Abstract
We examined the role of N-linked glycan structures of VWF on its interaction with ADAMTS13. PNGase F digestion followed by lectin analysis demonstrated that more than 90% of VWF N-linked glycan chains could be removed from the molecule (PNG-VWF) without disruption of its multimeric structure or its ability to bind to collagen. PNG-VWF had an approximately 4-fold increased affinity for ADAMTS13 compared with control VWF. PNG-VWF was cleaved by ADAMTS13 faster than control VWF and was also proteolysed in the absence of urea. Occupancy of the N-linked glycan sites at N1515 and N1574 and their presentation of ABO(H) blood group sugars were confirmed with an isolated tryptic fragment. Recombinant VWF was mutated to prevent glycosylation at these sites. Mutation of N1515 did not alter ADAMTS13 binding or increase rate of ADAMTS13 proteolysis. Mutation of N1574 increased the susceptibility of VWF to ADAMTS13 proteolysis and allowed cleavage in the absence of urea. Mutation of N1574 in the isolated recombinant VWF-A2 domain also increased binding and ADAMTS13 proteolysis. These data demonstrate that the N-linked glycans of VWF have a modulatory effect on the interaction with ADAMTS13. At least part of this effect is conformational, but steric hindrance may also be important.
Introduction
Von Willebrand factor (VWF) is a large multimeric plasma glycoprotein essential to normal hemostasis, first acting as the carrier molecule for procoagulant factor VIII (FVIII), extending its half-life within the circulation by protecting it from proteolytic degradation, and second, supporting platelet adhesion to thrombogenic surfaces at sites of vascular injury.1,2
Synthesis of VWF is limited to megakaryocytes and endothelial cells.3 The pre-pro-VWF molecule comprises a 22–amino acid signal peptide, a 741–amino acid propeptide, and the 2050–amino acid mature subunit. The pro-VWF monomer is composed of 4 types of domains (A-D) arranged as follows: NH2-D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK-COOH. VWF multimers are formed by C- and N-terminal intermolecular disulphide bonds, with the largest multimers exceeding 2 × 104 kDa and being the most hemostatically active. Within the circulation, the multimeric size of VWF is controlled by the plasma metalloprotease ADAMTS13, which cleaves VWF at the Y1605-M1606 bond within the A2 domain, reducing multimeric size and thus regulating its adhesive function.4
During synthesis, VWF undergoes extensive posttranslational modification resulting in the addition of 12 N-linked and 10 O-linked glycosylation sites per mature monomer.5 The overall structural composition of the glycans has been determined, but their exact functional significance is poorly understood. There is some evidence to suggest they protect the molecule from proteolytic degradation and are required for dimerization and subsequent multimerization.6-8 Significantly, a small proportion of the N-linked glycans on VWF present the ABO(H) blood group sugars,9,10 and the importance of this is highlighted by the well-established observation that ABO blood group status is a major determinant of plasma VWF levels: individuals of blood group O have 20% to 30% lower VWF levels than non-O blood groups.11-15 How the ABO sugars presented on VWF affect levels is unresolved. Recent evidence from Bowen demonstrated that ABO group altered susceptibility of VWF to ADAMTS13 proteolysis, with blood group O VWF the most susceptible.16 We confirmed and extended this observation by showing that VWF from individuals with the rare Bombay blood group was proteolysed faster by ADAMTS13 than group O VWF and that plasma levels of VWF were the lowest in Bombay individuals.15
Biochemical sequencing of the N-linked glycans has estimated that only 13% presented the blood group sugars.10 It is therefore not clear whether the ABO effect is a general effect of VWF glycans and their modifications, or if it is a specific effect in which particular modifications of selective glycans are crucial. So far, a detailed glycan map of VWF is not available and it is not known what sugar structures are present at specific sites and if, indeed, all the predicted sites are occupied. In this study, we show that N-linked glycosylation has a role in stabilizing the globular conformation of VWF that determines its ability to interact with ADAMTS13. Further, we demonstrate a critical role for the glycan at N1574 in modulating the VWF-ADAMTS13 interaction.
Methods
Purification of VWF
Plasma-derived VWF (pdVWF) was purified from Haemate P (ZLB, Behring, Germany) using a combination of gel filtration and heparin-Sepharose affinity chromatography. Haemate P is prepared from pooled human plasma via multiple precipitation steps followed by pasteurization at 60°C for 10 hours. It has previously been shown to contain a multimer content similar to that of normal plasma.17 Briefly, resuspended Haemate P was gel filtered through a Sephacryl-400 gel filtration column (GE Healthcare, Little Chalfont, United Kingdom) and VWF-containing fractions eluted with buffer 100 mM NaCl, 20 mM Tris-HCl, pH 7.4, were pooled and passed over a SK-16 chromatography column (GE Healthcare) packed with 30 mL heparin-Sepharose 6 fast flow (GE Healthcare). The column was eluted with 300 mM NaCl, 20 mM Tris-HCl, pH 7.4, and the resulting fractions were dialyzed into 20 mM Tris-HCl, pH7.4. Purified VWF appeared as single 250-kDa band under reducing conditions on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with both Coomassie and silver staining and exhibited normal multimeric composition and collagen binding. The concentration of VWF was determined by VWF enzyme-linked immunosorbent assay (ELISA) as previously described,18 and VWF multimer analysis and collagen-binding function were assessed using previously described assays.19,20
Trypsin digestion of VWF
A 55-kDa tryptic fragment of VWF was isolated by trypsin digestion of pdVWF and ion-exchange chromatography as previously described.21 Partial PNGase F digestion of the fragment (20 nM) was performed using 5 U PNGase F (NEB, Hitchin, United Kingdom) at 37°C for 2 hours and then analyzed by reducing SDS-PAGE in 10% gels followed by probing with either anti–VWF-HRP (Dako, Ely, United Kingdom) or biotinylated elderberry bark lectin (EBL; Vector Labs, Peterborough, United Kingdom) specific for terminal sialic acid residues and Ulex europaeus lectin specific for the H-antigen (Vector Labs). N-terminal sequencing was performed by Alta Biosciences (Birmingham, United Kingdom), and mass spectroscopy analysis was kindly performed by Prof Anne Dell (Imperial College London).
Expression of recombinant VWF
Plasmid pSVhVWF containing the wild-type (wt) human full-length (FL) cDNA sequence was kindly supplied by Dr Enayat (Birmingham Children's Hospital, United Kingdom). To facilitate efficient subcloning of VWF, primers were designed to amplify the final 900 bp of the VWF sequence, introducing a unique AgeI restriction site immediately after the stop codon (sequence available on request). The polymerase chain reaction (PCR) product was cloned into the pBlueScript cloning vector and the sequence confirmed by DNA sequencing. This vector was designated pBS-VWF3′. FL-VWF cDNA was then digested with BamHI and SphI, and the resulting fragment cloned into the BamHI and SphI sites of pBS-VWF3′ to form the vector pBS-VWF-BamHI-AgeI (the SphI site is located within the final 900 bp of the FL-VWF cDNA sequence). This vector was then used to introduce asparagine to glutamine mutations at sites N1515 and N1574, both individually and as a double mutant, using the QuickChange XL mutagenesis kit (Stratagene, Cambridge, United Kingdom) with primers 5′GGTGAAGCCGACTTCCAAA GGAGCAAGGAGTTC3′ and 5′GAACTCCTTGCTCCTTTGGAAGTCGGCTTCAC3′ for N1515Q and 5′CCGCTACCAGGGCGGCCAAAGGACCAACACTGGGC3′ and 5′GCCCAGTGTTGGTCCTTTGGCCGCCCTGGTAGCGG3′ for N1574Q (mutated codons underlined). To complete the FL-VWF sequence, the 5′ EcoRI to BamHI fragment was digested from pSVhVWF and cloned into the EcoRI and BamHI sites of pBS-VWF-BamHI-AgeI. The VWF sequence was confirmed by DNA sequencing and then digested from pBS with EcoRI and AgeI and cloned into the EcoRI and AgeI sites of the mammalian expression vector pcDNA3.1(A+) myc/His (Invitrogen, Paisley, United Kingdom).
Recombinant wt and mutated VWF were expressed in HEK293T cells as previously described using 10 mM polyethylenimine (PEI) as transfection reagent.22 At 2 days transfection, expression media were collected and concentrated 10-fold using 100-kDa cutoff spin filters (Amicon, Peterborough, United Kingdom) then dialyzed into 20 mM Tris-HCl, pH 7.8, overnight at 4°C.
Expression of recombinant A2 domain
The vector pcDNAVWFA1A2A3, constructed as previously described,23 was used as a template to generate pcDNAVWFA2 in 2 steps. An intermediary construct, pcDNAVWFA2A3, was obtained in the first step. A1 domain was removed by inverse PCR using pcDNAVWFA1A2A3 as template for DNA amplification with forward primer 5′-ATGGCACAAGTCACTGTGG-3′ and reverse primer 5′-ACAAAGGGTCCCTGGCAA-3′ by KOD HotStart DNA Polymerase (Novagen, Nottingham, United Kingdom). The amplified DNA fragment was gel-purified, kinased, and ligated overnight before transformation of Escherichia coli DH5α. In a second step, pcDNAVWFA2A3 was used as template to remove A3 domain using forward primer 5′-GAACAAAAACTCATCTCAGAAG-3′ and reverse primer 5′CCTCTGCAGCACCAGGT-3′ in an inverse PCR reaction as for step one. Recombinant A2 domain was expressed in HEK293T cells using PEI as transfection reagent. At 2 days after transfection, conditioned media were collected and A2 domain was purified by nickel affinity chromatography. The purity of recombinant A2 domain was assessed by SDS-PAGE in 16% Tris-tricine gels.
PNGase F digestion of VWF and VWF A2 domain
Purified VWF at a final concentration of 100 μg/mL or 20 μM purified A2 domain was incubated with or without 10 U PNGase F (NEB) overnight at 37°C. As a control to compare the removal of VWF N-linked glycans under nondenaturing and denaturing conditions, 100 μg/mL VWF was reduced and denatured with 1% β-mercaptoethanol for 20 minutes at 90°C. NP-40 (NEB) was added to a final concentration of 10% vol/vol and the reaction incubated with PNGase F overnight at 37°C. Removal of glycans was assessed using SDS-PAGE in either 7% or 12% gels followed by staining with anti–VWF-HRP to detect the expected shift in mobility and by a modified lectin ELISA, in which VWF was coated onto MaxiSorp Plates (Nunc, Loughborough, United Kingdom) overnight at 4°C and N-linked glycans were detected with concanavalin A (Vector Labs) diluted 1/1000 in Tris-buffered saline, pH 7.4, supplemented with 1 mM CaCl2.
ADAMTS13 activity assays
Recombinant ADAMTS13 with a C-terminal myc/His tag was stably expressed in HEK293 cells and purified as previously described.15 Purified ADAMTS13 at a final concentration of 10 nM was used to digest 5 μg/mL pdVWF in a reaction mixture containing 5 mM CaCl2, 50 mM NaCl, and 20 mM Tris-HCl (pH 7.8) in the absence or presence of 1.5 M urea. Subsamples were removed at designated time points and the reaction stopped with EDTA. The extent of VWF proteolysis was assessed by collagen-binding assay (CBA) and VWF multimer analysis. Cleavage of the purified VWF A2 domain was performed using the same reaction mixture with 5 μM A2 domain and cleavage assessed by SDS-PAGE in 16% Tris-tricine gels, followed by Coomassie staining.
ADAMTS13-binding assays
Binding of ADAMTS13 to VWF was performed using a modified version of a previously described equilibrium plate-binding assay.24 MaxiSorp plates (Nunc) were coated overnight at 4°C with 7.5 μg/mL multimeric VWF diluted in phosphate-buffered saline (PBS). Unbound VWF was removed and wells blocked with 2% BSA-PBS-0.1% Tween for 1 hour at room temperature. Following washing with phosphate-buffered saline containing 0.1% Tween (PBS-T), the wells were incubated with decreasing concentrations of ADAMTS13 diluted with 20 mM Tris-HCl, pH 7.8, 5 mM EDTA for 2 hours at room temperature. Unbound protein was removed and wells were washed 3 times with PBS-T. Bound ADAMTS13 was specifically detected with 2 antibody populations, a rabbit polyclonal anti-ADAMTS13 antibody, depleted of antibodies against the TSP2-4 domains (pAb(-Tsp2-4)) or a biotinylated anti-ADAMTS13 polyclonal directed against the TSP2-4 domains (pAb Tsp2-4).25 These 2 detecting antibodies were used to investigate whether results of binding were dependent upon domain-specific recognition of ADAMTS13 in this assay. Bound antibodies were detected with either polyclonal goat anti–rabbit Ig-HRP or streptavidin-horseradish peroxidase conjugate (GE Healthcare), Bound antibody was measured with Sigma color fast OPD substrate (Poole, United Kingdom) and the absorbance recorded at 492 nm. Binding curves were generated using Prism software (Droitwich, United Kingdom) fitting the data to the one binding site equation. In a modified version of this assay, VWF was captured on MaxiSorp plates by a monoclonal anti-VWF antibody, EsVWF-8 (American Diagnostica, Greenwich, CT), directed against the SpII fragment of VWF; all other steps were performed as described.
Results
pdVWF N-linked glycosylation sites are accessible to PNGase F digestion and do not affect multimeric structure or collagen binding
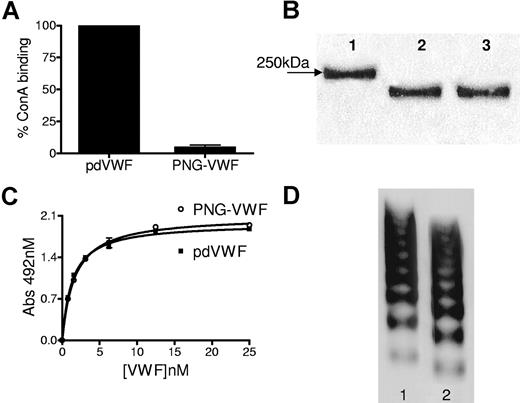
VWF was purified from the commercial VWF/FVIII concentrate Haemate P by gel filtration and heparin-Sepharose chromatography. Haemate P has previously been shown to contain a multimer content similar to that of normal plasma. pdVWF (100 μg/mL) was incubated with PNGase F under both denaturing and nondenaturing conditions and the extent of N-linked glycan removal determined by modified concanavalin A ELISA. Digestion with PNGase F resulted in the removal of more than 90% of VWF N-linked glycans, demonstrated by a more than 90% reduction in concanavalin A (ConA) binding (Figure 1A). The removal of the N-linked glycans did not affect the ability of VWF to bind to the microtiter plate (data not shown). Nondenatured and denatured pdVWF digested with PNGase F were analyzed by SDS-PAGE in 3% to 8% gels. The denatured and nondenatured samples migrated as discrete bands further in the gel compared with pdVWF. However, there was no observable difference in migration between the 2 samples, indicating that similar amounts of N-linked glycans were removed from both samples (Figure 1B). Multimer gel and collagen-binding analysis of PNG-VWF showed that despite almost complete N-linked glycan removal, it retained normal collagen-binding function and characteristic multimeric structure (Figure 1C,D). A shift in multimer band size was visible, consistent with the decrease in molecular weight due to removal of the sugar chains (Figure 1D).
Analysis of PNGase F digested VWF. (A) Equal amounts of digested and nondigested VWF diluted in PBS were coated overnight at 4°C onto microtiter plates and N-linked glycans detected with concanavalin A diluted 1/1000 in Tris-buffered saline, pH 7.4, supplemented with 1 mM CaCl2. Error bars represent mean and SD. (B) Reducing 7% SDS-PAGE analysis of denatured and nondenatured VWF digested with PNGase F. Nondigested VWF (lane 1), nondenatured VWF (lane 2), and denatured VWF (lane 3). (C) Binding of pdVWF and PNG-VWF to immobilized type III collagen. (D) Multimer analysis of PNGase F-digested VWF in 1.6% agarose multimer gels. pdVWF (lane 1); PNG-VWF (lane 2).
Analysis of PNGase F digested VWF. (A) Equal amounts of digested and nondigested VWF diluted in PBS were coated overnight at 4°C onto microtiter plates and N-linked glycans detected with concanavalin A diluted 1/1000 in Tris-buffered saline, pH 7.4, supplemented with 1 mM CaCl2. Error bars represent mean and SD. (B) Reducing 7% SDS-PAGE analysis of denatured and nondenatured VWF digested with PNGase F. Nondigested VWF (lane 1), nondenatured VWF (lane 2), and denatured VWF (lane 3). (C) Binding of pdVWF and PNG-VWF to immobilized type III collagen. (D) Multimer analysis of PNGase F-digested VWF in 1.6% agarose multimer gels. pdVWF (lane 1); PNG-VWF (lane 2).
PNG-VWF is more susceptible to proteolysis by ADAMTS13 and binds ADAMTS13 with increased affinity
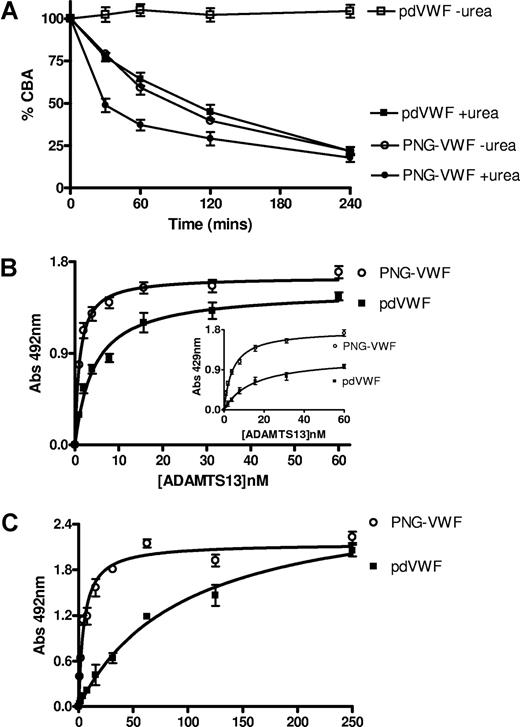
Because glycan composition is known to influence VWF susceptibility to ADAMTS13, the effect of removal of N-linked glycans was studied for this interaction. pdVWF and PNG-VWF were incubated with 20 nM ADAMTS13 in the presence or absence of 1.5 M urea, and the degree of proteolysis was determined by the reduction in collagen binding. In the presence of 1.5 M urea, PNG-VWF had a greater reduction in CBA at all time points (Figure 2A), indicating it had been cleaved appreciably faster than pdVWF. Interestingly, PNG-VWF treated with ADAMTS13 also had reduction of collagen binding in the absence of urea (in contrast to pdVWF), again demonstrating enhanced proteolysis, with the extent of proteolysis comparable with the cleavage of pdVWF in the presence of urea (Figure 2A). PNG-VWF incubated with 1.5 M urea without ADAMTS13 for the duration of the cleavage reaction showed no reduction in collagen binding (data not shown).
The interaction of ADAMTS13 with PNG-VWF. (A) VWF (5 μg/mL) was incubated with 10 nM ADAMTS13 in 5 mM CaCl2, 50 mM NaCl, and 20 mM Tris-HCl (pH 7.8) in the presence and absence of 1.5 M urea. At various time points, subsamples were taken and the reaction was stopped with EDTA, and the extent of proteolysis was determined by CBA. (B) MaxiSorp plates, coated with 7.5 μg/mL VWF, were incubated with decreasing concentration of ADAMTS13. Bound ADAMTS13 was detected with either pAb(-Tsp2-4) (main graph) or pAb Tsp2-4 (inset). (C) VWF was captured to the microtiter plate using a monoclonal anti-VWF antibody directed against the C-terminal SpII fragment. Captured VWF was incubated with decreasing concentration of ADAMTS13 and bound ADAMTS13 detected with pAb(-Tsp2-4). Error bars represent mean and SD.
The interaction of ADAMTS13 with PNG-VWF. (A) VWF (5 μg/mL) was incubated with 10 nM ADAMTS13 in 5 mM CaCl2, 50 mM NaCl, and 20 mM Tris-HCl (pH 7.8) in the presence and absence of 1.5 M urea. At various time points, subsamples were taken and the reaction was stopped with EDTA, and the extent of proteolysis was determined by CBA. (B) MaxiSorp plates, coated with 7.5 μg/mL VWF, were incubated with decreasing concentration of ADAMTS13. Bound ADAMTS13 was detected with either pAb(-Tsp2-4) (main graph) or pAb Tsp2-4 (inset). (C) VWF was captured to the microtiter plate using a monoclonal anti-VWF antibody directed against the C-terminal SpII fragment. Captured VWF was incubated with decreasing concentration of ADAMTS13 and bound ADAMTS13 detected with pAb(-Tsp2-4). Error bars represent mean and SD.
As PNG-VWF was cleaved more readily by ADAMTS13, we next investigated the effect of N-linked glycosylation on binding of ADAMTS13 to VWF. Taking advantage of the ability of VWF to adopt its extended conformation when bound to a microtiter plate,24 we used an equilibrium plate-binding assay. We determined that pdVWF bound ADAMTS13 with KD,app = 4.3 ± 0.5 nM (n = 7) using pAb(-Tsp2-4) (Figure 2B) which is approximately 3-fold higher affinity than the previously published value of 14 nM.24 This difference is almost certainly due to the different antibody used to detect bound ADAMTS13, as we have found that the precise KD,app value is dependent upon the nature of the detection antibody used. Indeed, when we used the alternative detection antibody pAb Tsp2-4, we determined a KD,app = 18.3 ± 2 nM (n = 7; Figure 2B inset). Interestingly, PNG-VWF bound ADAMTS13 with 4-fold increased affinity with both antibodies, KD,app = 1.1 ± 0.3 nM with pAb(-Tsp2-4) and 4.2 ± 0.2 nM with pAb Tsp2-4 (n = 7). Since PNG-VWF was cleaved without the need for a denaturant, we hypothesized that ADAMTS13 would be able to bind to PNG-VWF without the need for unfolding by its binding to the microtiter plate. To investigate this, the plate-binding assay was modified to capture VWF with a monoclonal anti-VWF antibody directed against the C-terminus of the molecule, so that VWF remained in an unextended conformation. Using pAb(-Tsp2-4) to detect bound ADAMTS13, we observed that pdVWF bound ADAMTS13 with KD,app = 90 ± 10 nM (n = 7; Figure 2C). Significantly, PNG-VWF bound ADAMTS13 with much higher affinity, KD,app = 4.3 ± 0.8 nM (n = 7; Figure 2C), demonstrating that VWF devoid of N-linked glycans adopts a configuration that allows high-affinity binding to ADAMTS13 without the need for induction of a conformational change by binding to the microtiter plate.
The predicted N-linked glycosylation sites in the A2 domain of pdVWF are both occupied and present blood group sugars
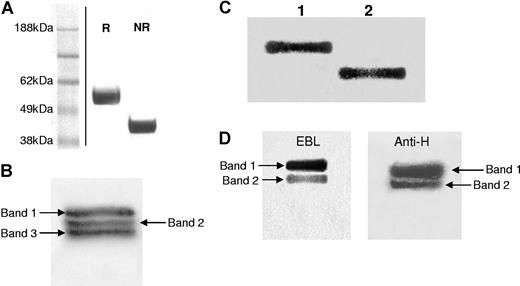
Since the removal of N-linked glycans from pdVWF increased its interaction with ADAMTS13, and the A2 domain is a major site involved in its interaction, it was hypothesized that the influence of N-linked glycans could be largely mediated by the 2 predicted N-linked glycan chains located within the A2 domain at N1515 and N1574, in close proximity to the ADAMTS13 cleavage site at Y1605-M1606. To confirm that both sites were indeed occupied by glycan chains, we isolated a 55-kDa fragment of pdVWF by trypsin digestion followed by purification with ion-exchange chromatography. One of the isolated fractions appeared as a 55-kDa band under reducing conditions and as 44-kDa band under nonreducing conditions (Figure 3A). N-terminal sequencing and mass spectroscopy analysis demonstrated that the fragment spanned residues 1493 to 1849, encompassing N1515 and N1574. Partial PNGase F digestion of the isolated fragment followed by Western blotting and probing with anti–VWF-HRP demonstrated the presence of 3 bands (Figure 3B): band 1 representing both glycans on the fragment, band 2 representing one glycan chain removed, and band 3 representing both glycan chains removed, indicating that both potential glycosylation sites are likely to be occupied by N-linked glycan chains. Complete digestion resulted in a single band (Figure 3C lane 2). Probing the partial PNGase F digest with EBL specific for terminal sialic acid residues demonstrated the presence of 2 bands, with the second band less intense than the first, implying that at least some of the glycan chains are complex and decorated with sialic acid residues (Figure 3D). Interestingly, probing with Ulex europaeus, specific for the H-antigen, also showed the presence of 2 bands, again indicating that at least some of the glycans present the ABO blood group sugars (Figure 3D).
Trypsin digestion of VWF. (A) Twelve percent SDS-PAGE analysis of the major fraction obtained by ion-exchange chromatography of trypsin-digested VWF under reducing (R) and nonreducing (NR) conditions followed by Coomassie staining. (Vertical line indicates a repositioned gel lane.) (B,C) Of the purified 55-kDa fragment, 20 nM was partially digested with PNGase F for 2 hours at 37°C (B) and completely digested with PNGase F overnight at 37°C (C), and analyzed in 12% SDS-PAGE gels followed by Western blotting to nitrocellulose membranes and probing with anti–VWF-HRP. (D) Partially PNGase F–digested VWF 55-kDa fragment was also detected with elderberry bark lectin (EBL) specific for terminal sialic acid residues (left panel) and Ulex europaeus specific for the H-antigen (right panel).
Trypsin digestion of VWF. (A) Twelve percent SDS-PAGE analysis of the major fraction obtained by ion-exchange chromatography of trypsin-digested VWF under reducing (R) and nonreducing (NR) conditions followed by Coomassie staining. (Vertical line indicates a repositioned gel lane.) (B,C) Of the purified 55-kDa fragment, 20 nM was partially digested with PNGase F for 2 hours at 37°C (B) and completely digested with PNGase F overnight at 37°C (C), and analyzed in 12% SDS-PAGE gels followed by Western blotting to nitrocellulose membranes and probing with anti–VWF-HRP. (D) Partially PNGase F–digested VWF 55-kDa fragment was also detected with elderberry bark lectin (EBL) specific for terminal sialic acid residues (left panel) and Ulex europaeus specific for the H-antigen (right panel).
Prevention of N-linked glycosylation at N1574 but not at N1515 increases susceptibility of recombinant VWF to ADAMTS13 cleavage
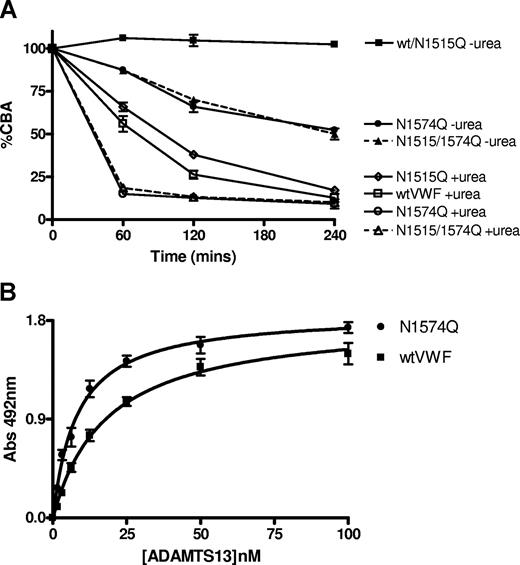
To determine the individual roles of the 2 A2 domain glycan chains, the respective asparagine residues were mutated in recombinant VWF to glutamine residues both individually and as a double mutant. The 3 mutants, N1515Q, N1574Q, and N1515/1574Q were expressed in HEK293T cells at a similar level to wtVWF, demonstrated normal collagen-binding activity (data not shown) and multimeric structure. Reducing SDS-PAGE in 7% gels showed that compared with wtVWF, N1515Q, and N1574Q, mutants migrated visibly further in the gel and the N1515/1574Q mutant further still, consistent with a decrease in molecular weight from the loss of N-linked glycosylation (Figure 4A). Recombinant wtVWF was also subjected to PNGase F digestion and analyzed by SDS-PAGE, migrating a similar distance through the gel as PNGase F–treated pdVWF (data not shown). ADAMTS13 cleavage of recombinant VWF was performed in the presence of 1.5 M urea. Multimer analysis demonstrated that mutant N1515Q was proteolysed slightly slower than wtVWF by ADAMTS13. Mutants N1574Q and N1515/1574Q were both proteolysed faster by ADAMTS13 (Figure 4B). Cleavage assays were repeated in the absence of urea with analysis by CBA. In the absence of urea, no cleavage of wtVWF or mutant N1515Q was observed. However, both N1574Q and N1515/1574Q mutants were proteolysed by ADAMTS13 in the absence of urea (Figure 5A).
Recombinant VWF glycan mutants. (A) The cDNA encoding full-length VWF was cloned into pcDNA3.1 as described and transfected into HEK293T cells using PEI. Recombinant VWF was analyzed by reducing 7% SDS-PAGE followed by Western blotting and probing with anti–VWF-HRP. wtVWF (lane 1), N1515Q (lane 2), N1574Q (lane 3), and N1515/1574Q (lane 4). (B) wt and mutated recombinant VWF (5 μg/mL) was cleaved by 10 nM ADAMTS13 in the presence of 1.5 M urea and cleavage assessed by multimer analysis in 1% gels. wtVWF (lanes 1,5), N1515Q (lanes 2,6), N1574Q (lanes 3,7), and N1515/1574Q (lanes 4,8). RP indicates reference plasma; vertical line indicates a repositioned gel lane.
Recombinant VWF glycan mutants. (A) The cDNA encoding full-length VWF was cloned into pcDNA3.1 as described and transfected into HEK293T cells using PEI. Recombinant VWF was analyzed by reducing 7% SDS-PAGE followed by Western blotting and probing with anti–VWF-HRP. wtVWF (lane 1), N1515Q (lane 2), N1574Q (lane 3), and N1515/1574Q (lane 4). (B) wt and mutated recombinant VWF (5 μg/mL) was cleaved by 10 nM ADAMTS13 in the presence of 1.5 M urea and cleavage assessed by multimer analysis in 1% gels. wtVWF (lanes 1,5), N1515Q (lanes 2,6), N1574Q (lanes 3,7), and N1515/1574Q (lanes 4,8). RP indicates reference plasma; vertical line indicates a repositioned gel lane.
Interaction of ADAMTS13 with VWF glycan mutants. (A) ADAMTS13 cleavage of recombinant VWF was repeated in the absence of 1.5 M urea and the extent of cleavage analyzed by CBA. Shown for comparison is the cleavage of recombinant VWF in the presence of 1.5 M urea. Cleavage of wtVWF and the N1515Q mutant in the absence of urea is represented by a single line. (B) Recombinant VWF was coated on to MaxiSorp plates at a final concentration of 7.5 μg/mL and incubated with decreasing concentration of ADAMTS13 and bound protein detected with pAb Tsp2-4. Error bars represent mean and SD.
Interaction of ADAMTS13 with VWF glycan mutants. (A) ADAMTS13 cleavage of recombinant VWF was repeated in the absence of 1.5 M urea and the extent of cleavage analyzed by CBA. Shown for comparison is the cleavage of recombinant VWF in the presence of 1.5 M urea. Cleavage of wtVWF and the N1515Q mutant in the absence of urea is represented by a single line. (B) Recombinant VWF was coated on to MaxiSorp plates at a final concentration of 7.5 μg/mL and incubated with decreasing concentration of ADAMTS13 and bound protein detected with pAb Tsp2-4. Error bars represent mean and SD.
The ability of ADAMTS13 to bind to the mutant VWF was assessed using pAb Tsp2-4 to detect bound ADAMTS13. Both wtVWF and mutant N1515Q bound ADAMTS13 with similar affinity: KD,app approximately 18.8 (± 1.7) nM. However, both N1574Q and N1515/1574Q mutants bound ADAMTS13 with approximately 2-fold increased affinity: KD,app 9.7 (± 0.4) nm (Figure 5B, data not shown for mutants N1515Q and N1515/1574Q). Thus, mutant N1574Q behaved in a similar way to PNG-VWF, but N1515Q did not.
N-linked glycosylation at N1574 has a localized effect on recombinant VWF A2 domain
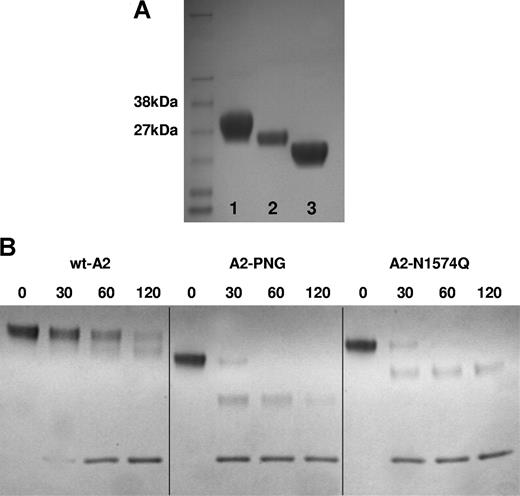
To further investigate the significance of glycosylation at N1574, wt and mutant recombinant A2 domains were expressed in HEK293T cells and purified by Nickel affinity chromatography. Analysis by SDS-PAGE in 4% to 12% gels showed the presence of a predominant band at approximately 29 kDa characteristic of recombinant A2 domain (Figure 6A lane 1). For A2-N1574Q, the band migrated slightly further in the gel due to the absence of the glycan at N1574 (Figure 6A lane 2). Recombinant A2 domain digested with PNGase F (A2-PNG) migrated further than A2-N1574Q, suggesting that both glycan sites are occupied in wtA2 and that the N1515 site is occupied in A2-N1574Q (Figure 6A lane 3). wtA2, A2-N1574Q, and A2-PNG were subjected to ADAMTS13 proteolysis and proteolysis was assessed by SDS-PAGE. Both A2-N1574Q and A2-PNG were proteolysed at a similar rate that was appreciably faster than wtA2 (Figure 6B).
Proteolysis of recombinant A2 domain fragments. (A) Purified recombinant A2 domain was analyzed by 12% SDS-PAGE and Coomassie staining. VWF A2 domain was visible as a single band of approximately 29 kDa corresponding to its predicted mass (lane 1). A2-N1574Q (lane 2) and PNGase F–digested A2 (lane 3) were also analyzed on the same gel for comparison. (B) wtA2, A2-N1574Q, and A2-PNG (5 μM) were incubated with 10 nM ADAMTS13 in 5 mM CaCl2, 50 mM NaCl, and 20 mM Tris-HCl (pH 7.8), and 1.5 M urea. At various time points subsamples were taken and the reaction was stopped with EDTA, and proteolysis was assessed by SDS-PAGE in 16% Tris-tricine gels and Coomassie staining.
Proteolysis of recombinant A2 domain fragments. (A) Purified recombinant A2 domain was analyzed by 12% SDS-PAGE and Coomassie staining. VWF A2 domain was visible as a single band of approximately 29 kDa corresponding to its predicted mass (lane 1). A2-N1574Q (lane 2) and PNGase F–digested A2 (lane 3) were also analyzed on the same gel for comparison. (B) wtA2, A2-N1574Q, and A2-PNG (5 μM) were incubated with 10 nM ADAMTS13 in 5 mM CaCl2, 50 mM NaCl, and 20 mM Tris-HCl (pH 7.8), and 1.5 M urea. At various time points subsamples were taken and the reaction was stopped with EDTA, and proteolysis was assessed by SDS-PAGE in 16% Tris-tricine gels and Coomassie staining.
Discussion
Glycan moieties, of which 70% are N-linked glycans, comprise approximately 20% of the molecular weight of VWF.26 Previous analysis of the N-linked glycan structures demonstrated that a small proportion (∼ 10%) present ABO(H) blood group sugars, which is likely to be of relevance with respect to the long-held observation that plasma VWF levels are significantly affected by ABO blood type.10 In previous studies, we and others have shown also that ABO/Bombay blood group alters susceptibility of VWF to ADAMST13 cleavage.15,16 To explore the influence of glycans further, we have investigated the general and specific influences of N-linked glycosylation on the interaction of VWF with ADAMTS13.
A previous study has investigated the accessibility of VWF N-linked glycans to PNGase F digestion. Fischer et al were able to remove appreciable amounts of N-linked glycan chains from recombinant VWF only following denaturation of the molecule.27 However, in the present study, we were able to remove more than 90% of VWF N-linked glycans with PNGase F digestion. This reduction was observed when both nondenatured and denatured VWF were digested with PNGase F. This demonstrates that either all the N-linked glycan sites are directly accessible to PNGase F cleavage or, possibly, that they become progressively accessible as a result of conformational changes after initial removal of the most exposed chains. While N-linked glycosylation of VWF is required for dimerization to occur,8 conflicting data exist as to whether the removal of VWF sugars affects its multimeric structure.7,28 In our hands, PNGase F treatment of pdVWF did not alter its multimeric structure, but caused only a shift in electrophoretic mobility due to the change in molecular weight. Maintenance of multimeric structure was confirmed by the lack of effect on collagen-binding activity of PNG-VWF compared with pdVWF. Incidentally, we note that there are no predicted N-linked glycan sites in close proximity to the known collagen-binding sites.
The N-linked glycans of other proteins have been shown to protect against proteolytic attack.29,30 It was therefore hypothesized that removal of VWF N-linked glycans would potentiate ADAMTS13 cleavage of VWF. Accordingly, PNG-VWF was shown to be proteolysed significantly faster than unmodified VWF by ADAMTS13 in the presence of 1.5 M urea. Interestingly, PNG-VWF was also cleaved by ADAMTS13 in the absence of urea and at a similar rate to unmodified VWF with urea. This observation suggests that removal of the N-linked glycans alters the globular conformation of VWF allowing ADAMTS13 access to the cleavage site even in the absence of a denaturant. To investigate the reason for enhanced proteolysis, we performed VWF:ADAMTS13-binding assays, exploiting the tendency of VWF to take on its elongated conformation when bound to a surface. When ADAMTS13 bound to VWF coated directly on a plate was detected with polyclonal anti-ADAMTS13 antibodies, we observed that PNG-VWF bound ADAMTS13 with 4-fold increased affinity compared with unmodified VWF. Increased cleavage of VWF devoid of N-linked glycans therefore arises in part by increased binding of ADAMTS13. Since PNG-VWF could be cleaved in the absence of urea/induced unfolding, we modified the VWF:ADAMTS13-binding assay to investigate the binding of ADAMTS13 to globular (unextended) VWF. In this assay, ADAMTS13 bound to pdVWF with much lower affinity (KD,app ∼ 90 nM), as predicted, but bound to PNG-VWF with high affinity (KD,app ∼ 4 nM). Together, these studies imply that removal of VWF N-linked glycans mimics the unraveling effect of urea or microtiter plate unfolding. However it also has a local effect on accessibility of ADAMTS13-binding sites even when VWF is unraveled.
To determine whether the effects of VWF glycans on ADAMTS13 binding and cleavage were due to general properties of the sugar chains or were attributable to specific N-linked chains, we investigated the 2 N-linked glycosylation sites located in the A2 domain (N1515Q and N1574Q) that lie in close proximity to the ADAMTS13 cleavage site at Y1605-M1606. First, and consistent with their hypothesized role in the ABO effect, we confirmed that, for pdVWF, both sites are indeed occupied by sugar chains and demonstrated that at least some of these contain sialic acid and H antigen.
We then removed the A2 domain N-linked glycan chains from recombinant VWF, separately and together, by site directed mutagenesis. Using transient transfection, the 3 mutants were expressed at a similar level to wtVWF, with similar proportions in cell lysates. Multimeric analysis demonstrated that the recombinant wtVWF had a similar size and multimer pattern to pdVWF. Lectin analysis with lectins specific for N-linked glycan chains, sialic acid, and galactose residues also demonstrated that recombinant wtVWF presented similar glycan structures to pdVWF (data not shown). Interestingly, when subjected to ADAMTS13 proteolysis, the N1515Q mutant was cleaved slightly slower than wtVWF. However, both N1574Q and the N1515/1574Q mutants were rapidly proteolysed by ADAMTS13 and were also cleaved in the absence of urea, albeit slower than wtVWF treated with urea. After treatment with PNGase F, the N1574Q mutant was cleaved in the absence of urea at a similar rate to wtVWF with urea (data not shown). The affinity of ADAMTS13 was increased approximately 2-fold by loss of the single N1574Q chain, compared with the approximately 4-fold increase resulting from loss of all N-linked glycans on PNG-VWF. Conceivably, the effect could also be due to the amino acid change rather than the loss of N-linked glycosylation, since many type 2A mutations within the A2 domain cause a similar effect to mutation of N1574Q. However, these are nonconservative changes and we have chosen to use a semiconservative mutation to try to ensure that there is minimal effect on protein structure. Furthermore, when we expressed the N1574Q mutation using the short bacterial-expressed VWF115 substrate, no difference in the rate of proteolysis was observed compared with wild-type VWF115, indicating the amino acid change is not part of the primary recognition sequence needed for ADAMTS13 (data not shown). We have also expressed the A2 domain in mammalian cells in the presence of the N-linked glycan pathway inhibitor tunicamycin. This produced recombinant A2 domain devoid of glycosylation without an underlying amino acid change. This molecule was also cleaved significantly faster than wtA2 (data not shown), demonstrating that it is most probably the removal of the N-linked glycan chain that is responsible for the increased susceptibility to ADAMTS13 proteolysis.
These results indicate that the N-linked glycan component of VWF has major effects in determining the conformation of VWF and its susceptibility to ADAMTS13 binding and cleavage. To further confirm a crucial role of N1574, we expressed in mammalian cells an isolated A2 domain fragment and a mutated A2 fragment (A2-N1574Q). Compared with wtA2, A2-N1574Q and also PNGase F treated A2 (PNG-A2) were proteolysed significantly faster by ADAMTS13.
The precise mechanisms by which VWF N-linked glycans modulate the interaction with ADAMTS13 remain unclear. It may be due to steric or conformation effects or a combination of both. Potentially, the N-linked glycans, specifically that at N1574, may sterically hinder ADAMTS13 access to its cleavage site within the A2 domain. The relative positions of the asparagine residues at 1574 and 1515 in the predicted tertiary structure of the A2 domain are shown in Figure 7. The asparagines are seen to project in different directions, consistent with different steric effects of the attached glycan structures on access to the ADAMTS cleavage site. However, the effects on interaction with ADAMTS13 are more pronounced with PNG-VWF, implicating a role for other N-linked glycosylation sites as well. Any hypothesis must therefore include an effect on VWF conformation, by removal of the N-linked glycans (especially that of N5174), altering the susceptibility of the molecule to cleavage. In this way, the normally hidden scissile bond Y1605-M1606 may become more exposed and accessible following removal of the N-linked glycans. This is supported by our observation that PNG-VWF and mutation of N1574 allows ADAMTS13 to cleave VWF in the absence of urea. The glycan moieties of other proteins, such as erythropoietin, have been shown to confer stability on the molecule by forming hydrophobic interactions between glycan and protein.31 It has been suggested that N-linked glycans form hydrophobic planes, where the degree of hydrophobicity is increased with increased branching of the glycan chain.31 This hypothesis would also provide an explanation for the observation made by ourselves15 and Bowen16 that ABO/Bombay blood group affects susceptibility to ADAMTS13 cleavage. The increased branching of the glycan chains due to the extra fucose, and galactose or N-acetylgalactosamine residues may increase the hydrophobic plane of the glycans, stabilizing VWF in its globular conformation. The observation that PNG-A2 and N1574Q-A2 are proteolysed faster than wtA2 suggests that a combination of local and long-range effects of VWF N-linked glycans maintain the correct structure of the molecule. In our experiments, while binding and cleavage are both altered by changing N-linked glycan structures, there is not always a strict quantitative link between these 2 factors. This probably reflects the discrepancy between Km and Kd for ADAMTS13 substrate binding and cleavage.32 Further investigation is also required to determine whether these effects occur under flow conditions, since only static assays have been used in this study. It is, however, attractive to speculate that the N-linked glycan moiety of VWF aids the maintenance of the globular VWF structure under flow conditions.
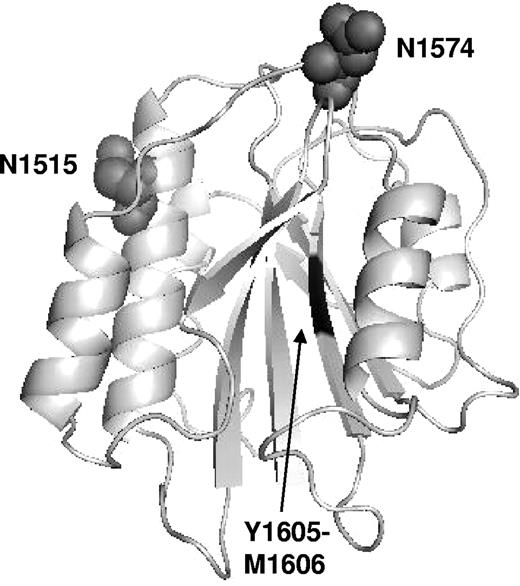
Predicted model of the VWF A2 domain showing the location of N1515 and N1574. A model of the VWF A2 domain was prepared as previously described32 and manipulated using Protein Explorer software (available from http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/, University of Massachusetts). The ADAMTS13 cleavage site, Y1605-M1606, is shown in the center of the domain. Both glycosylation sites, N1515 and N1574, are located on the same face of the A2 domain but on opposite sides of the ADAMTS13 cleavage site. Glycosylation sites are represented by ball structures and are not representative of the size of the glycan present at each site.
Predicted model of the VWF A2 domain showing the location of N1515 and N1574. A model of the VWF A2 domain was prepared as previously described32 and manipulated using Protein Explorer software (available from http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/, University of Massachusetts). The ADAMTS13 cleavage site, Y1605-M1606, is shown in the center of the domain. Both glycosylation sites, N1515 and N1574, are located on the same face of the A2 domain but on opposite sides of the ADAMTS13 cleavage site. Glycosylation sites are represented by ball structures and are not representative of the size of the glycan present at each site.
Together the data presented in this paper demonstrate that the total N-linked glycan population of VWF contributes to its tendency to adopt a globular configuration in free solution. N1574 also contributes to this but also plays a critical role in the binding interaction with ADAMTS13 and the accessibility of its cleavage site.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the British Heart Foundation (FS/03074/15912 and RG/02/008), and received support from the National Institute for Health Research Biomedical Research Centre Funding Scheme.
Authorship
Contribution: T.A.J.M. designed and performed research, analyzed data, and wrote the paper; A.C.K.C. designed and performed research and wrote the paper; A.J.M. performed research; D.A.L. wrote the paper and analyzed data; M.A.L. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. J. McKinnon, Haematology Department, Imperial College London, 5th Floor, Commonwealth Building, Hammersmith Hospital Campus, Du Cane Road, London W12 0NN, United Kingdom; e-mail: t.mckinnon03@imperial.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal