiden inactivation at R306.
Effect of protein S and protein S-C4BP on thrombin generation in factor VIII–neutralized plasma factor VLeiden plasma. (A) Thrombin generation in factor VLeiden plasma was measured without APC (▲), with 30 nM APC (△), with 30 nM APC and 1.87 μM antibodies against protein S (○) and 30 nM APC and 500 nM protein S-C4BP (●), and with 30 nM APC, 500 nM protein S-C4BP, and 1.87 μM antibodies against protein S (■). (B) Thrombin generation in factor VIII–neutralized factor VLeiden plasma was measured without APC (▲), with 30 nM APC (△), with 30 nM APC and 1.87 μM antibodies against protein S (○) and 30 nM APC and 500 nM protein S-C4BP (●).
Effect of protein S and protein S-C4BP on thrombin generation in factor VIII–neutralized plasma factor VLeiden plasma. (A) Thrombin generation in factor VLeiden plasma was measured without APC (▲), with 30 nM APC (△), with 30 nM APC and 1.87 μM antibodies against protein S (○) and 30 nM APC and 500 nM protein S-C4BP (●), and with 30 nM APC, 500 nM protein S-C4BP, and 1.87 μM antibodies against protein S (■). (B) Thrombin generation in factor VIII–neutralized factor VLeiden plasma was measured without APC (▲), with 30 nM APC (△), with 30 nM APC and 1.87 μM antibodies against protein S (○) and 30 nM APC and 500 nM protein S-C4BP (●).
Effect of protein S and protein S-C4BP on thrombin generation in factor VIII-neutralized factor VLeiden plasma
At 6.8 pM TF used in our experiments, the factor VIII(a)-route contributes to thrombin generation, and regulation of thrombin formation by APC might therefore include inactivation of FVIIIa. To study the possibility that observed effects were caused by cofactor activity of protein S-C4BP in the APC-catalyzed inactivation of FVIIIa,31 we performed an experiment in factor VLeiden plasma to which inhibitory antibodies against factor VIII(a) were added that completely inhibited intrinsically activated thrombin generation in plasma. In the presence of these antibodies, the effect of protein S-C4BP was studied by adding 500 nM C4BP. As a control, factor VIII–neutralized factor VLeiden plasma was incubated for 15 minutes with antiprotein S antibodies prior to initiation of thrombin generation in the presence or absence of 30 nM APC (Figure 3B). In factor VIII(a)–neutralized factor VLeiden plasma, protein S-C4BP was able to reduce thrombin generation in the presence of APC to a similar extent as in plasma without anti–factor VIIIa antibodies, indicating that the observed protein S-C4BP cofactor activity for APC was located at the FVa-inactivation level (Figure 3B).
The differences in inhibition of protein S anticoagulant activity by antibodies against protein S and by C4BP indicated that the protein S-C4BP complex was active as a cofactor for APC and prompted us to further investigate the reported inhibition of APC-cofactor activity of protein S by C4BP.25-27
APC-catalyzed inactivation of purified factor Va and factor VaLeiden
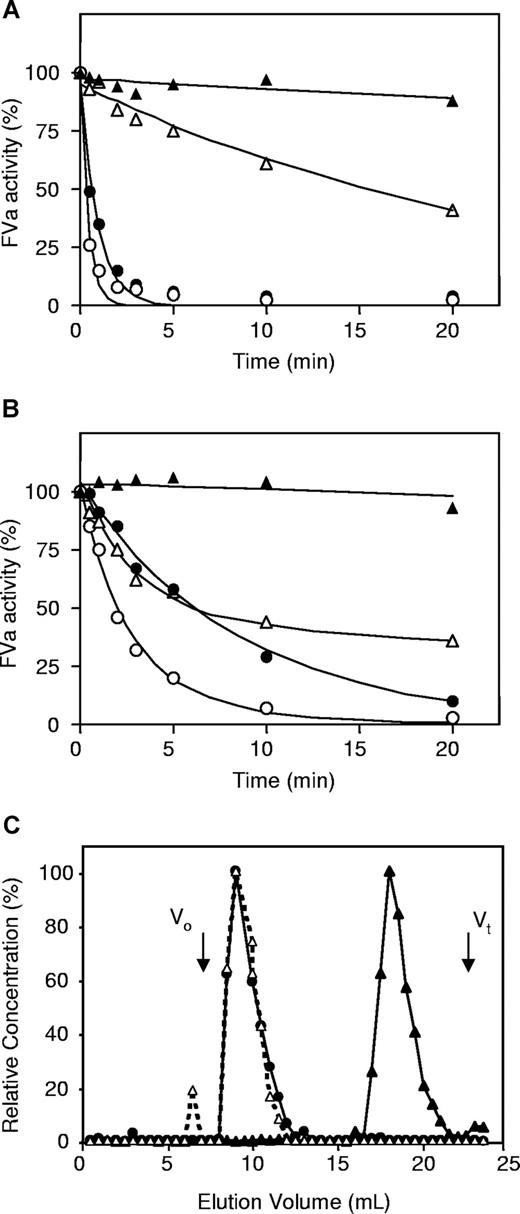
To characterize the APC-cofactor activity of protein S-C4BP, purified factor VaLeiden or normal factor Va was inactivated by APC in the absence or presence of protein S or protein S-C4BP in model systems containing purified proteins. In the absence of protein S or protein S-C4BP, factor VaLeiden, which cannot be cleaved at R506, was slowly inactivated by APC via a monophasic inactivation reaction (Figure 4A open triangles). Addition of protein S or protein S-C4BP resulted in an increased rate of inactivation of factor VaLeiden, which indicated that both protein S and protein S-C4BP stimulated APC-catalyzed proteolysis at R306 (Figure 4A circles).
Effect of protein S and protein S-C4BP on APC-mediated inactivation of factor Va and factor VaLeiden in a reconstituted model system. (A) Inactivation of factor VaLeiden by APC (△), APC and 200 nM protein S (○), and APC and 200 nM protein S-C4BP (●). Loss of factor Va activity (stability) in the absence of APC (▲). (B) Inactivation of factor Va with APC (△), with APC and 200 nM protein S (○) and with APC and 200 nM protein S-C4BP (●). Loss of factor Va Leiden activity (stability) in the absence of APC (▲). (C) Superose-6 size-exclusion chromatography of incubation mixtures from B: factor Va, APC, and 200 nM protein S: protein S was measured by ELISA (▲); factor Va, APC, and 200 nM protein S-C4BP: protein S-C4BP (●) and protein S (△) were measured by ELISA.
Effect of protein S and protein S-C4BP on APC-mediated inactivation of factor Va and factor VaLeiden in a reconstituted model system. (A) Inactivation of factor VaLeiden by APC (△), APC and 200 nM protein S (○), and APC and 200 nM protein S-C4BP (●). Loss of factor Va activity (stability) in the absence of APC (▲). (B) Inactivation of factor Va with APC (△), with APC and 200 nM protein S (○) and with APC and 200 nM protein S-C4BP (●). Loss of factor Va Leiden activity (stability) in the absence of APC (▲). (C) Superose-6 size-exclusion chromatography of incubation mixtures from B: factor Va, APC, and 200 nM protein S: protein S was measured by ELISA (▲); factor Va, APC, and 200 nM protein S-C4BP: protein S-C4BP (●) and protein S (△) were measured by ELISA.
In the absence of protein S or protein S-C4BP, inactivation of normal factor Va by APC showed the typical biphasic pattern representing rapid cleavage at R506 yielding a factor Va intermediate with reduced factor Xa cofactor activity followed by slow cleavage at R306 and complete inactivation of factor Va (Figure 4B open triangles). The presence of protein S as well as protein S-C4BP accelerated factor Va inactivation by APC resulting in an apparent monophasic inactivation curve (Figure 4B). In a control experiment it was observed that C4BP alone had no effect on the inactivation of factor Va by APC (data not shown). Notably, close inspection of the factor Va inactivation curve in the presence of protein S-C4BP complex (Figure 4B filled circles) shows an enhancement of the second slow phase inactivation of factor Va by APC (R306-cleavage) but an inhibition of the first rapid phase of factor Va inactivation (R506-cleavage).
To quantitate the effect of protein S-C4BP complex on APC-catalyzed factor Va inactivation, the curves from Figure 4 were fitted according to the model proposed by Nicolaes et al,16 and the second-order rate constants for cleavage at R506 and R306 obtained in the absence and presence of protein S and protein S-C4BP are presented in Table 1. At 200 nM protein S-C4BP complex, an 11-fold stimulation of the APC-catalyzed cleavage at R306 was observed. While protein S had only minor influence on the proteolysis at R506, protein S-C4BP (200 nM) inhibited APC-catalyzed proteolysis at R506 3- to 4-fold (Table 1). In addition, analysis of factor VLeiden inactivation revealed a 13-fold stimulation of APC-catalyzed inactivation by protein S-C4BP compared with 26-fold by protein S.
Rate constants of proteolysis at R506 and R306 in plasma factor Va by APC
| . | n . | k506, mol−1 sec−1 . | Fold-stimulation . | k306, mol−1 sec−1 . | Fold-stimulation . |
|---|---|---|---|---|---|
| Normal factor Va | |||||
| APC | 3 | 8.03 ± 1.50 × 107 | — | 3.77 ± 1.27 × 106 | — |
| APC plus protein S | 4 | 6.56 ± 0.44 × 107 | 0.82 | 6.76 ± 0.25 × 107 | 18 |
| APC plus protein S-C4BP | 4 | 2.31 ± 0.89 × 107 | 0.29 | 4.25 ± 0.58 × 107 | 11 |
| Factor VaLeiden | |||||
| APC | 6 | — | — | 2.79 ± 1.04 × 106 | — |
| APC plus protein S | 6 | — | — | 7.38 ± 2.94 × 107 | 26 |
| APC plus protein S-C4BP | 6 | — | — | 3.62 ± 1.13 × 107 | 13 |
| . | n . | k506, mol−1 sec−1 . | Fold-stimulation . | k306, mol−1 sec−1 . | Fold-stimulation . |
|---|---|---|---|---|---|
| Normal factor Va | |||||
| APC | 3 | 8.03 ± 1.50 × 107 | — | 3.77 ± 1.27 × 106 | — |
| APC plus protein S | 4 | 6.56 ± 0.44 × 107 | 0.82 | 6.76 ± 0.25 × 107 | 18 |
| APC plus protein S-C4BP | 4 | 2.31 ± 0.89 × 107 | 0.29 | 4.25 ± 0.58 × 107 | 11 |
| Factor VaLeiden | |||||
| APC | 6 | — | — | 2.79 ± 1.04 × 106 | — |
| APC plus protein S | 6 | — | — | 7.38 ± 2.94 × 107 | 26 |
| APC plus protein S-C4BP | 6 | — | — | 3.62 ± 1.13 × 107 | 13 |
Concentrations of protein S and protein S-C4BP were 200 nM. Rate constants were determined as described.16 Data are presented as means plus or minus SD.
— indicates not applicable.
From these experiments, especially those with purified factor VaLeiden in which only the cleavage site at R306 is available, we can conclude that protein S-C4BP acts as a cofactor for APC and that it stimulates APC-catalyzed proteolysis at R306.
To verify that the added protein S-C4BP complex did not dissociate during the time course of factor Va inactivation (Figure 4B), the reaction mixtures remaining at the end of factor Va inactivation were quantitatively subjected to Superose-6 size-exclusion chromatography, and the fractions eluting from the column were tested for the presence of protein S and protein S-C4BP complex with specific ELISAs (Figure 4C). The reaction mixture containing only free protein S was analyzed by a protein S ELISA, and the retention volume of free protein S was approximately 18 mL. In the inactivation mixture to which protein S-C4BP complex was added, all protein S appeared to be associated with C4BP because protein S and protein S-C4BP co-eluted at a retention volume of 9 mL just after the void volume of the Superose-6 column, and no protein S was detected in the subsequent column fractions, indicating that during factor Va inactivation in the presence of protein S-C4BP, no free protein S was present (Figure 4C).
Inactivation of recombinant factor Va (R306Q/R679Q and R506Q/R679Q)
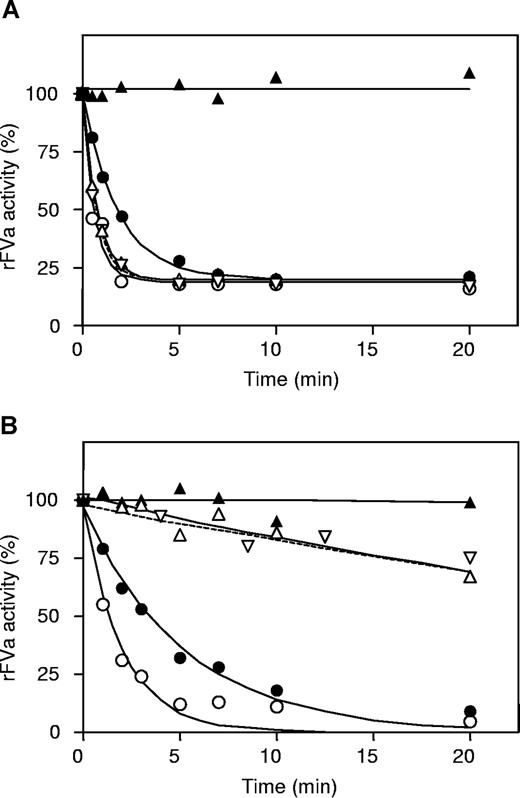
Kinetic analysis of inactivation of factor Va by APC in the presence of protein S-C4BP showed that the protein S-C4BP complex inhibited cleavage at R506 in factor Va and stimulated cleavage at R306 in factor Va. To confirm the effects of protein S-C4BP on APC-catalyzed proteolysis of R506 and R306, recombinant factor Va R306Q/R679Q and factor Va R506Q/R679Q were used, in which arginines at positions 306/679 and 506/679 were replaced by glutamines, respectively. These factor Va variants could only be inactivated by APC via cleavage at either R506 (in factor Va R306Q/R679Q) or R306 (in factor Va R506Q/R679Q). The recombinant factor Va molecules were inactivated by APC in the absence and presence of protein S or protein S-C4BP (Figure 5). Time courses of factor Va inactivation were fitted as described.16
Effect of protein S and protein S-C4BP on APC-mediated inactivation of recombinant factor Va (R306/R679Q and R506Q/R679Q) in a reconstituted model system. (A) Inactivation of recombinant factor Va (R306Q/R679Q) with APC (△), with APC and 200 nM C4BP (▽ dashed line) with APC and 200 nM protein S (○), with APC and 200 nM protein S-C4BP (●), and without APC (▲). (B) Inactivation of recombinant factor Va (R506Q/R679Q) with APC (△), with APC and 200 nM C4BP (▽ dashed line), with APC and 200 nM protein S (○), with APC and 200 nM protein S-C4BP (●), and without APC (▲).
Effect of protein S and protein S-C4BP on APC-mediated inactivation of recombinant factor Va (R306/R679Q and R506Q/R679Q) in a reconstituted model system. (A) Inactivation of recombinant factor Va (R306Q/R679Q) with APC (△), with APC and 200 nM C4BP (▽ dashed line) with APC and 200 nM protein S (○), with APC and 200 nM protein S-C4BP (●), and without APC (▲). (B) Inactivation of recombinant factor Va (R506Q/R679Q) with APC (△), with APC and 200 nM C4BP (▽ dashed line), with APC and 200 nM protein S (○), with APC and 200 nM protein S-C4BP (●), and without APC (▲).
For R306Q/R679Q factor Va, protein S hardly influenced the rate constant of cleavage at R506 (Figure 5A; Table 2).21 In contrast, the proteolysis at R506 by APC was inhibited 3-fold by protein S-C4BP (Figure 5A; Table 2). Experiments performed with recombinant factor Va (R506Q/R679Q), in which APC can only cleave the peptide bond at R306, showed that protein S and protein S-C4BP stimulated APC-catalyzed proteolysis at R306 31-fold and 14-fold, respectively (Figure 5B; Table 2). Addition of C4BP alone to APC had no influence on the inactivation of recombinant factor Va (R506Q/R679Q and R306Q/R679Q; Figure 5).
Rate constants of proteolysis at R306 or R506 in recombinant factor Va by APC
| FVa (R306Q/R769Q) . | k506, mol−1 sec−1 . | Fold-stimulation . | FVa (R506Q/R679Q) . | k306, mol−1 sec−1 . | Fold-stimulation . |
|---|---|---|---|---|---|
| APC | 1.89 × 108 | — | APC | 2.41 × 106 | — |
| APC plus protein S | 2.40 × 108 | 1.27 | APC + protein S | 7.58 × 107 | 31 |
| APC plus protein S-C4BP | 6.72 × 107 | 0.36 | APC + protein S-C4BP | 3.39 × 107 | 14 |
| FVa (R306Q/R769Q) . | k506, mol−1 sec−1 . | Fold-stimulation . | FVa (R506Q/R679Q) . | k306, mol−1 sec−1 . | Fold-stimulation . |
|---|---|---|---|---|---|
| APC | 1.89 × 108 | — | APC | 2.41 × 106 | — |
| APC plus protein S | 2.40 × 108 | 1.27 | APC + protein S | 7.58 × 107 | 31 |
| APC plus protein S-C4BP | 6.72 × 107 | 0.36 | APC + protein S-C4BP | 3.39 × 107 | 14 |
Concentrations of protein S and protein S-C4BP were 200 nM. Average rate constants from 2 separate experiments were determined as described.16
— indicates no data.
Finally, the effect of C4BP was tested in normal pooled plasma. After initiation of coagulation with tissue factor, the ETP in normal pooled plasma was determined to be 588 nM IIa.min. When APC was present during thrombin generation, the ETP was reduced to approximately 10% of the ETP determined in the absence of APC (51 nM IIa.min). Addition of antibodies against protein S in normal pooled plasma in the presence of APC resulted in a dose-dependent increase of the ETP to a level that was more than 95% of the ETP determined in the absence of APC (data not shown). Addition of C4BP to normal pooled plasma in the presence of APC resulted in a dose-dependent inhibition of APC anticoagulant activity, shown by an increase in ETP in the presence of APC to more than 75% (452 nM IIa.min) compared with the ETP in the absence of APC (Figure 6 filled circles, insert). However, C4BP was unable to restore the ETP to values that were observed in titrations with polyclonal antibodies against protein S in the presence of APC.
The effect of C4BP on thrombin generation in normal pooled plasma. Thrombin generation curves in normal pooled plasma without APC (▲), with 4 nM APC (△), and with 4 nM APC and 500 nM C4BP (●). Inset: percentage of ETP as a function of C4BP concentration in normal pooled plasma in the absence of APC (○) or in the presence of APC (●).
The effect of C4BP on thrombin generation in normal pooled plasma. Thrombin generation curves in normal pooled plasma without APC (▲), with 4 nM APC (△), and with 4 nM APC and 500 nM C4BP (●). Inset: percentage of ETP as a function of C4BP concentration in normal pooled plasma in the absence of APC (○) or in the presence of APC (●).
Discussion
It has long been accepted that the complex of protein S and C4BP is inactive as a cofactor for APC in the inactivation of factor Va in model systems containing purified proteins or in plasma.26,30 In contrast, the data presented in the current paper show that protein S-C4BP complex constitutes a cofactor for APC that enhances APC-catalyzed proteolysis at R306 more than 10-fold. In addition, complex formation between protein S and C4BP resulted in the generation of a specific inhibitor of APC-catalyzed cleavage at R506 of factor Va.
These observations offer a rationale for why it was originally concluded that C4BP inhibited the APC-cofactor activity of protein S. The assumption that C4BP inhibited the APC-cofactor activity of protein S could still be supported at first sight by the observation that addition of C4BP to normal plasma reversed the inhibition of thrombin generation by APC/protein S, apparently by counteracting the APC-cofactor activity of free protein S (Figure 6). However, further observations offer an alternative explanation. Addition of C4BP to plasma still results in factor Va cleavage at R306 by APC, but specifically results in inhibition of factor Va cleavage at R506 by APC. The inhibition of the fast cleavage at R506 by protein S-C4BP and stimulation of the slow cleavage at R306 by protein S-C4BP creates a situation in which the initial rate of factor Va inactivation by APC is decreased (Figures 4B,7).
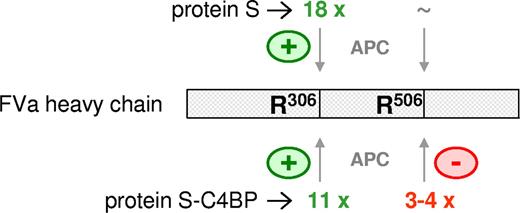
Effects of protein S and protein S-C4BP on APC-mediated proteolysis of factor Va at R306 and R506. Both protein S and protein S-C4BP are cofactors for APC that stimulate APC-mediated proteolysis at R306 in factor Va 18-fold and 11-fold, respectively. While protein S has no effect on APC-mediated inactivation at R506 in factor Va, the protein S-C4BP complex specifically inhibits APC-mediated cleavage at R506 3- to 4-fold.
Effects of protein S and protein S-C4BP on APC-mediated proteolysis of factor Va at R306 and R506. Both protein S and protein S-C4BP are cofactors for APC that stimulate APC-mediated proteolysis at R306 in factor Va 18-fold and 11-fold, respectively. While protein S has no effect on APC-mediated inactivation at R506 in factor Va, the protein S-C4BP complex specifically inhibits APC-mediated cleavage at R506 3- to 4-fold.
When factor VIII in plasma was neutralized by inhibitory antibodies, the APC-cofactor activity of protein S-C4BP remained, identifying APC-catalyzed factor Va-inactivation at R306 as the target for the anticoagulant activity of the protein S-C4BP complex.
It should be realized that differences between previous findings and new data can be explained by different experimental conditions under which experiments were performed. Original findings were done using activated partial thromboplastin time (APTT)–based clotting-assay to study the effect of protein S and protein S-C4BP on thrombin generation. APTT clotting times correspond to the lag times of CAT assay thrombin generation curves, on top of which the CAT assay allows interpretation of coagulation processes beyond initial clot formation. It is possible that the fast cleavage of factor Va at R506 might play a role in the initial phase of thrombin generation, that is, clot formation, whereas cleavage at R306 might be important after clot formation. In this case, inhibition of APC-catalyzed cleavage at R506 by protein S-C4BP might be responsible for the observed inhibitory activity of C4BP added to plasma in clotting assays.
In a previous study it was suggested that C4BP alone protects factor Va from inactivation by APC.30 However, the conditions of these experiments were different from our experimental conditions. Especially the low phospholipid concentrations used in the study might have contributed to the observed protective effect of C4BP-α and C4BP-β in factor Va-inactivation by APC. It has been proposed that for binding of coagulation proteins of approximately 75 kDa to phospholipid vesicles (20:80 PS/PC), approximately 1% of total phospholipid concentration represents the available protein binding sites (ratio protein/PL = 1.0 w/w).38 In the mentioned study, a phospholipid concentration of 5 μM PS/PC was used, which will result in a concentration of total protein binding sites of 50 nM for 75-kDa proteins. If C4BP (570 kDa) binds to phospholipids, even fewer binding sites will be available, and concentrations of 200 nM C4BP and more could easily inhibit phospholipid-dependent reactions in general, in the present case caused by competition for phospholipid binding sites with factor Va and APC. It was reported that C4BP can bind to phospholipid surfaces through protein S,39-41 but also in the absence of protein S.40 Using ellipsometry studies,13 we also have observed that C4BP alone is able to bind to 20:80 PS/PC phospholipid vesicles (T.M.H. and L.F.A.M., unpublished results, 2007). Taken together, we propose that the use of low phospholipid concentration will promote nonspecific inhibition of phospholipid surface-dependent enzymatic processes, which might offer an explanation for the fact that in the previous report inhibition of APC-catalyzed factor Va-inactivation at low phospholipid concentrations was not observed in an activated partial thromboplastin time-based plasma clotting assay (at high phospholipid concentrations).30,42
The size of C4BP might contribute to inhibition of proteolysis at R506 by APC. Protein S is a cofactor for APC-catalyzed inactivation of factor Va, and it reportedly causes a translocation of the active site of APC by binding to APC near the R506 site of factor Va, which has been proposed to optimize factor Va-inactivation.28,43 If binding of C4BP to protein S does not alter the binding properties of protein S-C4BP to factor Va, it might result in hindrance of the docking of APC to the FVa/protein S/C4BP complex, resulting in less effective proteolysis at R506 by APC. In line with this, it has been reported previously that interaction between APC and FVa around R506 is different from that around R306, with the interaction at R506 requiring more pronounced molecular contacts between APC and factor Va than the interaction at R306.44 Covering of these molecular contact sites at R506 by protein S-C4BP might well offer a rationale for the observed inhibition of APC-catalyzed factor Va-inactivation by protein S-C4BP.
We propose that protein S-C4BP is a cofactor for APC that, although at a 2-fold lower activity than free protein S, stimulates proteolytic inactivation of factor Va at R306 more than 10-fold. In contrast, protein S-C4BP specifically inhibits proteolysis at R506 by APC, most likely explained by interfering with R506 susceptibility for APC by C4BP. However, the net effect of C4BP on protein S–enhanced APC-catalyzed factor Va-inactivation will be a 2-fold inhibition of R306 cleavage and a 3- to 4-fold inhibition of R506 cleavage, resulting in an overall 6- to 8-fold inhibition of factor Va inactivation by APC (Figure 7).
These results are of interest for carriers of the factor VLeiden mutation, the most common inherited risk factor for venous thrombosis, in which factor V is characterized by a R506 to Q replacement.17,45 In this case, an inhibitory effect of protein S-C4BP on APC-cleavage at amino acid position 506 is absent, and especially in the case of homozygotes, effective concentrations protein S will be closer to that of total protein S concentrations instead of established free protein S concentrations.
In conclusion, our observations indicate that C4BP cannot be simply be considered as an inhibitor of APC-cofactor activity of protein S anymore. The reason that free protein S is a better cofactor for APC than protein S-C4BP lies in the fact that protein S-C4BP selectively impairs proteolysis at R506 by APC and not by inhibition of the protein S–dependent enhancement of APC-catalyzed proteolysis at R306. The selective impairment of APC-catalyzed proteolysis at R506 by protein S-C4BP will be the main reason for the observed effects of addition of C4BP to plasma. This suggests an active role for the protein S-C4BP complex in the regulation of thrombin formation, which is in concert with an observation in which protein S-C4BP is active as a cofactor for TFPI in the regulation of tissue factor activity.14
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported in part by a research grant from The Netherlands Organisation for Scientific Research (NWO; VIDI 917.36.372 to T.H. and VIDI 917.46.330 to G.N.).
Authorship
Contribution: L.F.A.M. performed research and wrote the paper. M.C.L.G.D.T. performed research. G.A.F.N. analyzed data and contributed vital new reagents. B.D. analyzed data and contributed vital new reagents. G.T. analyzed data and wrote the paper. J.R. designed research, analyzed data, and wrote the paper. T.M.H. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tilman M. Hackeng, Department of Biochemistry, Cardiovascular Research Institute Maastricht, University Maastricht, 6200 MD Maastricht, The Netherlands; e-mail: t.hackeng@bioch.unimaas.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal