Abstract
Effective administration of flavopiridol in advanced-stage chronic lymphocytic leukemia (CLL) is often associated with early biochemical evidence of tumor cell lysis. Previous work using other cell types showed that flavopiridol impacts mitochondria, and in CLL cells flavopiridol down-regulates the mitochondrial protein Mcl-1. We therefore investigated mitochondrial structure and function in flavopiridol-treated CLL patient cells and in the lymphoblastic cell line 697 using concentrations and times at which tumor lysis is observed in treated patients. Mitochondrial membrane depolarization was detected in flavopiridol-treated CLL cells by 6 hours, well before the onset of cell death. Flavopiridol-induced mitochondrial depolarization was not blocked by caspase inhibitors or by the calcium chelator EGTA, but was reduced by Bcl-2 overexpression. Intracellular calcium mobilization was noted at early time points using fluorescence microscopy. Furthermore, electron paramagnetic resonance oximetry showed a gradual but significant reduction in cellular oxygen consumption rate by 6 hours, corresponding with ultrastructural mitochondrial damage detected by electron microscopy. These observations suggest that in CLL and 697 cells, flavopiridol mediates its cytotoxic effects via induction of the mitochondrial permeability transition and changes in intracellular calcium.
Introduction
Flavopiridol is a semisynthetic flavone (N-methylpiperidinyl chlorophenyl flavone) that is considered to act broadly as a cyclin-dependent kinase (CDK) inhibitor.1 However, the in vivo mechanism of action of flavopiridol is not well understood, and may involve actions other than or inclusive of CDK inhibition. Our group recently demonstrated significant clinical efficacy of flavopiridol in patients with refractory chronic lymphocytic leukemia (CLL) using a novel schedule of administration.2 Approximately 50% of CLL patients who receive flavopiridol using this schedule exhibit biochemical signs of tumor lysis (elevated potassium and phosphate levels, reduced calcium levels) occurring as early as 4.5 hours after treatment initiation. In limited cases with highly elevated peripheral white blood cell counts, this tumor lysis can be severe enough to require dialysis. This observation suggests that flavopiridol, when effectively administered, induces very rapid cell death that is atypical of classical apoptosis. However, more work is needed to understand this process and to better predict which patients may experience severe tumor lysis
Multiple previous studies in different cell types have incriminated mitochondrial mechanisms in flavopiridol-induced apoptosis, although conditions and time points under which this is noted vary widely and are often reported in combination with other agents.3-11 Several reports showed that flavopiridol induces mitochondrial membrane disruption and release of cytochrome c in the U937 human monoblastic leukemia cell line and that these effects were potentiated by phorbol myristate acetate (PMA).4,9,10 This process in U937 cells was noted to be partially caspase independent, as a general caspase inhibitor blocked flavopiridol-induced loss of mitochondrial membrane potential (ΔΨm) but not cytochrome c release. Using human glioma cell lines, Alonso et al also reported that flavopiridol induces caspase-independent cell death, and although cytochrome c release was not observed in these cells, apoptosis-inducing factor (AIF) was translocated into the nucleus.12 In contrast, this same group also showed that, in murine glioma cells, flavopiridol induced mitochondrial outer membrane permeability as evidenced by both cytochrome c release and AIF translocation.13 Furthermore, in human lung carcinoma cells lacking pro–caspase 8, flavopiridol caused early mitochondrial depolarization in the absence of cytochrome c release. Interestingly, Bcl-2, an inhibitor of the mitochondrial permeability transition pore (PTP), was irrelevant to this process.3 In a different study also using lung cancer cell lines, inhibition of caspases blocked apoptosis but not early loss of ΔΨm.11 Thus, although there is substantial evidence for the involvement of mitochondria in flavopiridol-induced cytotoxicity, the mechanism is still unclear and differs by cell type.
Several groups have demonstrated that the antiapoptotic protein Mcl-1 is rapidly down-regulated following flavopiridol treatment at both the mRNA and protein levels.14-16 Mcl-1 contributes significantly to mitochondrial membrane stability, both through sequestering proapoptotic factors such as Bax17 and through inhibition of mitochondrial calcium signaling.18 Mitochondria play a major role in calcium homeostasis through the rapid uptake of calcium released from intracellular stores such as the endoplasmic reticulum (ER).19 Increased calcium uptake via the calcium uniporter favors the opening of the PTP, resulting in movement of ions and solutes along their respective electrochemical gradients and expansion of the mitochondrial matrix space, culminating in disruption of the outer mitochondrial membrane and the liberation of proapoptotic factors from the intramembranous space.19,20 This sequence of events is referred to as the mitochondrial permeability transition (MPT). Thus, the indirect inhibition of intracellular calcium flux by Mcl-1 provides an additional explanation of its antiapoptotic effect. Down-regulation of Mcl-1 is known to potentiate the effects of anticancer agents in several tumor types, and is sufficient to cause apoptosis in leukemic cell lines and CLL patient cells.21
Because of these factors, we sought to determine whether the ability of flavopiridol to induce rapid cell death in CLL patient cells involves mitochondria and to understand the cellular events surrounding this effect. Determining the mechanism of mitochondrial perturbation will be essential to understanding flavopiridol's activity in CLL and may help to predict which patients are at increased risk for acute tumor lysis syndrome with treatment.
Methods
CLL patient samples and cell culture
Written informed consent to collect blood was obtained from CLL patients in accordance with the Declaration of Helsinki under a protocol approved by The Ohio State University Institutional Review Board. In vitro studies were carried out using either whole blood or CD19+ cells obtained from CLL patients with elevated leukocyte counts. Patients were untreated for at least 6 weeks at the time of sample collection. CLL cells were isolated from peripheral blood using B-cell Rosette-Sep (StemCell Technologies, Vancouver, BC). Isolated cells were resuspended in Hybridoma SFM (Invitrogen, Carlsbad, CA) supplemented with l-glutamine, penicillin and streptomycin (Sigma-Aldrich, St Louis, MO), and 10% human serum. The acute lymphoblastic leukemia cell line 697 was obtained from DSMZ (Braunschweig, Germany). The Bcl-2–overexpressing 697 cell line 697-Bcl2 and its empty-vector control 697-Neo22 were the kind gift of Drs Shinichi Kitada and John Reed (Burnham Institute for Medical Research, La Jolla, CA). Immediately prior to the experiment, 697-Bcl2 cells were verified to have a 10-fold higher expression of Bcl-2 relative to 697-Neo cells (data not shown). Cell lines were maintained in RPMI 1640 supplemented with 10% human serum, plus glutamine and antibiotics. Cells were maintained in a humidified incubator at 37°C, 5% CO2. Flavopiridol was obtained from the National Cancer Institute and used at either 1.0 or 1.5 μM as stated in the text, based on pharmacokinetic data from treated CLL patients.2 Experiments conducted with both 1.0 and 1.5 μM flavopiridol showed very similar results. In whole blood experiments, flavopiridol was used at 3.0 μM due to its extensive binding to human plasma proteins. The pan-caspase inhibitor Z-VAD-fmk and caspase-8 (Z-IETD-fmk) and caspase-9 (Z-LEHD-fmk) inhibitors were obtained from Calbiochem (San Diego, CA). Inhibitors were used at 100 μM and added 1 hour prior to flavopiridol treatment. The active metabolite of fludarabine, 2-fluoroadenine-9-β-D-arabinofuranoside (F-ara A), was used as a positive control (Sigma-Aldrich).
Apoptosis and mitochondrial membrane depolarization studies
Apoptosis and cytotoxicity were assessed using annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) (BD Biosciences, San Diego, CA) and an EPICS-XL flow cytometer (Beckman Coulter, Fullerton, CA). Mitochondrial membrane potential changes were assessed using the voltage-sensitive lipophilic cationic dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine iodide (JC-1; Molecular Probes, Eugene, OR) as described.21 In these analyses, a gate was drawn around the population with aggregated JC-1 (intact mitochondria) in untreated cells. Using this gate, the percentage of cells with intact mitochondria in treated samples was then calculated relative to the untreated sample, set at 100%.
Protein fractionation and immunoblotting
Cytosolic and mitochondrial fractions were prepared using a kit from BioVision (Mountain View, CA). BioVision also supplied the cytochrome c antibody. Antibody to voltage-dependent anion channel (VDAC; Calbiochem) was used to check the purity of mitochondrial fractions. Other antibodies used were anti–Mcl-1 (sc-19), anti–β-actin (sc-1616), anti–α-tubulin (Tu-02) (Santa Cruz Biotechnology, Santa Cruz CA), and anti–Bcl-XL (Chemicon, Temecula, CA).
Calcium studies
Intracellular calcium changes were assessed using confocal laser scanning microscopy (LSM) and Fluo-4/AM dye. CLL cells and 697 cells were treated with 1.5 μM flavopiridol for 1 to 5 hours in dye-free media supplemented with 10% human serum. Cells were resuspended in calcium- and magnesium-free phosphate-buffered saline, and loaded with Fluo-4/AM dye. Cells were centrifuged and washed prior to resuspending in calcium-free modified Tyrode buffer (145 mM NaCl, 5.6 mM KCl, 100 μM EGTA, 1.0 mM MgCl2, 10 mM glucose, and 5.0 mM HEPES, pH 7.2; all from Sigma) and incubated for an additional 30 minutes on polylysine-coated cover slips, which were then mounted on a custom-made perfusion chamber to keep the cells hydrated. Fluorescence was measured at room temperature using a Zeiss 510 confocal imaging system (Carl Zeiss, Peabody, MA) with a 63×/1.2 NA water-immersion lens. The excitation wavelength was 488 nm and emission channel 1 or 3 was used with a bandpass filter of 500 to 550 nm. The calcium ionophore ionomycin (10 ng/μL; Sigma-Aldrich) was used as positive control for release of calcium from internal stores. To allow comparative quantification between treatment groups, laser beam intensity and photodetector sensitivity were kept constant. Fluorescence was quantified over all the cells in 2 randomly selected fields using ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij/).
Measurement of cellular respiration
Electron paramagnetic resonance (EPR) oximetry allows simple and reproducible measurement of oxygen consumption rate (OCR) in live cells in real time.23 This assay was performed using lithium 5,9,14,18,23,27,32,36-octa-n-butoxy-2,3-naphthalocyanine (LiNc-BuO) microcrystals as the oximetry probe as described previously by members of our group.24-26 CLL cell suspensions (5 × 106/mL) were saturated with room air (pO2 160 mmHg). LiNc-BuO microcrystals were added to these suspensions and the mixture transferred into 50-μL microcapillary tubes. The tubes were then sealed and EPR spectral measurements immediately started. OCRs of untreated CLL cells were compared with CLL cells incubated for 3 to 6 hours with 1.5 μM flavopiridol. The mitochondrial oxidative uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 50 μM) and the respiratory inhibitor potassium cyanide (KCN, 100 μM) were used as control agents to determine the contribution of mitochondrial respiration. EPR spectra were analyzed by fitting the data into a Lorentzian lineshape by a least-squares algorithm as described.26 Data are presented as mean ± standard deviation (SD). OCR was calculated by the equation: (nmol/min/106 cell) = [(1.59 nmol) × (pO2/min)]/ [(5 × 106 cells/mL) × 50 μL], where 1.59 nmol is solubility of oxygen in water.
Transmission electron microscopy
Whole blood from patients with CLL was treated with flavopiridol for 6 hours at 37°C with mixing to provide a more physiologically relevant situation to examine mitochondria structural alterations. Flavopiridol was used at 3.0 μM due to its extensive binding to human plasma proteins. CD19+ CLL cells were quickly isolated from treated blood and resuspended in fixative (2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, containing 2% polyvinylpyrrolidone (PVP; Sigma-Aldrich) overnight at 4°C. Cells were resuspended in warm 2% low-temperature gelling agarose and quickly centrifuged. Agarose blocks (1 mm) were fixed in 1% osmium tetroxide in phosphate buffer, then rinsed and stained in 1% uranyl acetate for 1 hour. Blocks were dehydrated in graded ethanols followed by propylene oxide, then embedded in Spurr resin and polymerized overnight at 60°C. Sections were cut on a Leica EM UC6 ultramicrotome (Bannockburn, IL) at a thickness of 70 nm and stained for 15 minutes in 2% uranyl acetate and 5 minutes in Reynold lead citrate. Grids were examined in a FEI Technai G2 Spirit transmission electron microscope (Hillsboro, OR) at 80 kV and images captured using an AMT digital camera (Danvers, MA).
Statistical methods
All experiments were analyzed using the raw data in random effects models to allow for correlations among measures from the same patient or cell line. If a particular group in an experiment was used in multiple comparisons, P values were adjusted by Holm method. All tests were 2 sided and the significance level was set at α = .05.
Results
Flow cytometric analysis of flavopiridol-treated CLL cells
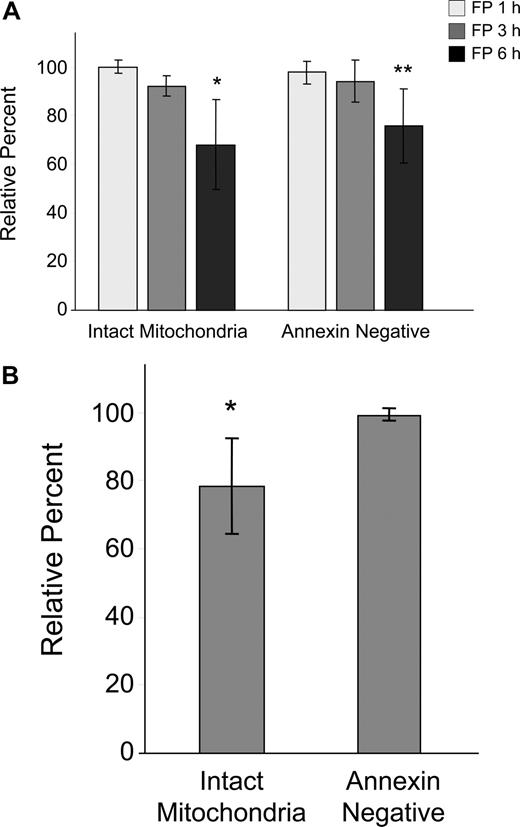
Because of the early and sometimes dramatic evidence of tumor cell death in CLL patients treated with flavopiridol, we conducted in vitro kinetic studies to determine the time course of flavopiridol activity on CLL cells using media containing human serum and a flavopiridol concentration similar to what is achieved in treated patients.2 We observed a gradual but significant loss in ΔΨm by 6 hours as evidenced by disaggregation of JC-1 (Figure 1A). This effect was concurrent with increased annexin binding indicative of early stages of apoptosis, although no increase in PI uptake was noticed during this period. We also studied drug washout after 4 hours, measuring effects on viability and mitochondrial depolarization at 6 hours. There was no difference between the continuous exposure for 6 hours versus washout, likely due to rapid uptake and/or short in vitro half-life of flavopiridol (data not shown). To better understand the situation in CLL patients treated with flavopiridol, we then incubated whole blood from patients with or without 3.0 μM flavopiridol for up to 6 hours. CD19+ cells were then quickly isolated and analyzed as above (Figure 1B). In these experiments, mitochondrial depolarization occurred by 6 hours as in isolated cells, but to a lesser extent. Furthermore, mitochondrial depolarization was consistently observed prior to increases in annexin binding.
Effects of flavopiridol (FP) on mitochondrial membrane potential and annexin binding in CLL patient cells. (A) CD19+ CLL patient cells were isolated from peripheral blood and treated with 1.5 μM FP for 1, 3, and 6 hours (N = 5) in media with 10% human serum. Mitochondrial membrane potential was quantified by flow cytometric determination using JC-1, and data are given as the percentage treated cells with intact mitochondria (aggregated JC-1) relative to untreated cells at the same time points. Annexin binding was assessed by flow cytometry using FITC-labeled annexin, and data are shown as the percentage of annexin-negative cells in treated samples relative to time-matched untreated samples in each case. Error bars represent ± standard deviation. The average percentage of intact mitochondria at 6 hours in FP-treated cells was significantly less than in untreated cells (*P < .001). Likewise, annexin positivity was significantly less in treated cells at 6 hours relative to untreated (**P < .001). (B) Whole peripheral blood from CLL patients (N = 7) was incubated for 6 hours with or without 3.0 μM FP. CD19+ cells were rapidly isolated and assessed as above. Data are shown relative to untreated, time-matched samples in each case. The average percentage of intact mitochondria in whole blood treated with FP was significantly less than in untreated cells (*P = .007). In contrast, there was no significant change in annexin positivity between treated and untreated cells (P = .13).
Effects of flavopiridol (FP) on mitochondrial membrane potential and annexin binding in CLL patient cells. (A) CD19+ CLL patient cells were isolated from peripheral blood and treated with 1.5 μM FP for 1, 3, and 6 hours (N = 5) in media with 10% human serum. Mitochondrial membrane potential was quantified by flow cytometric determination using JC-1, and data are given as the percentage treated cells with intact mitochondria (aggregated JC-1) relative to untreated cells at the same time points. Annexin binding was assessed by flow cytometry using FITC-labeled annexin, and data are shown as the percentage of annexin-negative cells in treated samples relative to time-matched untreated samples in each case. Error bars represent ± standard deviation. The average percentage of intact mitochondria at 6 hours in FP-treated cells was significantly less than in untreated cells (*P < .001). Likewise, annexin positivity was significantly less in treated cells at 6 hours relative to untreated (**P < .001). (B) Whole peripheral blood from CLL patients (N = 7) was incubated for 6 hours with or without 3.0 μM FP. CD19+ cells were rapidly isolated and assessed as above. Data are shown relative to untreated, time-matched samples in each case. The average percentage of intact mitochondria in whole blood treated with FP was significantly less than in untreated cells (*P = .007). In contrast, there was no significant change in annexin positivity between treated and untreated cells (P = .13).
Investigation of apoptosis pathways activated by flavopiridol
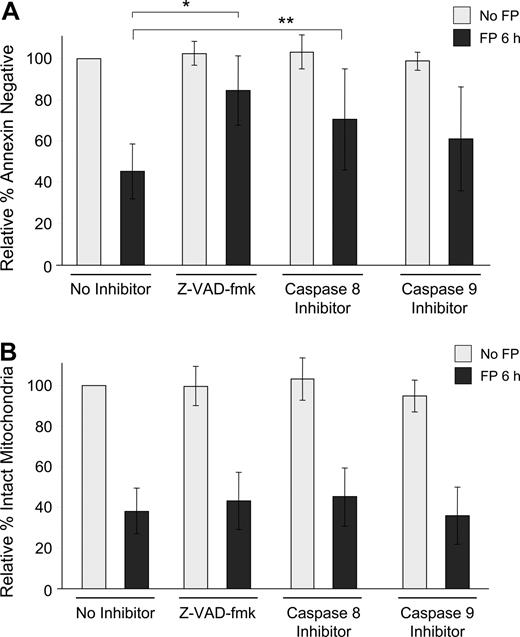
To determine whether this observed mitochondrial depolarization is dependent on caspase activation, isolated CLL cells were exposed to 1.5 μM flavopiridol for 6 hours in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk or by specific inhibitors of caspase-8 (Z-IETD-fmk) or caspase-9 (Z-LEHD-fmk). In each case, loss of ΔΨm was similar, although apoptosis as measured by annexin binding was significantly prevented (Figure 2A,B). As is typical with CLL cells ex vivo, the degree of spontaneous apoptosis in these samples was variable, and thus data are presented as relative to each sample's untreated value at that time point. For example, in these experiments, the average percentage of annexin-negative cells without any treatment was 81% at 6 hours. Similarly, using the 697 lymphoblastic cell line, flavopiridol-mediated loss of ΔΨm was not reduced by caspase inhibitors, although apoptosis was effectively blocked by Z-VAD-fmk and to a lesser extent by both caspase-8 and caspase-9 inhibitors (data not shown).
Influence of caspase inhibitors on flavopiridol-mediated effects in CLL patient cells. (A) CD19+ CLL patient cells in media with 10% human serum were incubated with or without 1.5 μM FP for 6 hours in the presence or absence of 100 μM pan-caspase inhibitor (Z-VAD-fmk), caspase-8 inhibitor (Z-IETD-fmk), or caspase-9 inhibitor (Z-LEHD-fmk). CLL samples (N = 7) were analyzed for annexin binding as above. Data are shown relative to untreated cells, set at 100%. The effect of FP with Z-VAD-fmk was significantly less than with no inhibitor (*adjusted P < .001) as was the case with caspase-8 (**adjusted P = .04). The effect of FP with caspase-9 also tended to be less, although not significant (adjusted P = .08). (B) Samples (N = 8) were then assessed for mitochondrial membrane depolarization at 6 hours. Error bars represent plus or minus standard deviation. None of the caspase inhibitors blocked FP-mediated mitochondrial depolarization (adjusted P > .3 for each).
Influence of caspase inhibitors on flavopiridol-mediated effects in CLL patient cells. (A) CD19+ CLL patient cells in media with 10% human serum were incubated with or without 1.5 μM FP for 6 hours in the presence or absence of 100 μM pan-caspase inhibitor (Z-VAD-fmk), caspase-8 inhibitor (Z-IETD-fmk), or caspase-9 inhibitor (Z-LEHD-fmk). CLL samples (N = 7) were analyzed for annexin binding as above. Data are shown relative to untreated cells, set at 100%. The effect of FP with Z-VAD-fmk was significantly less than with no inhibitor (*adjusted P < .001) as was the case with caspase-8 (**adjusted P = .04). The effect of FP with caspase-9 also tended to be less, although not significant (adjusted P = .08). (B) Samples (N = 8) were then assessed for mitochondrial membrane depolarization at 6 hours. Error bars represent plus or minus standard deviation. None of the caspase inhibitors blocked FP-mediated mitochondrial depolarization (adjusted P > .3 for each).
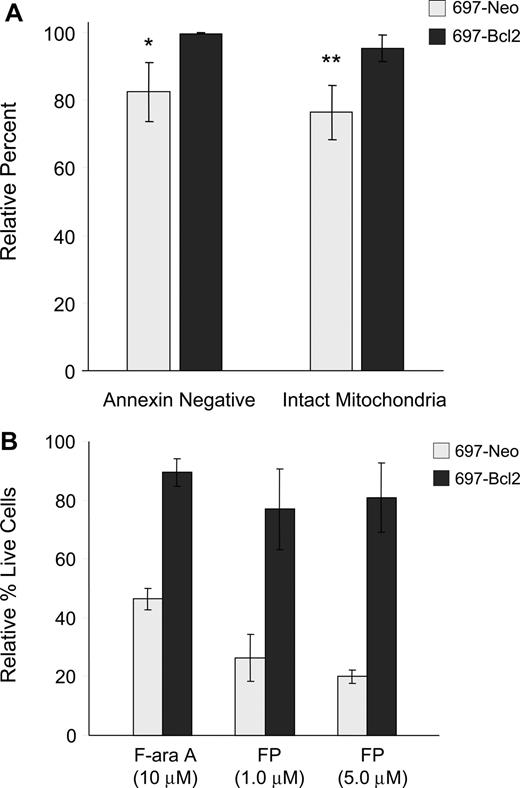
Apoptosis is generally reported to occur either through the intrinsic pathway, which is regulated by Bcl-2 family members and involves early induction of the MPT and consequent caspase-9 activation, or the extrinsic pathway, in which death receptor components CD95/Fas, FADD adapter protein, and caspase-8 associate to form the death-inducing signaling complex (DISC).27,28 To further understand the pathway of flavopiridol-mediated cell death, we used the 697 lymphoblastic cell line overexpressing Bcl-2. Flow cytometry was conducted on flavopiridol-treated 697 cells to assess both annexin binding and mitochondrial effects. In these experiments, we observed that overexpression of Bcl-2 in the 697 cell line prevented both the induction of apoptosis and depolarization of mitochondrial membrane relative to the vector-only control cell line (Figure 3A). To confirm whether this correlated with cytotoxicity, these same cell lines were incubated with flavopiridol and assessed using a standard MTT assay. In these experiments, Bcl-2 overexpression reduced but did not eliminate flavopiridol-mediated cell death (Figure 3B). These results are in contrast to several reports indicating the Bcl-2 independence of flavopiridol,3,29 and support a role for the intrinsic pathway in flavopiridol activity.
Effects of Bcl-2 overexpression on flavopiridol-induced mitochondrial depolarization and viability. (A) 697 cell lines with normal (697-Neo) or 10-fold Bcl-2 overexpression (697-Bcl2) were treated with 1.0 μM FP in media with 10% human serum for 6 hours (N = 3). Annexin binding and mitochondrial depolarization were assessed as for CLL patient cells. Results are shown relative to untreated cells at the same time point. Error bars represent plus or minus standard deviation. The difference in FP-mediated annexin binding between 697-Neo and 697-Bcl2 at this time point was significant (*P = .03) as was the difference in mitochondrial depolarization (**P = .01). (B) 697-Neo and 697-Bcl2 cells were treated with FP in media with 10% human serum, or F-ara A in media with 10% fetal bovine serum as a positive control for cytotoxicity prevented by Bcl-2. Viability was assessed at 24 hours by MTT assay. The averages of 4 individual experiments, each run in quadruplicate, are shown relative to the untreated control for each, set at 100%. Error bars represent plus of minus standard deviation.
Effects of Bcl-2 overexpression on flavopiridol-induced mitochondrial depolarization and viability. (A) 697 cell lines with normal (697-Neo) or 10-fold Bcl-2 overexpression (697-Bcl2) were treated with 1.0 μM FP in media with 10% human serum for 6 hours (N = 3). Annexin binding and mitochondrial depolarization were assessed as for CLL patient cells. Results are shown relative to untreated cells at the same time point. Error bars represent plus or minus standard deviation. The difference in FP-mediated annexin binding between 697-Neo and 697-Bcl2 at this time point was significant (*P = .03) as was the difference in mitochondrial depolarization (**P = .01). (B) 697-Neo and 697-Bcl2 cells were treated with FP in media with 10% human serum, or F-ara A in media with 10% fetal bovine serum as a positive control for cytotoxicity prevented by Bcl-2. Viability was assessed at 24 hours by MTT assay. The averages of 4 individual experiments, each run in quadruplicate, are shown relative to the untreated control for each, set at 100%. Error bars represent plus of minus standard deviation.
Flavopiridol induces mobilization of intracellular calcium
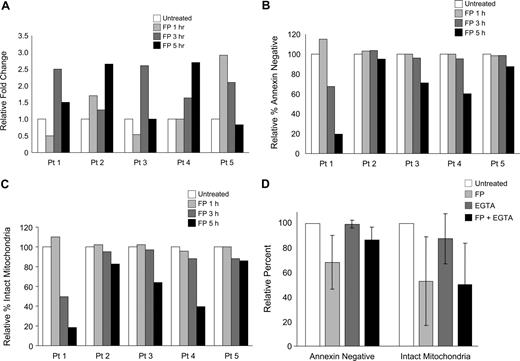
To determine whether calcium flux is involved in flavopiridol-mediated mitochondrial depolarization, we studied changes in intracellular calcium levels in flavopiridol-treated CLL cells and in 697 cells using confocal laser-scanning microscopy. Cells were loaded with Fluo-4/AM, which increases fluorescence upon binding free calcium.30 Cells were incubated in calcium-free media with EGTA to reduce potentially confounding effects of extracellular calcium, and incubated with or without 1.5 μM flavopiridol for 1, 3, and 5 hours. Increases in intracellular free calcium were noted in each sample following treatment (Figure 4A), although the time at which maximal fluorescence occurred varied and data are therefore shown by individual sample. Changes in ΔΨm and annexin positivity were also determined at the same time points for each sample (Figure 4B,C). As seen previously, annexin positivity and the loss of ΔΨm occurred nearly concurrently, and to similar extents. Using this same technique, we also noted increased free calcium in 697 cells 3 to 5 hours after addition of flavopiridol (data not shown). Successful staining and calcium release was confirmed in each sample by spiking the cells with the calcium ionophore ionomycin, which in each case caused a 5- to 20-fold increase in fluorescence within 30 seconds.
Modulation of intracellular calcium with flavopiridol treatment. (A) Calcium flux in isolated CD19+ cells from 5 individual patients was measured using fluorescent confocal microscopy. Cells were incubated with or without 1.5 μM FP for 1, 3, or 5 hours, then transferred to calcium-free buffer and plated on polylysine-coated coverslips for examination. Data represent a mean fluorescent intensity of all cells in 2 randomly selected fields. Ionomycin was used as a positive control for calcium release from intracellular stores (not shown). Data are shown as fold increases in fluorescence intensity of treated cells relative to the untreated controls. These same samples were concurrently analyzed for (B) annexin binding and (C) mitochondrial depolarization. (D) Effect of EGTA on apoptosis and mitochondrial membrane potential of FP-treated CLL patient cells. CLL cells (N = 6) were preincubated with 2 mM EGTA for 1 hour prior to adding 1.5 μM FP for an additional 6 hours. Annexin binding, PI uptake, and JC-1 aggregation were assessed by flow cytometry. Data are shown relative to the untreated sample at the same time point. By itself, EGTA did not impact annexin binding or mitochondrial depolarization (P = .85 and P = .17, respectively). Differences in FP-induced annexin binding with and without EGTA did not reach significance (P = .07). FP-mediated mitochondrial depolarization was unaffected by the addition of EGTA (P = .59). EGTA at 4 mM produced similar results (data not shown).
Modulation of intracellular calcium with flavopiridol treatment. (A) Calcium flux in isolated CD19+ cells from 5 individual patients was measured using fluorescent confocal microscopy. Cells were incubated with or without 1.5 μM FP for 1, 3, or 5 hours, then transferred to calcium-free buffer and plated on polylysine-coated coverslips for examination. Data represent a mean fluorescent intensity of all cells in 2 randomly selected fields. Ionomycin was used as a positive control for calcium release from intracellular stores (not shown). Data are shown as fold increases in fluorescence intensity of treated cells relative to the untreated controls. These same samples were concurrently analyzed for (B) annexin binding and (C) mitochondrial depolarization. (D) Effect of EGTA on apoptosis and mitochondrial membrane potential of FP-treated CLL patient cells. CLL cells (N = 6) were preincubated with 2 mM EGTA for 1 hour prior to adding 1.5 μM FP for an additional 6 hours. Annexin binding, PI uptake, and JC-1 aggregation were assessed by flow cytometry. Data are shown relative to the untreated sample at the same time point. By itself, EGTA did not impact annexin binding or mitochondrial depolarization (P = .85 and P = .17, respectively). Differences in FP-induced annexin binding with and without EGTA did not reach significance (P = .07). FP-mediated mitochondrial depolarization was unaffected by the addition of EGTA (P = .59). EGTA at 4 mM produced similar results (data not shown).
To understand the mitochondrial effect of calcium mobilized by flavopiridol treatment, we then tested calcium chelators EGTA and BAPTA-AM and the ER ATPase inhibitor thapsigargin, which raises cytosolic calcium levels, under conditions that show flavopiridol-induced calcium flux. Concentrations of these reagents were selected based both on published reports as well as our own preliminary experiments. EGTA alone at 2 mM had no significant effect on annexin binding or mitochondrial depolarization of CLL patient cells at 6 hours (Figure 4D). In flavopiridol-treated cells, 2 mM EGTA showed a minor but consistent protective effect against flavopiridol-mediated apoptosis as measured by annexin binding, but had no effect on flavopiridol-mediated mitochondrial depolarization as assessed by JC-1 flow cytometry. This suggests that although extracellular calcium may have a role in apoptosis induction following flavopiridol treatment, it does not play a role in early flavopiridol-mediated mitochondrial destabilization. We also tested BAPTA-AM (20 μM) and determined that it too was unable to prevent flavopiridol-mediated loss of mitochondrial membrane potential at 6 hours (data not shown). However, BAPTA-AM by itself notably induced both apoptosis and mitochondrial depolarization within 6 hours in CLL patient cells, preventing firm conclusions from being drawn. In addition, Hyrc et al31 demonstrated that the calcium-binding moiety of BAPTA also has substantial affinity for Zn+2 ions, and this dual specificity therefore further complicates experimental interpretation. Similar to BAPTA-AM, thapsigargin at 0.5 or 1.0 μM potently induced mitochondrial depolarization by 6 hours, and there was no difference in this effect when this reagent was used in combination with flavopiridol.
Mechanism of mitochondrial membrane permeabilization
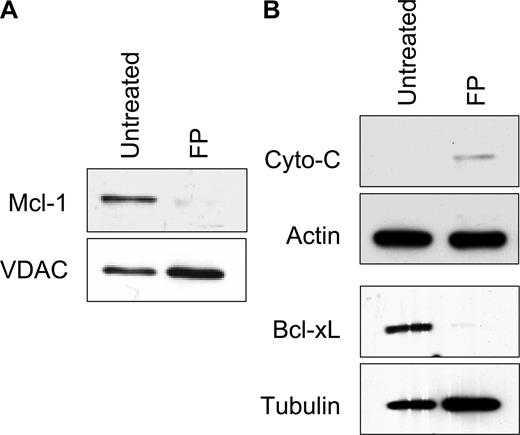
While many reports support the importance of Bcl-2 family members in modulating mitochondrial stability, work from several groups also indicates there are important functional interactions of PTP components and Bcl-2 family proteins including Bax, Bcl-2, and Bcl-XL.32,33 In addition, Bcl-XL inhibits translocation of Bax and Bak to the mitochondrial membrane,34 and its expression level has been associated with changes in cytosolic calcium.35 Bcl-XL regulates the release of calcium from the ER, modulating mitochondrial membrane permeability by altering the expression of inositol 1,4,5 triphosphate receptor (IP3R).36,37 Thus, calcium flux in flavopiridol-treated cells may both affect, or be affected by, Mcl-1 or Bcl-XL levels. We therefore examined the effects of flavopiridol on these key Bcl-2 family members in CLL patient cells by immunoblot analysis. Mcl-1 is markedly depleted from mitochondria within 5 hours of flavopiridol treatment, relative to the mitochondrial control protein VDAC (Figure 5A). Likewise, cytosolic fractions of flavopiridol-treated CLL cells showed down-regulation of Bcl-XL expression (Figure 5B). The timing of reduced expression of these antiapoptotic proteins corresponded with the loss of ΔΨm, similar to that reported by Pei et al.38 In 2 separate experiments, we did not observe translocation of the cytosolic protein Bax into the mitochondrial fraction of CLL patient cells, as reported previously using epithelial and fibroblast cell lines.39 In addition, we noted only minor increases in cytochrome c in cytoplasm following flavopiridol treatment (Figure 5B), without a significant decrease in the mitochondria fraction (not shown).
Mechanistic analysis of mitochondrial damage in CLL cells. (A) CLL cells were treated with or without 1.0 μM FP (6 hours), and mitochondrial and cytosolic fractions were isolated. (B) Mcl-1 levels in mitochondria relative to the control protein VDAC, and (C) Bcl-XL and cytochrome c in cytosolic fractions were analyzed by immunoblot. Data represent 3 independent experiments.
Mechanistic analysis of mitochondrial damage in CLL cells. (A) CLL cells were treated with or without 1.0 μM FP (6 hours), and mitochondrial and cytosolic fractions were isolated. (B) Mcl-1 levels in mitochondria relative to the control protein VDAC, and (C) Bcl-XL and cytochrome c in cytosolic fractions were analyzed by immunoblot. Data represent 3 independent experiments.
Flavopiridol effects on oxygen consumption in CLL cells
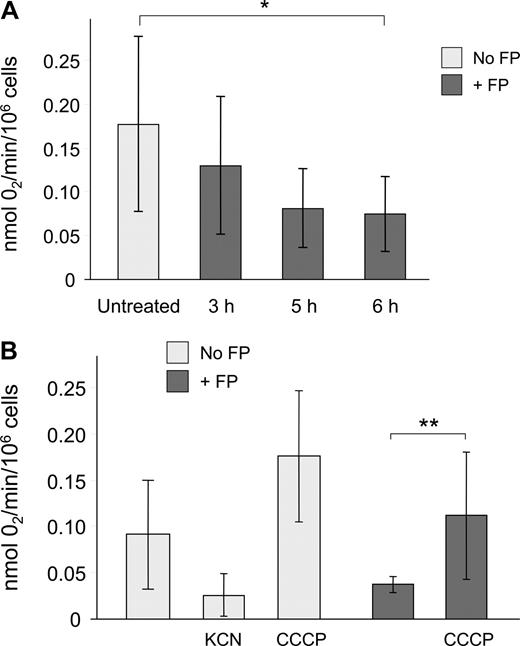
Mitochondrial respiration is crucial in energy production and cell survival, and mitochondria account for the majority of oxygen consumption in most cells. Thus, ADP-dependent oxygen consumption serves as a direct indicator of mitochondrial function. We therefore sought to determine whether flavopiridol causes a time-dependent effect on oxygen consumption rate (OCR). We used a highly oxygen-sensitive microparticulate spin probe (LiNc-BuO) for measuring oxygen concentration in cellular suspensions.24,25 CLL cells incubated in 10% human serum with or without 1.5 μM flavopiridol were analyzed as described in “Methods.” Under these conditions, we observed that CLL patient samples exhibited lower baseline OCR in comparison with human solid tumor cell lines. For example, the radiation-induced fibrosarcoma cell line RIF-1, although hypoxic in nature, shows an approximately 5-fold greater OCR (P.K., unpublished observation, March 2006). However, oxygen consumption was still substantially reduced in flavopiridol-treated CLL cells by 5 to 6 hours relative to untreated controls (Figure 6). To determine the contribution of mitochondrial metabolic processes on OCR, CLL cells were pretreated with either KCN, an inhibitor of cytochrome c oxidase that effectively blocks ADP-dependent mitochondrial respiration, or CCCP, a molecule that uncouples electron transport and oxygen consumption from ATP formation, thereby maximizing mitochondrial oxygen consumption. As expected, KCN caused an inhibition of respiration, whereas CCCP strongly increased OCR relative to untreated cells. When CLL cells incubated with flavopiridol for 6 hours were treated with CCCP, oxygen consumption was preserved as evidenced by a significant increase in OCR, indicating that flavopiridol does not permanently disrupt the mitochondrial electron transport mechanism.
Flavopiridol effects on respiration in CLL patient cells. Oxygen consumption rate (OCR) of CLL cells was determined using electron paramagnetic resonance (EPR) as described in “Measurement of cellular respiration.” (A) CLL samples (N = 5) were incubated with or without 1.5 μM FP for 3, 5, or 6 hours. Cells (250 000) plus probe were transferred to a sealed microcapillary and analyzed for 20 minutes. Untreated cells (N = 8) were analyzed at several intervals to ensure that incubation alone up to 6 hours had no effect on OCR. Data are presented as mol O2 consumed per minute per million cells. The average oxygen consumption of CLL cells treated with FP for 6 hours was significantly less than in untreated cells (*P < .001). (B) In an independent experiment, KCN (100 μM, N = 3) and CCCP (50 μM, N = 5) were used as negative and positive controls for oxygen utilization, respectively. The OCR of CLL cells treated with CCCP following 6 hours of FP treatment (N = 5) was significantly increased relative to cells treated with FP alone (**P = .03).
Flavopiridol effects on respiration in CLL patient cells. Oxygen consumption rate (OCR) of CLL cells was determined using electron paramagnetic resonance (EPR) as described in “Measurement of cellular respiration.” (A) CLL samples (N = 5) were incubated with or without 1.5 μM FP for 3, 5, or 6 hours. Cells (250 000) plus probe were transferred to a sealed microcapillary and analyzed for 20 minutes. Untreated cells (N = 8) were analyzed at several intervals to ensure that incubation alone up to 6 hours had no effect on OCR. Data are presented as mol O2 consumed per minute per million cells. The average oxygen consumption of CLL cells treated with FP for 6 hours was significantly less than in untreated cells (*P < .001). (B) In an independent experiment, KCN (100 μM, N = 3) and CCCP (50 μM, N = 5) were used as negative and positive controls for oxygen utilization, respectively. The OCR of CLL cells treated with CCCP following 6 hours of FP treatment (N = 5) was significantly increased relative to cells treated with FP alone (**P = .03).
Flavopiridol induces early morphologic changes in CLL patient cells
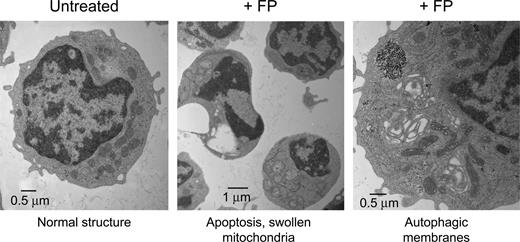
Transmission electron microscopy (TEM) is a powerful technique to document subcellular structural changes that may be the result of processes such as apoptosis or autophagy. To determine whether changes in specific organelles could be associated with flavopiridol, we collected images of CLL cells treated with flavopiridol for 6 hours. Cells were treated in whole blood to minimize effects on mitochondria due to extraneous or unknown factors in standard culture conditions, then quickly isolated and fixed before being processed for microscopy. The TEM images reveal multiple changes in flavopiridol-treated cells (Figure 7). While some cells in the flavopiridol-treated samples showed nuclei similar to the untreated control, others exhibited a lobed or segmented appearance typical of apoptotic nuclei. Also visible in some but not all cells was substantial swelling of mitochondria and loss of cristae definition, although in other cells mitochondria appeared ultrastructurally similar to the control. We additionally observed an increased frequency of mitochondria surrounded by double membrane structures, which is consistent with the lysosomal clearance of mitochondria (autophagy).40 It is important to note that although these differences in mitochondrial structure were increased in flavopiridol-treated samples, they were variable among cells within the sample. This may be simply due to variations in the rate of apoptosis among the population of treated cells, although mitochondria are also known to be heterogeneous within cells in both morphology and function.41 Evidence of mitochondrial swelling and loss of cristae definition were also noted in untreated specimens, albeit at a lower frequency, suggesting that apoptosis was under way in these samples as well. Also detectable in some flavopiridol-treated cases was “cristae remodeling,” as described by Kroemer et al.36 This appears under TEM as electron-dense regions within otherwise intact mitochondria (data not shown). This reorganization of cristae is thought to promote mobilization of proteins such as cytochrome c in the mitochondrial intramembrane space. Permeabilization of the mitochondrial membrane may then be the final event that would allow the release of these proteins into cytoplasm.36,42
Transmission electron microscopy (TEM) of flavopiridol-treated CLL cells. Peripheral blood from CLL patients was incubated with or without 3.0 μM FP for 6 hours. CD19+ cells were then isolated, fixed, and processed for TEM. Data shown are from 1 representative patient sample of 4 tested.
Transmission electron microscopy (TEM) of flavopiridol-treated CLL cells. Peripheral blood from CLL patients was incubated with or without 3.0 μM FP for 6 hours. CD19+ cells were then isolated, fixed, and processed for TEM. Data shown are from 1 representative patient sample of 4 tested.
Discussion
Flavopiridol has achieved recent success in the treatment of refractory, advanced-stage CLL using a new schedule of administration. However, challenges remain to understanding not only its mechanism of action but the reason for the acute tumor lysis syndrome reported in some patients. Our group has observed substantial and sometimes dramatic biochemical evidence of tumor cell lysis as early as 4 to 6 hours into treatment2 as indicated by decreased calcium levels as well as elevations in potassium, phosphate, urea, and lactate dehydrogenase, consistent with tumor lysis syndrome. Furthermore, a patient who succumbed to tumor lysis syndrome was found to have necrosis in intra-abdominal lymph nodal tissue, but not in other organ sites. Based on these clinical observations as well as published reports using tumor cell lines, we postulated that the mode of action of flavopiridol in CLL patient cells involves early severe perturbation of mitochondria, leading to rapid cell death atypical of classical apoptosis.
Collapse of ΔΨm, required for mitochondrial functions including ATP generation, is a standard indicator of mitochondrial damage. We detected loss of ΔΨm in flavopiridol-treated cells as early as 5 hours, prior to the onset of PI positivity. Earlier studies showed that the mitochondrial depolarization induced by flavopiridol in the U937 cell line is blocked by caspase inhibitors, suggesting it may be a downstream event to caspase activation.4 On the contrary, our experiments using primary CLL tumor cells suggest that since flavopiridol-induced loss of ΔΨm is not rescued by either a pan-caspase inhibitor or specific inhibitors of caspase-8 and caspase-9, this process is either caspase-independent or lies upstream of caspase activation. Furthermore, Puppo et al43 showed that treatment of human neuroblastoma cells with flavopiridol causes an early activation of caspase-3 prior to extensive cell death, similar to what we reported previously in CLL patient cells.44 However, in this report, caspase-8 and caspase-9 were not activated, suggesting that in flavopiridol-mediated cytotoxicity caspase-3 may be acting upstream of these activator caspases. While we have not addressed this directly, our data do not support a role for calpains in flavopiridol-mediated mitochondrial membrane depolarization, although calpains may be involved in cell death subsequent to flavopiridol treatment.45,46 We were unable to prevent flavopiridol-induced loss of mitochondrial membrane potential using the caspase inhibitor Z-VAD-fmk, which also inhibits calpains by preventing caspase-mediated activation.47,48 In addition, Kitada et al14 detected no loss of expression of the calpain substrate Bax49 in flavopiridol-treated CLL patient cells, even at extended incubations.
Multiple lines of evidence suggest that flavopiridol causes increased permeability of the outer mitochondrial membrane by depleting labile Bcl-2 family members such as Mcl-1 and liberating proapoptotic factors such as Bax that are then available to associate with mitochondrial membranes to promote pore formation.36 Our data partially support this hypothesis in that Mcl-1 and Bcl-XL are rapidly depleted from CLL cells following flavopiridol treatment; however, Bax levels were not substantially different in mitochondrial fractions from treated and untreated cells. This suggests that Bax is not a key mediator of mitochondrial swelling in flavopiridol-treated CLL cells. However, it is possible that another proapoptotic Bcl-2 family member is separately involved (eg, Bid, Bak), as in our experiments the overexpression of Bcl-2 blocked flavopiridol-induced mitochondrial depolarization and cell death.
Intracellular calcium flux was reproducibly induced by flavopiridol in all CLL patient samples tested, but the extent and time of these changes were variable. Although the reason for this variability is unknown, it may in part explain the different flavopiridol sensitivity seen between samples. Although we were able to determine that the calcium source was intracellular using EGTA, further attempts to precisely identify this source were unsuccessful. In our experiments, both thapsigargin and BAPTA-AM, which alter intracellular calcium levels via different mechanisms, potently induced early mitochondrial depolarization. This depolarization was followed by substantial apoptosis as measured by annexin binding. As intracellular calcium is known to affect a large array of factors including mitochondrial stability and cell survival,50 this is perhaps not unexpected, but the rate and extent of the effects of thapsigargin and BAPTA-AM on CLL patient cells was striking. These results may indicate that CLL cells are particularly sensitive to disruptions of intracellular calcium homeostasis. Although this hypothesis requires further study, it may present an additional strategy to target these tumor cells in patients, as suggested in part by Vilpo et al.51
Modulation of calcium flux also may provide an explanation for the mitochondrial stabilizing effects of Bcl-2. Previously it has been shown that Bcl-2 is expressed in both mitochondria and ER, and its overexpression can lower the amount of calcium that is released from ER. This leads to a reduction in mitochondrial calcium uptake, promoting preservation of ΔΨm and thus cell survival.52 Our findings are in contrast to several reports indicating the Bcl-2 and/or Bcl-XL independence of flavopiridol,3,53 and also suggest there is not a direct effect of flavopiridol on APAF-1/apoptosome assembly, as Bcl-2 family proteins likely do not affect this process.28 It is possible that the discrepancy in the involvement of Bcl-2 in CLL versus lung carcinoma cells3 may be because in CLL cells caspase-8 appears to play a minor role, with release of cytochrome c in the cytosolic fraction. In contrast, flavopiridol activity in lung carcinoma cells was found to be caspase-8 dependent without cytochrome c release. Therefore, it is possible that in carcinoma cell lines the effect of Bcl-2 overexpression may be compensated by cross-talk between the mitochondrial and extrinsic apoptotic pathways. Although in our studies Bcl-2 family proteins appear to be involved in flavopiridol-mediated cytotoxicity in CLL cells, we have not yet examined the relationship between pretreatment expression levels of Bcl-2 family members and the response of CLL patients to flavopiridol treatment. However, in contrast to our in vitro findings, Pepper et al29 noted that changes in Bcl-2/Bax expression ratios, which influence the sensitivity of the PTP to opening, were not associated with CLL cell sensitivity to flavopiridol in vitro. Rather than protecting against the PTP, it is interesting to speculate that overexpression of Bcl-2 compensates for Mcl-1 suppression, thereby normalizing mitochondrial calcium homeostasis.54
The interrelations between calcium flux, ΔΨm, and OCR are varied and complex. Billard et al55 showed that in CLL cells, inducible nitric oxide synthase is constitutively expressed and can be inhibited with 3 to 9 hours of flavopiridol treatment. As nitric oxide is a potent regulator of mitochondrial function,56 we sought to determine whether flavopiridol could affect OCR in CLL cells at times when induction of mitochondrial depolarization and calcium flux are seen. We observed that untreated CLL cells had moderately low OCR relative to solid tumor cell lines. OCR was significantly reduced by flavopiridol treatment over time, concurrent with calcium release and mitochondrial depolarization. The low baseline OCR of CLL patient cells may explain why these cells have an increased sensitivity to flavopiridol, in that further reduction of mitochondrial function in cells with weak respiration is less tolerable than in cells with highly active mitochondria. Classical apoptosis is generally believed to be an energy-dependent process, and to allow the execution of apoptosis mitochondria must maintain ATP synthesis.56 Partial restoration of respiration when CCCP was added to flavopiridol-treated CLL cells ex vivo suggests that the electron transport system is fundamentally intact at the early stages of flavopiridol-induced cell death. Nonetheless, the observed collapse of ΔΨm within 6 hours of treatment and inhibition of mitochondrial respiration indicates that flavopiridol, either directly or indirectly, causes impaired mitochondrial function prior to the onset of CLL cell death.
Ultrastructural analyses of CLL cells revealed mitochondrial changes in the flavopiridol treatment group, which have interesting mechanistic implications. Untreated cells are irregularly round with large nuclei; the nuclei are often convoluted or even segmented. The cytoplasmic volume is low and CLL cells typically exhibit numerous mitochondria. Although many treated cells were similar to untreated controls, a substantial subgroup of flavopiridol-treated CLL cells assumed a phenotype indicative of apoptotic cell death within 6 hours. However, unlike apoptosis induced by chlorambucil, which is typically associated with mitochondrial shrinkage,57 the mitochondria of flavopiridol-treated CLL cells tended to be swollen with signs of autolysis, similar to that recently shown by Sun et al.58 Furthermore, we observe increased prevalence of mitochondrial clusters surrounded by double membranes, consistent with an autophagic phenotype. In this regard, recent studies indicate that collapse of ΔΨm triggers activation of intrinsic mitochondrial calcium-independent lipase (iPLA2), which promotes self-degradation (autolysis)59 and influences cytochrome c release.60 Autolysis ultimately leads to mitochondrial clearance by lysosomes (autophagy), and when many mitochondria are involved, this process may trigger apoptotic cell death.61 As noted in many of our experiments, at this early time point (6 hours), annexin binding was minimal and PI uptake is the same as untreated controls. This indicates that flavopiridol has a negligible effect on plasma membrane integrity at this early stage. Instead, it is possible that activation of mitochondrial calcium efflux pathways (Na+/Ca2+ and Na+/H+ exchangers) consequent to suppression of Mcl-1 and attendant calcium overload could dissipate ΔΨm, which would suppress the activity of K+/H+ exchange, leading finally to mitochondrial swelling. Thus, mitochondrial swelling and de-energization may occur independent of the PTP.62
In conclusion, our data indicate that mitochondrial de-energization triggered by Bcl-2–sensitive changes in calcium flux promote flavopiridol-induced CLL cell death. These mitochondrial events were contemporaneous with the onset of tumor cell lysis in CLL patients treated with flavopiridol using an effective new schedule. The mechanism of mitochondrial de-energization is unclear, but our data indicate that suppression of Mcl-1, inhibition of mitochondrial electron transport, and changes in calcium flux precede collapse of mitochondrial ΔΨm and may significantly contribute to this phenomenon. Interestingly, Bcl-2 family proteins including Mcl-1 and Bcl-XL appear to suppress these mitochondrial events, apparently by maintaining calcium homeostasis and mitochondrial respiration. Additional studies will be needed to establish how flavopiridol causes the inhibition of mitochondrial respiration in CLL cells and to better define the role of Bcl-2 family proteins in this process. Finally, the ex vivo techniques for assessing sensitivity of CLL cells to flavopiridol described here may prove useful for identifying patients who will have a response to flavopiridol, as well as those who are at increased risk of acute tumor lysis syndrome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors especially thank Kathy Wolken of the OSU Campus Microscopy and Imaging Facility for her TEM expertise. We also are grateful to our laboratory members for experimental assistance and helpful comments throughout this work, and to the many CLL patients who donated blood.
This work was supported by the National Cancer Institute (CA PO1 CA81534), The Leukemia and Lymphoma Society of America, the Samuel Waxman Cancer Research Foundation, and the D. Warren Brown Foundation.
National Institutes of Health
Authorship
Contribution: S.-R.A.H. designed and conducted experiments and authored the paper; D.M.L. designed experiments and authored the paper; A.J.J. contributed to experimental design and interpretation and provided data regarding CLL patients; T.S.L. is an investigator on the flavopiridol clinical trial and provided CLL patient samples; A.P.B. performed calcium mobilization experiments together with S.-R.A.H.; V.X.D. conducted OCR experiments under direction of P.K.; S.V.-K. oversaw design and interpretation of calcium experiments; A.S.R. performed the statistical analyses and edited the paper; J.C.B. contributed to experimental design and interpretation, provided CLL patient samples, oversaw clinical trial that led to observations described here, and is coprincipal investigator of the laboratory in which this work was conducted; P.K. oversaw design and interpretation of OCR experiments and assisted in paper writing; E.D.C. provided expertise on experimental design and interpretation, interpreted TEM images, and authored the paper; M.R.G. provided research concept, oversaw project design and experimental interpretation, authored the paper, and is principal investigator of the laboratory in which this work was conducted; all authors approved the final paper.
Conflict-of-interest disclosure: Two of the authors (J.C.B. and M.R.G.) are included in an application for a patent related to the clinical use of flavopiridol. All other authors declare no competing financial interests.
Correspondence: David M. Lucas, OSU CCC Bldg, Rm 455, 410 W 12th Ave, Columbus, OH 43210; e-mail: david.lucas@osumc.edu.
References
Author notes
S.-R.A.H. and D.M.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal