Abstract
Graft-versus-host disease (GVHD) is initiated after activation of donor T cells by host antigen-presenting cells (APCs). The immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by APCs and parenchymal cells and is further inducible by inflammation. We investigated whether lethal conditioning and GVHD induce IDO and if IDO prevents tissue injury by suppressing immune responses at the induction site. We determined that IDO is a critical regulator of GVHD, most strikingly in the colon, where epithelial cells dramatically up-regulated IDO expression during GVHD. IDO−/− mice died more quickly from GVHD, displaying increased colonic inflammation and T-cell infiltration. GVHD protection was not mediated by control of T-cell proliferation, apoptosis, or effector mechanisms in lymphoid organs, nor did it require donor T regulatory cells. Instead, T cells in IDO−/− colons underwent increased proliferation and decreased apoptosis compared with their wild-type counterparts. This evidence suggests that IDO can act at the site of expression to decrease T-cell proliferation and survival, diminishing colonic inflammation and reducing disease severity. These studies are the first to identify a function for IDO in GVHD lethality and indicate that modulation of the IDO pathway may be an effective strategy for treatment of this disease.
Introduction
Wider application of allogeneic bone marrow transplantation (BMT) is restricted due to graft-versus-host disease (GVHD), the most significant cause of morbidity and mortality after transplantation. Despite extensive investigations of GVHD pathophysiology, the environmental effects that influence GVHD injury and the natural regulatory mechanisms by which GVHD is controlled at the tissue level are incompletely understood. Indoleamine 2,3-dioxygenase (IDO) catalyzes the first and rate-limiting step of tryptophan catabolism. Decreased tryptophan and/or increased metabolite concentrations elicit a stress response in nearby responding T cells, leading to anergy or apoptosis.1,2 Several aspects of IDO biology make it an intriguing target for transplantation. IDO expression has been shown to be critical for allogeneic fetal tolerance,3 tumor tolerance,4 and down-regulation of autoimmunity.5 The lung and intestine, which are GVHD target organs, constitutively express IDO and can up-regulate expression during inflammation. Ectopic IDO expression in lung allografts prolongs acceptance.6,7 In the gut, inhibition of functional IDO activity during immune-mediated colitis markedly worsens disease.8 Antigen-presenting cells (APCs) can be induced to express IDO by interferons,9,10 lipopolysaccharide,11 and ligation of costimulatory molecules,12,13 all of which are prevalent during the intense inflammation of GVHD. High IDO expression in APCs in tumor draining lymph nodes is associated with poor tumor immune responses. In addition, IDO activity has been linked to the generation of T regulatory cells.14 Given the expression pattern of IDO and the function of IDO in the local environment to control the immune response and reduce tissue injury in the colon, we sought to determine whether IDO expression in the host would regulate GVHD-induced tissue injury and lethality.
To date, IDO often has been studied in vivo by transfer of IDO-expressing cells. Whereas these studies have highlighted the immunosuppressive and tolerance-promoting effects of IDO, the physiologic role of IDO in disease processes has been less well explored. The small molecule 1-methyltryptophan (1-MT) competitively inhibits IDO. This compound has been administered through slow-release capsules implanted subcutaneously or by supplementation of drinking water. Both of these delivery approaches pose particular problems for models of GVHD because GVHD colitis prevents reliable absorption of orally administered agents, whereas implantation of pumps or pellets causes significant inflammation, which can accelerate the lethality process. To circumvent these issues, we have investigated the role of IDO in GVHD using genetically IDO-deficient mice as BMT recipients.
We show here that IDO deficiency accelerates GVHD lethality. Allogeneic bone marrow (BM) plus supplemental donor T-cell infusion, but not BM alone, transferred into lethally irradiated recipients up-regulates IDO in the colon. IDO−/− recipients suffered significantly worse inflammation and T-cell infiltration in the colon. GVHD suppression was not dependent on donor T-regulatory cells (Tregs) and did not affect donor T-cell proliferation or apoptosis in secondary lymphoid organs or the acquisition of effector molecules. Instead, T cells in colons of IDO−/− recipients underwent increased rates of proliferation and decreased rates of apoptosis. These results suggest IDO induced by GVHD is capable of reducing ongoing donor T-cell proliferation and survival at the site of IDO expression and suggest that IDO up-regulation may be useful for preventing or treating GVHD.
Methods
Mice
Recipient C57BL/6 (H2b; termed B6) and Balb/c (H2d) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Donor Balb/c and B6 mice were purchased from the National Institutes of Health (Bethesda, MD). B6 and Balb/c IDO−/− mice were generated as previously described15 and bred at the University of Minnesota. Mice were housed in a specific pathogen-free facility in microisolator cages and used at 6 to 16 weeks of age.
GVHD induction
Transplantations were performed according to protocols approved by the University of Minnesota Institutional Animal Care and Use Committee. B6 or B6.IDO−/− mice were irradiated with 8.0 to 9.0 Gy total body irradiation by x-ray on day −1. A total of 10 million Balb/c BM cells with or without purified T cells were infused on day 0. T cells were isolated from lymph nodes (LNs) and purified by incubation with phycoerythrin-labeled antibodies to CD19, γδ TCR, and DX5 (BD Biosciences PharMingen, San Diego, CA) followed by incubation with anti-phycoerythrin beads and magnetic column depletion (Miltenyi Biotec, Auburn, CA). Experiments using CD4+ T cells included anti-CD8 antibody in the depletion, and Treg-depleted cells were obtained with the addition of anti-CD25 antibody. Cell preparations were verified by flow cytometric phenotyping as more than 99% of the desired phenotype. In experiments using T cell–depleted BM (TCD BM), BM was incubated with antibodies to CD4 and CD8 on ice for 20 minutes followed by addition of rabbit complement at 37°C for 30 minutes. Mice were monitored daily for survival and weighed twice weekly for the first month, then once weekly thereafter. In some experiments, recipients were electively killed, and hematoxylin and eosin-stained cryosections of liver, lung, colon, small intestine, thymus, skin, and spleen were histologically assessed using a semiquantitative GVHD scoring system (0-4.0 grades in 0.5 increments) as published.16 Coded sections were graded by one of us (A.P.-M.) without knowledge of the treatment.
Frozen tissue preparation
Tissues, including colon, small intestine, liver, lung, thymus, mesenteric LN, skin, and spleen, were taken at indicated days after transplantation, embedded in Optimal Cutting Temperature compound (Bayer, Emeryville, CA), snap-frozen in liquid nitrogen, and stored at −80°C.
Immunohistochemistry
After acetone fixation, cryosections (6 μm) were immunoperoxidase-stained using avidin-biotin blocking reagents, ABC-peroxidase conjugate, and DAB chromogenic substrate, all purchased from Vector Laboratories (Burlingame, CA). Antibodies used were biotinylated mAbs to CD4 (clone GK1.5) and CD8 (clone 2.43), both purchased from BD Biosciences PharMingen. Sections were counterstained with methyl green.
For IDO staining, tissues were fixed in formalin and paraffin embedded. Sections underwent high-temperature antigen retrieval using Target Retrieval Solution (Dako North America, Carpinteria, CA) and stained using rabbit anti-IDO polyclonal antibody (prepared by Southern Biotech, Birmingham, AL, based on the peptide sequence previously described17 ). Biotinylated antirabbit secondary antibody was incubated for 1 hour, followed by ABC-peroxidase conjugate and AEC chromogenic substrate, all from Biogenex (San Ramon, CA). Sections were counterstained with hematoxylin.
Images were obtained on an Olympus BX51 microscope (Center, Valley, PA) using 20×/0.70 or 10×/0.40 objective lens and Permant medium (Sigma-Aldrich, St Louis, MO) and acquired using a Spot CCD digital camera and Spot software (Diagnostic Instruments, Sterling Heights, MI) and then processed with Adobe Photoshop CS2, version 9.0.2 (Adobe Systems, San Jose, CA).
CFSE experiments
Splenocytes were labeled 10 minutes with 2.5μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) at room temperature followed by quenching with fetal calf serum; 60 × 106 labeled splenocytes were infused into lethally irradiated recipients. Spleens and LNs were harvested on indicated days, and single-cell suspensions were made. Cells were stained with fluorochrome-labeled antibodies to CD4, CD8, and H-2b (BD Biosciences PharMingen) and assessed for apoptosis using annexin V staining kit (BD Biosciences PharMingen) and acquired on a FACSCalibur flow cytometer (BD Biosciences). Analysis was performed using FlowJo software (TreeStar, Ashland, OR).
BrdU incorporation assays
GVHD was induced as described and drinking water was supplemented with 0.8 mg/mL bromodeoxyuridine (BrdU; Sigma-Aldrich) for indicated periods. BrdU was made fresh daily. Recipients were killed on indicated days. Spleens and mesenteric LN were harvested and single-cell suspensions were stained with fluorochrome-labeled antibodies to CD4, CD8, and H-2b (BD Biosciences PharMingen). BrdU incorporation was assessed using FITC BrdU Flow Kit (BD Biosciences PharMingen) and analyzed using FlowJo software (TreeStar).
T-cell isolation
Livers were harvested and mashed into a single-cell suspension. Colons from cecum to rectum were harvested, cut into 5-mm pieces, and incubated 3 times with 5 mM EDTA in RPMI with 5% serum for 15 minutes at 37°C. Supernatants containing intraepithelial lymphocytes were discarded. Colons were then incubated twice with 0.5 mg/mL and once with 1 mg/mL Collagenase D (Roche, Indianapolis, IN) for 1 hour at 37°C with shaking. Remaining colon pieces were mashed, and cells from livers and colons were isolated on a 40/80 Percoll (Sigma-Aldrich) gradient. Some cells were stained with antibodies for CD4 and CD8 (eBioscience, San Diego, CA) and annexin V (BD Biosciences PharMingen) according to manufacturer's instructions.
Intracellular cytokine staining
Isolated cells were incubated 4 hours on αCD3-coated plates at 37°C. Cells were stained for CD4 and CD8 (eBioscience) and stained intracellularly with antibodies to interleukin-10 (IL-10), IL-4, IFN-γ, and granzyme B using a fixation/permeabilization kit (BD PharMingen).
Immunofluorescence
After acetone fixation, cryosections (6 μm) were incubated with monoclonal rat anti-CD4 and polyclonal rabbit anti-Ki-67 (Abcam, Cambridge, MA) antibodies for 1 hour at room temperature. Fluorochrome-labeled secondary antibodies to rat and rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) were incubated for 30 minutes. Slides were mounted with DAPI slow fade gold (Invitrogen). Confocal images were acquired on an Olympus FluoView 500 Confocal Laser Scanning Microscope under 40×/0.9 oil-immersion objective lens using FluoView 3.2 software (Olympus) and processed with Adobe Photoshop CS2, version 9.0.2 (Adobe Systems).
Statistics
Survival data were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. Group comparisons of cell counts and flow cytometry data were analyzed by Student t test. P values less than .05 were considered significant in all tests.
Results
Host IDO expression is up-regulated during GVHD and is associated with decreased lethality
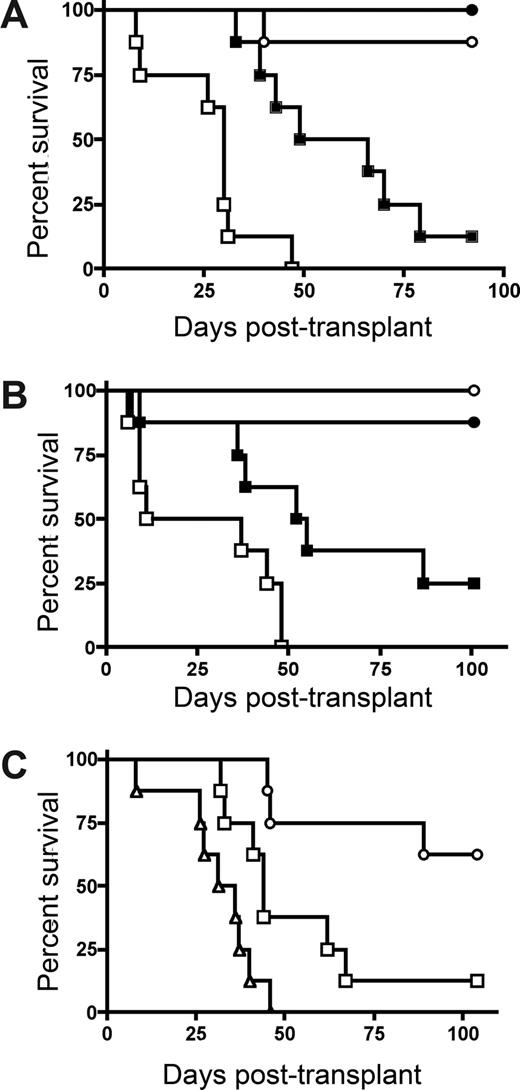
To determine whether host IDO expression would regulate GVHD lethality, we transplanted lethally irradiated wild-type (wt) and IDO−/− B6 mice with BM from allogeneic BALB/c donors with or without purified donor CD4+ T cells to induce GVHD. CD4+ T-cell–mediated GVHD lethality was markedly accelerated in IDO−/− versus wt recipients (Figure 1A). The absence of host IDO expression did not result in an increase in post-BMT lethality in recipients given BM without supplemental CD4+ T cells, although IDO−/− recipients of BM alone that were long-term survivors were not GVHD-free by clinical appearance, in contrast to wt recipients of BM alone. Nonetheless, together, these results indicated that host IDO expression was needed to slow the GVHD lethality process in this model system.
Donor T cells cause accelerated GVHD in IDO−/− versus wt recipients. (A) B6 or IDO−/− mice were lethally irradiated (9.0 Gy) and infused with 107 MHC-mismatched Balb/c non–T cell–depleted BM with or without 3 × 106 Balb/c CD4+ T cells. Survival plots of B6 versus IDO−/− recipients are shown. ● indicates BM→B6; ■, CD4+ T cells→B6; ○, BM→IDO−/−; □, CD4+ T cells→IDO−/− (P < .03). Data are from one experiment; n = 8 mice/group. (B) B6 or IDO−/− mice were lethally irradiated and given Balb/c TCD BM with or without 3 × 106 donor CD4+ and CD8+ T cells. Survival plots of B6 vs IDO−/− recipients are shown. ● indicates BM→B6; ■, CD4+ and CD8+ T cells→B6; ○, BM→IDO−/−; □, CD4+ and CD8+ T cells→IDO−/− (P < .04). Data are from one experiment; n = 8 mice/group. (C) Balb/c or IDO−/− mice were lethally irradiated (7.0 Gy) and infused with 107 B6 TCD BM with or without 2 × 106 CD4+ and CD8+ T cells. Survival plots of Balb/c versus IDO−/− recipients are shown. ● indicates BM→Balb/c; ■, CD4+ and CD8+ T cells→Balb/c; ○, BM→IDO−/−; □, CD4+ and CD8+ T cells→IDO−/−. Data are pooled from 2 experiments; n = 16 mice/group (P < .001).
Donor T cells cause accelerated GVHD in IDO−/− versus wt recipients. (A) B6 or IDO−/− mice were lethally irradiated (9.0 Gy) and infused with 107 MHC-mismatched Balb/c non–T cell–depleted BM with or without 3 × 106 Balb/c CD4+ T cells. Survival plots of B6 versus IDO−/− recipients are shown. ● indicates BM→B6; ■, CD4+ T cells→B6; ○, BM→IDO−/−; □, CD4+ T cells→IDO−/− (P < .03). Data are from one experiment; n = 8 mice/group. (B) B6 or IDO−/− mice were lethally irradiated and given Balb/c TCD BM with or without 3 × 106 donor CD4+ and CD8+ T cells. Survival plots of B6 vs IDO−/− recipients are shown. ● indicates BM→B6; ■, CD4+ and CD8+ T cells→B6; ○, BM→IDO−/−; □, CD4+ and CD8+ T cells→IDO−/− (P < .04). Data are from one experiment; n = 8 mice/group. (C) Balb/c or IDO−/− mice were lethally irradiated (7.0 Gy) and infused with 107 B6 TCD BM with or without 2 × 106 CD4+ and CD8+ T cells. Survival plots of Balb/c versus IDO−/− recipients are shown. ● indicates BM→Balb/c; ■, CD4+ and CD8+ T cells→Balb/c; ○, BM→IDO−/−; □, CD4+ and CD8+ T cells→IDO−/−. Data are pooled from 2 experiments; n = 16 mice/group (P < .001).
Subsequent studies were performed to determine whether GVHD lethality would be augmented in IDO−/− versus wt recipients of combined CD4+ and CD8+ T cells (Figure 1B). As seen with donor CD4+ T cell supplementation, IDO−/− recipients of CD4+ and CD8+ T cells had markedly accelerated GVHD lethality compared with wt recipients. In addition, lethality was not increased in IDO−/− versus wt recipients of BM alone, and long-term survivors in both groups had regained their pre-BMT body weight (not shown). To determine whether these findings were unique to the strain combination used, a second GVHD model was used. Again, GVHD lethality was accelerated in Balb/c IDO−/− versus wt recipients of B6 BM and combined CD4+ and CD8+ T cells (Figure 1C).
To determine whether IDO expression was up-regulated by GVHD, lymphoid and GVHD target tissues of mice were analyzed on day 7 after transplantation. Figure 2A shows expression of IDO in colonic and small intestinal cells with a morphology and location consistent with epithelial cells. IDO expression was dramatically up-regulated in colons of GVHD mice. Interestingly, mice given BM alone expressed far less IDO in the small intestine and colon, similar to nontransplanted controls, indicating that gut toxicity caused by irradiation was not sufficient for IDO induction and that donor T cells were required for IDO up-regulation. IDO was not significantly expressed or up-regulated by GVHD in liver or skin (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), nor was IDO up-regulation significant in lung, mesenteric LN, or thymus (not shown). IDO expression in the spleen was also up-regulated by GVHD (Figure S2), although the origin of these cells (host or donor) cannot be derived by this assessment. Histopathologic examination of tissues taken 27 days after transplantation showed increased inflammation and tissue destruction in colon and spleen of IDO−/− recipients (Figure 2B). Liver, lung, and skin inflammation and destruction were not significantly different (Figure S3). Quantitative grading of day 27 tissues confirmed significantly worse GVHD of the colon and spleen in IDO−/− recipients, with a mean colon score for IDO−/− versus wt of 3.88 plus or minus 0.25 versus 2.50 plus or minus 0.79 (P < .02) and a mean spleen score of 3.88 plus or minus 0.25 versus 3.00 plus or minus 0.41 (P < .02) on a 4-point scale (n = 3-5/group). No significant differences were found in GVHD scores of liver, lung, skin, or small intestine (not shown). Quantification of T-cell infiltration showed increased numbers of CD4+ (Figure 2Ci-iv) and CD8+ (Figure 2Di-iv) cells in IDO−/− colons, which were associated with increased GVHD scores in this organ. Numbers of infiltrating cells were not significantly different in small intestine or liver, and few infiltrating cells were seen in skin (not shown). IHC for CD4 and CD8 in spleens demonstrated the disorganization in IDO−/− spleens undergoing GVHD, with smaller and disrupted follicles and T-cell zones (Figure 2Cv-viii,Dv-viii). Total spleen cellularity was significantly lower in IDO−/− versus wt GVHD mice at day 20 (not shown).
Donor T cells up-regulate host IDO expression and worsen GVHD in IDO−/− vs wt recipients. (A) Wt or IDO−/− mice were lethally irradiated and infused with 3 × 106 CD4+ and CD8+ T cells and killed on day 7. Cryosections of colon (left) and small intestine (right) were stained by immunohistochemistry for IDO. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (B) Hematoxylin and eosin staining of wt and IDO−/− colons (i-iv) and spleens (v-viii) 27 days after transplantation with 3 × 106 CD4+ and CD8+ Balb/c T cells. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (C) IHC staining of tissues in B for CD4. Cell numbers in colon are quantitated below (*P < .002). (D) IHC staining of tissues in B for CD8. Cell numbers in colon are shown below (*P < .02). Original magnification ×200, except spleens, which were ×100. Images are representative of 3 to 5 mice per group.
Donor T cells up-regulate host IDO expression and worsen GVHD in IDO−/− vs wt recipients. (A) Wt or IDO−/− mice were lethally irradiated and infused with 3 × 106 CD4+ and CD8+ T cells and killed on day 7. Cryosections of colon (left) and small intestine (right) were stained by immunohistochemistry for IDO. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (B) Hematoxylin and eosin staining of wt and IDO−/− colons (i-iv) and spleens (v-viii) 27 days after transplantation with 3 × 106 CD4+ and CD8+ Balb/c T cells. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (C) IHC staining of tissues in B for CD4. Cell numbers in colon are quantitated below (*P < .002). (D) IHC staining of tissues in B for CD8. Cell numbers in colon are shown below (*P < .02). Original magnification ×200, except spleens, which were ×100. Images are representative of 3 to 5 mice per group.
Host IDO up-regulation and protection from GVHD lethality occur independently of donor Tregs
Evidence suggests that IDO may have a role in activating CD4+25+ Tregs.18 To determine whether IDO-mediated reduction of GVHD lethality required the presence of donor Tregs in our system, we compared survival of wt and IDO−/− mice receiving grafts containing CD25-depleted T cells to eliminate donor Tregs. Both donor CD4+ alone (Figure 3A) and CD4+ and CD8+ T cells (Figure 3B) depleted of CD25+ cells caused accelerated GVHD in IDO−/− hosts compared with wt. In both experiments, there were comparable (0-2 g difference) mean body weights between wt and IDO−/− recipients of BM alone (not shown). Therefore, the acceleration of GVHD lethality in IDO−/− mice is not dependent on donor Tregs given at the time of BMT. Conversely, in some experimental systems, Tregs have been shown to mediate suppression by up-regulating IDO through CTLA-4 ligation of B7 molecules.19 Although 3 of 8 lethally irradiated IDO−/− recipients of BM alone failed to survive long-term, supplemental donor CD4+ or CD4+25− T cells clearly accelerated GVHD lethality compared with BM alone. Moreover, in IDO−/− hosts, CD25-depleted CD4+ T cells accelerated GVHD over that caused by nondepleted (ie, Treg replete) CD4+ T cells (Figure 3C), indicating that Tregs in the donor graft were still capable of slowing the course of GVHD lethality even in the absence of IDO. In addition, CD4+CD25− T cells were able to induce IDO expression in colon (Figure 4A), suggesting a suppressive mechanism other than induction of IDO up-regulation by naturally occurring donor CD4+25+ Tregs in these GVHD target organs. Consistent with the pathology seen in recipients of CD4+ and CD8+ T cells, CD4+CD25− T cells caused worse colon and spleen pathology (Figure 4B) and showed greater colon T-cell infiltration (Figure 4Ci-iv) in IDO−/− hosts, and IDO−/− spleens again had sparser T cells and disrupted architecture (Figure 4Cv-viii). Increased colon inflammation and T-cell infiltration were also observed in a transplant using a 2-fold lower dose of donor CD4+CD25− T cells (not shown). Collectively, these results indicate the GVHD-sparing effects of IDO are not dependent on the presence of Tregs in the donor graft.
IDO-mediated Inhibition of GVHD lethality occurs independently of the presence of donor Tregs. (A) Balb/c TCD BM with or without 3 × 106 Balb/c CD25-depleted CD4+ and CD8+ T cells were infused into lethally irradiated B6 or IDO−/− recipients. Survival plots are shown. ● indicates BM→B6; ■, CD25-depleted T cells→B6; ○, BM→IDO−/−; □, CD25-depleted T cells→IDO−/−. T cell groups of B6 vs IDO−/− (P < .005). Data are from one experiment; n = 8 per group. (B) Balb/c TCD BM with or without 2 × 106 Balb/c CD4+CD25− T cells were infused into lethally irradiated B6 or IDO−/− recipients. Survival plots of B6 vs IDO−/− are shown. ● indicates BM→B6; ■, CD4+CD25− T cells→B6; ○, BM→IDO−/−; □, CD4+CD25− T cells→IDO−/−. T cell groups (P < .02). Data are from one experiment; n = 8 mice/group. (C) Balb/c TCD BM with or without 1 × 106 Balb/c CD4+ T cells or CD4+CD25− T cells were infused into IDO−/− recipients. Survival plots of CD25-depleted versus nondepleted are shown. ○ indicates BM→IDO−/−; □, CD4+ T cells→IDO−/−; △, CD4+CD25− T cells→IDO−/−. T cell groups (P < .02). Data are from one experiment; n = 8 mice/group.
IDO-mediated Inhibition of GVHD lethality occurs independently of the presence of donor Tregs. (A) Balb/c TCD BM with or without 3 × 106 Balb/c CD25-depleted CD4+ and CD8+ T cells were infused into lethally irradiated B6 or IDO−/− recipients. Survival plots are shown. ● indicates BM→B6; ■, CD25-depleted T cells→B6; ○, BM→IDO−/−; □, CD25-depleted T cells→IDO−/−. T cell groups of B6 vs IDO−/− (P < .005). Data are from one experiment; n = 8 per group. (B) Balb/c TCD BM with or without 2 × 106 Balb/c CD4+CD25− T cells were infused into lethally irradiated B6 or IDO−/− recipients. Survival plots of B6 vs IDO−/− are shown. ● indicates BM→B6; ■, CD4+CD25− T cells→B6; ○, BM→IDO−/−; □, CD4+CD25− T cells→IDO−/−. T cell groups (P < .02). Data are from one experiment; n = 8 mice/group. (C) Balb/c TCD BM with or without 1 × 106 Balb/c CD4+ T cells or CD4+CD25− T cells were infused into IDO−/− recipients. Survival plots of CD25-depleted versus nondepleted are shown. ○ indicates BM→IDO−/−; □, CD4+ T cells→IDO−/−; △, CD4+CD25− T cells→IDO−/−. T cell groups (P < .02). Data are from one experiment; n = 8 mice/group.
Host IDO induction and IDO-mediated spleen and colon protection do not require donor Tregs. (A) IHC for IDO in colon 7 days after transplantation with Balb/c TCD BM with or without 2 × 106 CD4+CD25− T cells. (B) Hematoxylin and eosin staining of colon (i-iv) and spleen (v-viii) 7 days after transplantation with Balb/c TCD BM with or without 2 × 106 CD4+CD25− T cells. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (C) Tissues from panel B stained for CD4 by IHC. Original magnification ×200 for colons, ×100 for spleens.
Host IDO induction and IDO-mediated spleen and colon protection do not require donor Tregs. (A) IHC for IDO in colon 7 days after transplantation with Balb/c TCD BM with or without 2 × 106 CD4+CD25− T cells. (B) Hematoxylin and eosin staining of colon (i-iv) and spleen (v-viii) 7 days after transplantation with Balb/c TCD BM with or without 2 × 106 CD4+CD25− T cells. (i,v) Wild-type BM only. (ii,vi) Wild-type GVHD. (iii,vii) IDO−/− BM only. (iv,viii) IDO−/− GVHD. (C) Tissues from panel B stained for CD4 by IHC. Original magnification ×200 for colons, ×100 for spleens.
IDO−/− hosts do not have increased radiation sensitivity
As a heme-containing protein, IDO might function as a free-radical scavenger. We therefore wanted to exclude the possibility that radiation injury was more severe in IDO−/− mice. Sublethally irradiated (5.0 Gy) IDO−/− mice showed no morbidity or mortality, and maintained weight similarly to sublethally irradiated wild-type mice (Figure S4A). To prove that IDO−/− mice could recover after lethal irradiation in the absence of the confounding effect of GVHD on survival, we transplanted lethally irradiated (8.0 Gy) wt and IDO−/− recipients with TCD or non–T cell–depleted syngeneic BM cells (107). All mice survived and maintained weight (Figure S4B). This was also true using a higher radiation dose (9.0 Gy) along with a lower syngeneic BM dose (5 × 106) (not shown). Therefore, the absence of host IDO did not adversely affect the survival of lethally irradiated, BM-rescued recipients, consistent with the fact that BM transplantation alone, in the absence of supplemental donor T cells, did not induce IDO in allogeneic recipients (Figure 2A). Taken together, these results indicate that the increased mortality in IDO−/− recipients requires allogeneic T-cell infusion.
Host IDO deficiency does not affect proliferation, apoptosis, or effector mechanisms of donor T cells in spleen or LN
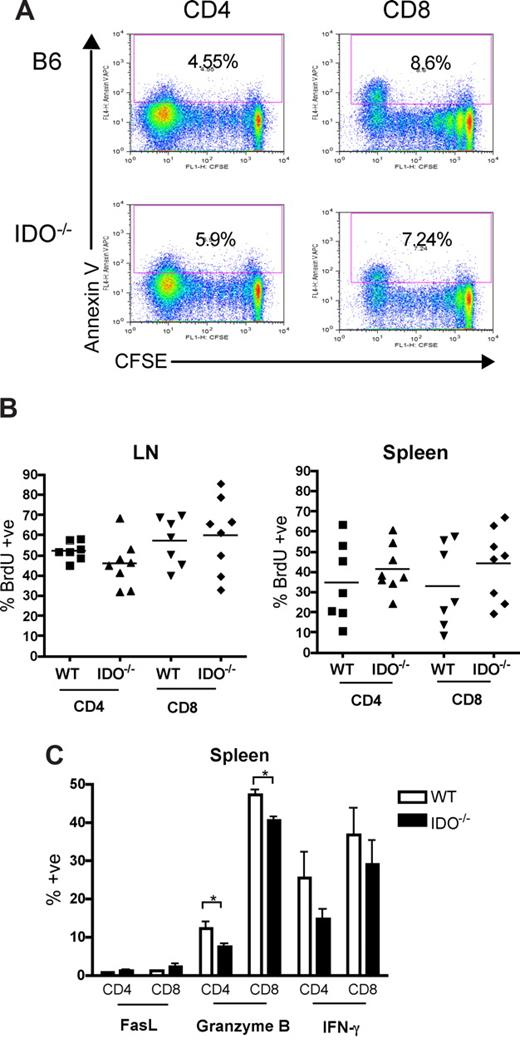
Naive donor T cells infused into irradiated recipients travel to LN and spleen, where they are activated by host APC and proliferate for 3 to 4 days before leaving for the periphery.20,21 In other models, reduced numbers of alloreactive T cells were recovered after transfer into mice in which DCs were systemically induced to express IDO.22 We therefore reasoned that transplanted T cells first may encounter IDO+ APC in the LN and spleen, halting their proliferation and/or leading to apoptosis. Because the colon is the first and most severely affected target organ in this model, T-cell proliferation or survival effects of IDO deficiency may result in increased colon infiltration, where the absence of IDO amplifies damage and recruits more T cells, leading to increased GVHD. To investigate the proliferation and survival of donor T cells, allogeneic splenocytes were labeled with the dye CFSE to track cell division and then transferred into lethally irradiated wt or IDO−/− hosts. Spleen and LN were analyzed on day 4 for CFSE dilution as a measure of proliferation and for annexin V staining as a marker of apoptosis. Figure 5A shows representative CFSE/annexin V profiles for gated donor CD4+ and CD8+ spleen cells in wt and IDO−/− recipients 4 days after transplantation. Neither responder frequency (percent of original cells that divided) nor proliferation index (average number of divisions of divided cells) was significantly increased in IDO−/− recipients compared with wt. Percentages of annexin V–positive cells in both groups were similar as well. Similar results were obtained in identical experiments evaluated at days 2 and 3 (not shown). In addition, whereas donor T-cells in mesenteric LN proliferated significantly more than in spleen or peripheral (inguinal) LN, no differences were observed between wt and IDO−/− recipients (not shown).
Proliferation, survival, and effector function of allogeneic T cells in secondary lymphoid organs are not increased in IDO−/− mice. (A) 60 × 106 CFSE-labeled Balb/c splenocytes were transferred into lethally irradiated B6 or IDO−/− recipients. Mice were killed on day 4, and spleen was examined by flow cytometry for CFSE dilution and annexin V positivity. Spleen data shown are representative of 4 mice/group per day. Cells were gated on CD4+ or CD8+, H-2Kb-negative events. Numbers indicate percentage of annexin V–positive cells. (B) Lethally irradiated B6 or IDO−/− mice were infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. Drinking water was supplemented with BrdU at days 15 to 20 after transplantation. Spleen and mesenteric LN were harvested and analyzed by flow cytometry for BrdU incorporation. Data are pooled from 2 identical experiments; n = 3 or 4 mice/group. No significant differences were observed. Cells were gated on CD4+ or CD8+, H-2Kb–negative events. (C) Lethally irradiated B6 or IDO−/− mice were infused with Balb/c TCD BM and 4 × 106 CD4+ and CD8+ T cells. Seven days after transplantation, spleens were harvested and analyzed by flow cytometry for FasL, granzyme B, and IFN-γ expression; n = 4 mice/group. Cells were gated on CD4+ or CD8+, H-2Kb-negative events (*P < .05).
Proliferation, survival, and effector function of allogeneic T cells in secondary lymphoid organs are not increased in IDO−/− mice. (A) 60 × 106 CFSE-labeled Balb/c splenocytes were transferred into lethally irradiated B6 or IDO−/− recipients. Mice were killed on day 4, and spleen was examined by flow cytometry for CFSE dilution and annexin V positivity. Spleen data shown are representative of 4 mice/group per day. Cells were gated on CD4+ or CD8+, H-2Kb-negative events. Numbers indicate percentage of annexin V–positive cells. (B) Lethally irradiated B6 or IDO−/− mice were infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. Drinking water was supplemented with BrdU at days 15 to 20 after transplantation. Spleen and mesenteric LN were harvested and analyzed by flow cytometry for BrdU incorporation. Data are pooled from 2 identical experiments; n = 3 or 4 mice/group. No significant differences were observed. Cells were gated on CD4+ or CD8+, H-2Kb–negative events. (C) Lethally irradiated B6 or IDO−/− mice were infused with Balb/c TCD BM and 4 × 106 CD4+ and CD8+ T cells. Seven days after transplantation, spleens were harvested and analyzed by flow cytometry for FasL, granzyme B, and IFN-γ expression; n = 4 mice/group. Cells were gated on CD4+ or CD8+, H-2Kb-negative events (*P < .05).
Because weight loss curves begin to show a divergence between wt and IDO−/− recipients starting 2 weeks after transplantation (data not shown), we sought to determine whether later stages of T-cell proliferation were affected by host IDO expression. Mice were given drinking water supplemented with the thymidine analog BrdU, which is incorporated into DNA in replicating cells. T cells labeled from days 5 to 9 after transplantation showed very low levels of proliferation in splenic T cells and increased but equivalent proliferation in wt and IDO−/− mesenteric LN (not shown). The percentages of donor CD4+ and CD8+ T cells were equivalent in both spleen and LNs in wt versus IDO−/− hosts, and BrdU labeling from days 15 to 20 (Figure 5B) and days 20 to 25 (not shown) also showed no significant differences in BrdU+ CD4+ or CD8+ T cells in mesenteric LN or spleen.
An alternate possibility was that IDO might limit the ability of T cells to become fully activated. Therefore, donor T cells present in secondary lymphoid organs were analyzed 7 days after transplantation for the expression of FasL, IFN-γ, and granzyme B, which are effector molecules known to be employed in GVHD. Lethally irradiated B6 or IDO−/− mice were infused with Balb/c TCD BM and 4 × 106 CD4+ and CD8+ T cells. Spleens (Figure 5C) and mesenteric LN (not shown) were harvested on day 7 and analyzed by flow cytometry for FasL, granzyme B, and IFN-γ expression. Cells obtained from the spleen 7 days after transplantation showed little FasL expression. Granzyme B expression was in fact slightly higher in T cells from wt recipients. IFN-γ expression was similar in wt and IDO−/−. Mesenteric LN cells revealed similar findings (not shown). These results indicated that host IDO was not decreasing proliferation, survival, or acquisition of effector molecules during this initial phase of donor T-cell activation under the conditions tested.
These data are consistent with the fact that maximal IDO expression in this GVHD model is seen in the colon and not in lymphoid tissues. Therefore, IDO effects on donor T-cell responses may not be measurable until these cells reach GVHD tissues, especially the gastrointestinal tract.
T cells in IDO−/− versus wt recipients proliferate more and survive better in the colon
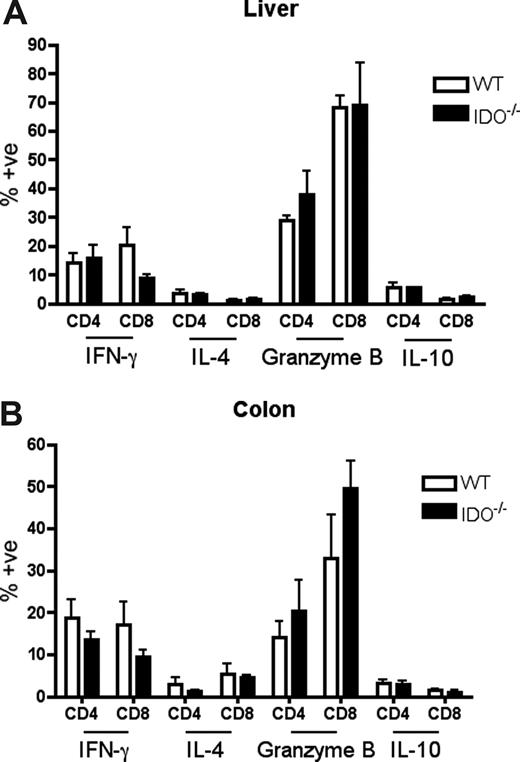
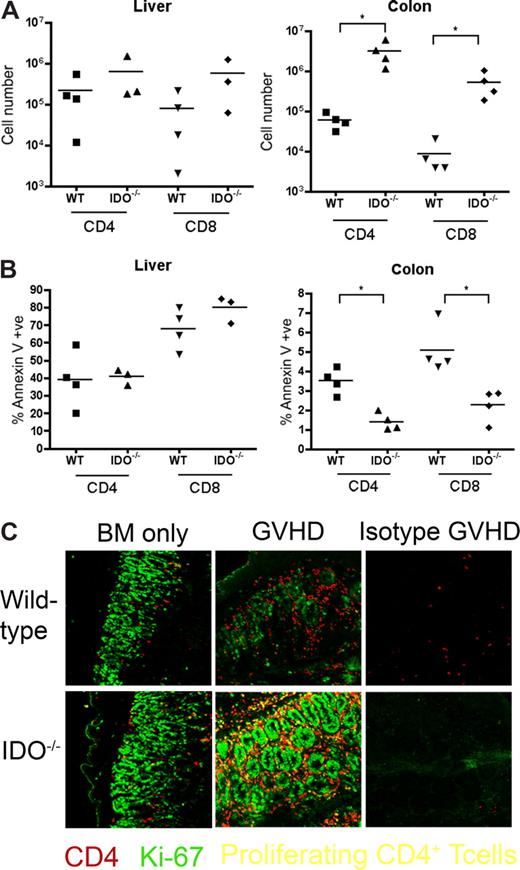
It was possible that IDO was acting on T cells not in secondary lymphoid organs but in GVHD target tissues. To assess the phenotype of T cells in target tissues, we transplanted lethally irradiated B6 or IDO−/− mice with CD4+ and CD8+ T cells and isolated cells from the colon and liver 21 days later. Intracellular staining for IFN-γ, IL-4, IL-10, and granzyme B revealed no differences between wt and IDO−/− hosts in either colon or liver (Figure 6). However, IDO−/− colons contained significantly more T cells than their wt counterparts, with mean CD4+ and CD8+ counts 50-fold higher in IDO−/− than wt, whereas cell counts in the livers were similar (Figure 7A). Moreover, significantly fewer T cells in IDO−/− colons were annexin V–positive, suggesting a decrease in the rate of local apoptosis in the colon in the absence of IDO (Figure 7B). No differences in annexin V staining were seen in the liver. These data suggest that IDO decreases T-cell apoptosis without affecting their effector phenotype. Consistent with this, day 7 sera showed no differences in IL-6, IL-12, TNFα, or IFN-γ levels (not shown).
Effector phenotype of donor T cells in target organs is not changed by IDO. B6 or IDO−/− mice were lethally irradiated and infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. At 21 days after transplantation, T cells were isolated from colons and livers, stimulated with plate-bound anti-CD3 for 4 hours, and stained for IFN-γ, IL-4, granzyme B, and IL-10; n = 4 mice/group. No significant differences were observed.
Effector phenotype of donor T cells in target organs is not changed by IDO. B6 or IDO−/− mice were lethally irradiated and infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. At 21 days after transplantation, T cells were isolated from colons and livers, stimulated with plate-bound anti-CD3 for 4 hours, and stained for IFN-γ, IL-4, granzyme B, and IL-10; n = 4 mice/group. No significant differences were observed.
T-cell proliferation and survival are increased in colons of IDO−/− recipients. (A) B6 or IDO−/− mice were lethally irradiated and infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. At 21 days after transplantation, T cells were isolated from colons and livers and counted; n = 4 mice/group (*P < .04). (B) T cells isolated from colon and liver were stained for annexin V; n = 4 mice/group (*P < .01). (C) Day 27 tissues from the transplant in Figure 2 were stained for CD4 in Cy5 (shown in red) and Ki-67 in FITC (shown in green), a nuclear marker of proliferation. Proliferating CD4+ cells are yellow. Original magnification ×400. Images are representative of 2 similar experiments, 2 to 5 fields/mouse, 3 to 5 mice/group.
T-cell proliferation and survival are increased in colons of IDO−/− recipients. (A) B6 or IDO−/− mice were lethally irradiated and infused with Balb/c TCD BM and 3 × 106 CD4+ and CD8+ T cells. At 21 days after transplantation, T cells were isolated from colons and livers and counted; n = 4 mice/group (*P < .04). (B) T cells isolated from colon and liver were stained for annexin V; n = 4 mice/group (*P < .01). (C) Day 27 tissues from the transplant in Figure 2 were stained for CD4 in Cy5 (shown in red) and Ki-67 in FITC (shown in green), a nuclear marker of proliferation. Proliferating CD4+ cells are yellow. Original magnification ×400. Images are representative of 2 similar experiments, 2 to 5 fields/mouse, 3 to 5 mice/group.
We next examined the tissues for proliferating T cells. IDO−/− and wt hosts were infused with BM with or without 3 million CD4+ and CD8+ T cells. On day 27, tissue sections were stained for Ki-67 (a marker for proliferating cells) and CD4. Double-positive cells, representing proliferating CD4+ cells, were detected in spleen, LN, and thymus, but few double-positive cells were evident in liver, lung, small intestine, or skin (not shown). In contrast, colons of IDO−/− mice with GVHD were filled with CD4 and Ki-67 double-positive cells (Figure 7C). These proliferating CD4+ cells were not prevalent in colons of wt mice under the same conditions, even in the most densely infiltrated and inflamed regions. Isotype controls were negative, ruling out nonspecific staining resulting from tissue destruction and inflammation. Colons of IDO−/− given BM alone contained more CD4+ cells than their wt counterparts, and occasional cells were also positive for Ki-67.
Collectively, these findings reveal a role for IDO induction by allogeneic T cells in the colon. In the absence of IDO expression,T cells continue to proliferate and survive late into disease, accumulating in the colon. Increased colonic infiltration is correlated with accelerated GVHD lethality.
Discussion
These findings uncover an important function for IDO during GVHD. Without host IDO expression, mice experience markedly more severe disease. Infused donor T cells cause striking up-regulation in gut epithelial cells, and the absence of IDO results in increased gut GVHD, as evidenced by histopathology and CD4+ and CD8+ T-cell infiltration. In line with other studies investigating GVHD colitis,23,24 mice with worse colon GVHD had higher lethality. As compared with wt recipients, the increased severity of colonic GVHD in IDO−/− mice correlated with prolonged or increased proliferation and survival of T cells later into disease. CD4+ T cells in IDO−/− GVHD colons expressed Ki-67 several weeks after transplantation, whereas wt colons had few, if any, of these cells. Colons of IDO−/− recipients also contained fewer annexin V–positive T cells than wt colons. These 2 effects resulted in massively increased numbers of T cells in IDO−/− colons. No differences in T-cell number, proliferation, or survival could be detected in spleen or LN, nor were any differences detected in effector function or cytokine profiles in any site, suggesting that T-cell effects are confined to control of proliferation and survival in the colon, the site of maximal host IDO expression.
An immunologic role for IDO was first suggested by the finding that acute inhibition of IDO by 1-MT caused rejection of allogeneic fetuses.3 The likelihood of fetal rejection in 1-MT–treated pregnant mothers increased with greater histocompatibility disparity between mother and fetus, although IDO−/− animals do not reject allogeneic fetuses. These data suggest that, whereas 1-MT acutely disrupts IDO activity and leads to allogeneic fetal rejection in wt animals, chronic genetic deficiency of IDO leads to compensatory suppressive mechanisms that allow an allogeneic fetus to survive despite the absence of IDO. Likewise, IDO−/− mice do not develop spontaneous autoimmunity, even though 1-MT administration worsens existing autoimmunity in several models.8,25,26 Despite these potential compensatory immunologic changes in IDO−/− mice, we see an effect of host IDO deficiency in GVHD. It is therefore possible that IDO is even more potent in suppressing GVHD than demonstrated here. This reasoning predicts that 1-MT would result in even more dramatic GVHD acceleration if it could be reliably absorbed at sufficient levels to block IDO activity. Consistent with this hypothesis and despite likely malabsorption defects that may have limited the amount of 1-MT present in the recipient, we did observe that wt mice given 1-MT in drinking water suffered significantly accelerated GVHD lethality (not shown).
Most immunologic studies have focused on the role of IDO-expressing APCs, usually DC, in modulating T-cell responses. Our results instead point to expression of IDO in gut epithelial cells as the main regulator of lethality in our GVHD model. It is possible, however, that IDO-expressing APCs in spleen or other organs contributed to the suppressive effect as well. Although IDO expression was difficult to accurately quantify in the spleen of mice undergoing a GVHD reaction resulting from tissue necrosis, we did observe markedly increased GVHD in the spleen in IDO−/− hosts, suggesting that IDO+ APCs contained within the spleen may have contributed to the inhibition of donor T cell–mediated destruction of the spleen. In support of these possibilities, ongoing preliminary chimera experiments, in which BMT recipients contain wt tissue and IDO−/− BM-derived cells, or vice versa, have indicated that both APC and tissue expression of IDO contribute to GVHD suppression such that maximum GVHD lethality is observed only when both host APCs and host tissues lack IDO expression.
Although the frequency of Ki-67+ CD4 cells was increased in the colon of IDO−/− versus wt mice at 4 weeks after transplant, staining with BrdU, which is incorporated into the DNA of dividing cells, was not increased in T cells in mesenteric LN in IDO−/− versus wt recipients. Because Ki-67 marks proliferating cells in any phase of the cell cycle, including those that have either proliferated in situ or that have proliferated just before reaching the gut, taken together, these data could indicate that increased T-cell proliferation occurs only on arrival of T cells in IDO−/− versus wt colon and not in the LN compartment before their migration to the colon. Alternatively, increased proliferation may be occurring but not be detectable in IDO−/− LNs, as proliferating T cells may down-modulate LN homing receptors and up-regulate gut homing receptors, resulting in increased Ki-67+ T cells in the colon but not accumulation of proliferating T cells in the LN. Increased T-cell infiltration leading to more severe tissue damage may cause an increase in inflammatory chemokines, further amplifying the response and increasing T-cell infiltration into the colon from the LN. Despite the clear requirement for allogeneic donor T cells in up-regulating IDO expression in the gastrointestinal tract and the lack of such induction in wt recipients of BM alone, in some experiments, lethally irradiated, BM-rescued IDO−/− recipients had significantly more weight loss than wt recipients, suggesting a potential role of host IDO expression in the gastrointestinal tract as modifying T-cell responses or tissue recruitment after transplantation. The cause of these findings is unknown and will be the subject of future investigations.
The gut is a complex immunologic milieu. Epithelial cells are in constant contact with foreign dietary antigens and with commensal bacteria expressing immunostimulatory toll-like receptor ligands. Suppressive mechanisms, including transforming growth factor-β and Tregs, are prevalent in the gut to counteract the stimulatory environment. The constitutive expression of IDO fits with this strategy of protecting epithelial cells against environmental triggers that might set off an overly aggressive and destructive immune response. Induction of IDO by inflammatory stimuli may be a mechanism for quenching immune responses that otherwise may cause tissue damage. GVHD creates much stronger inflammation than the gut has evolved to control, and the IDO that is induced can only delay, but not prevent, disease mortality. IDO is induced and suppresses injury in the gut in other colitis models. A murine model of trinitrobenzene sulfonic acid colitis showed up-regulation of IDO in the colon, and inhibition with 1-MT significantly increased mortality,8 and human inflammatory bowel disease patients showed highly up-regulated IDO in monocytes in colonic lesions.27 GVHD of the intestine is a particularly important therapeutic target. Not only is the gut a frequent and severely affected organ, dramatically decreasing the quality of patients' lives, but it is also an amplifier of systemic disease. Gut injury allows for translocation of endotoxin and other immunostimulatory molecules from commensal gut flora into the bloodstream, leading to the cytokine storm responsible for tissue injury and subsequently more endotoxin translocation. Strategies aimed at gut protection, such as epithelial cytoprotection or blockade of gut-homing molecules on T cells, are being pursued as therapies. Similarly, IDO may be a useful tool for gut protection alone or in combination with such other therapies. Strategies to up-regulate IDO may be specifically useful for GVHD, as a clinical study showed that peripheral blood mononuclear cells from patients with severe acute GVHD were less able to up-regulate IDO on exposure to IFN-γ than healthy donors or those with milder GVHD.28 Therefore, suboptimal IDO up-regulation may predispose patients to more severe GVHD, similar to our findings that IDO−/− mice have more severe GVHD than wt mice.
In conclusion, IDO is a critical suppressor of GVHD. It is induced in the colon and exerts its effects there, regulating T-cell proliferation and survival. This local control of the immune response results in gut protection and a marked delay of GVHD lethality. Enhanced IDO activity or induction of IDO before transplantation may suppress disease, and we speculate that the administration of tryptophan catabolites may mimic IDO activity and dampen disease. Our studies show that IDO does not require donor Tregs for its effect, suggesting that Treg infusional therapy may be additive with IDO therapy. Future studies will examine methods for using the IDO pathway for GVHD prevention or treatment.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joyce Wilson and Christine Vogtenhuber for excellent technical assistance and Mitzi Lewellen for animal husbandry.
This work was supported by grants R01 AI34495 (B.R.B.), R01 CA 72 669 (B.R.B), R37 HL56067 (B.R.B.), CA103320 (D.H.M.), and AI063402 (A.L.M.). The confocal microscope was made available through a National Center for Research Resources Shared Instrumentation Grant (1 S10 RR16851).
National Institutes of Health
Authorship
Contribution: L.K.J. designed experiments, performed research, analyzed data, designed the figures, and wrote the paper; C.B. performed research, analyzed data, and provided advice; A.P.-M. provided advice, analyzed data, performed pathologic analysis, and edited the paper; P.A.T. performed research, provided advice, and edited the paper; A.L.M. provided reagents and advice; D.H.M. provided reagents, technical support, and advice and edited the paper; and B.R.B. designed research, advised on experimental design, and edited the paper.
Conflict-of-interest disclosure: D.H.M. and A.L.M. have intellectual property interests in the therapeutic use of IDO and IDO inhibitors, and receive consulting income from NewLink Genetics, which holds a license to develop the technology for clinical trials. All other authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota Cancer Center and Department of Pediatrics, Division of BMT, MMC 109, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal