Abstract
The relative contribution of yolk sac (YS)–derived cells to the circulating definitive hematopoietic progenitor cell (HPC) pool that seeds the fetal liver remains controversial due to the presence of systemic circulation and the onset of hematopoiesis within the embryo proper (EP) before liver seeding. Ncx1−/− embryos fail to initiate a heartbeat on embryonic day (E) 8.25, but continue to develop through E10. We detected normal numbers of primitive erythroid progenitors in Ncx1−/− versus wild type (WT) YS, but primitive erythroblasts did not circulate in the Ncx1−/− EP. While there was no significant difference in the number of definitive HPCs in Ncx1−/− versus WT YS through E9.5, the Ncx1−/− EP was nearly devoid of HPCs. Thus, primitive erythroblasts and essentially all definitive HPCs destined to initially seed the fetal liver after E9.5 are generated in the YS between E7.0-E9.5 and are redistributed into the EP via the systemic circulation.
Introduction
The predominant sites and patterns of hematopoiesis change during murine ontogeny. Hematopoietic cells emerge de novo from mesoderm precursors within the yolk sac (YS) and paraaortic splanchnopleura (PSp) and possibly the allantois and placenta.1-3 Other organs, including the fetal liver, spleen, and bone marrow, are subsequently colonized by cells distributed via the circulation.4,5 The sites of origin of hematopoietic progenitor cells have been a subject of longstanding debate and intense investigation.6-9
The first blood cells, generated in the YS beginning at embryonic day (E) 7.0, are a unique population of primitive erythroid progenitors (EryP) that mature into large primitive erythroblasts expressing both embryonic and adult hemoglobins. This first phase of YS blood cell production by convention has been termed primitive hematopoiesis.10-12 Beginning at E8.25 (4-6 somite pairs [sp]), high proliferative potential, mixed lineage, and myeloerythroid hematopoietic progenitor cells (HPCs) emerge in the YS. This second phase, termed definitive hematopoiesis, is defined by its erythroid component, which produces smaller cells that express only adult hemoglobins.13 Definitive HPCs expand in the YS but are found in the embryo proper soon after the onset of cardiac contractions at E8.25 (4-6 sp).1,8,10,14 The PSp contains multilineage hematopoietic potential beginning at E8.5, but it is unclear if this potential is realized before the emergence of hematopoietic stem cells (HSCs) at E10.5.9,13 Because these 2 regions are connected by the systemic circulation, the relative role that each site plays in generating the definitive HPCs that seed the fetal liver at E10.0 (28 sp) remains elusive.6,9,15-17
Because the accurate determination of the sites of origin of hematopoietic progenitor cells is complicated by the development of the cardiovascular function, we have developed a novel approach to examine the contribution of the YS and PSp in generating the definitive HPCs that initially seed the fetal liver. Ncx1−/− embryonic mice die at E11 due to the lack of cardiac contractions, but developmental hematopoiesis from E7.0 to E9.5 remains intact.18 The total number of EryP and definitive HPCs emerging from the Ncx1−/−, Ncx1+/− and WT YS is not significantly different, but the Ncx1−/− PSp region lacks primitive erythroblasts and definitive HPCs from E8.25 to E9.5. Our study indicates that the primitive erythroblasts that circulate in the conceptus and the definitive HPCs that seed the liver at the 28 sp stage are all ultimately derived from the YS.
Study design
Ncx1 analysis
LacZ detection via X-gal staining and Ncx1 genotyping were performed as previously described.18 Reverse transcription–polymerase chain reaction (RT-PCR) detection of Ncx1 was performed as previously described (forward/exon1 5′-TTCAGAGCTGGTCGGTTTCT-3′; reverse/exon2 5′-GCAATTTTGTCCCCAAAAGA-3′, 295bp).19
Progenitor assays
Single cell suspensions of E8.25 to E9.5 YS and PSp were plated in either primitive erythroid colony or definitive HPC assays as previously described.1 The primitive erythroid assay medium contained 44% ES-Cult (StemCell Technologies, Vancouver, BC) and 5 U/mL erythropoietin (Besse Medical, West Chester, OH). The definitive HPC assay medium contained MethoCult (StemCell Technologies) with 4 U/mL erythropoietin (Besse Medical),100 ng/mL murine stem-cell factor (mSCF), 100 U/mL interleukin-3, and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, NJ). Plates were incubated at 37°C (5% CO2) for 5 to 7 days and scored by light microscopy.
ζ-globin in situ hybridization
Benzidine staining
Tissues were fixed in 1 mL 0.2% benzidine at room temperature for 10 minutes, then 20 μL 30% H202 was added and incubated for 30 to 60 minutes at room temperature. Stained samples were imaged by light microscopy.
Results and discussion
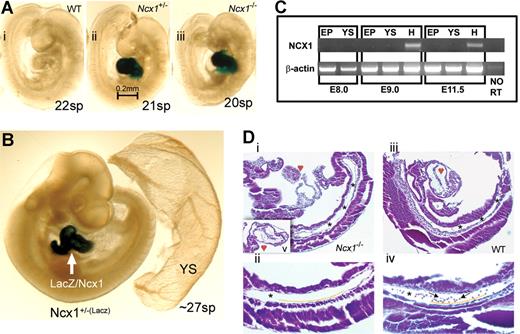
To interrogate Ncx1 expression patterns in the YS and blood cells, embryos were dissected from Ncx1+/− timed matings at E8.5 to E11.5, and whole embryo X-gal staining as well as Ncx1 RT-PCR were performed. X-gal staining was detected in all Ncx1+/− and Ncx1−/− embryos, with expression restricted to cardiac tissue throughout the developmental ages analyzed (Figure 1A,B).18,22 No X-gal staining was detected in the PSp, YS, or blood cells in any tissue (Figure 1B). cDNA was prepared from E8.0 to E11.5 embryo proper (minus the heart), YS, and heart tissue. Ncx1 transcripts were restricted to the heart, confirming the X-gal staining results (Figure 1C). The aorta, foregut, heart (hypoplastic), and YS were clearly present in the E9.25 Ncx1−/− embryo, but no blood cells were detected in the null dorsal aorta (Figure 1D). As we have reported, the post-E10 Ncx1−/− embryo demonstrates higher levels of apoptosis and overall diminished cell growth in extra-cardiac tissues.18 We therefore restricted our comparison of hematopoiesis between WT, Ncx1+/−, and Ncx1−/− embryos from 0 to 32 sp (E8.0 to early E10).
The Ncx1−/− mouse embryo provides an in vivo circulation-free environment.Ncx1 mutants were generated via insertion of LacZ reporter into exon 2 of the Ncx1 gene, thus all cells that normally express Ncx1 can be labeled with X-gal staining. (A) E9.5 embryos (WT(i), Ncx1+/−(ii), and Ncx1−/−(iii)) demonstrate that development continues well past the onset of circulation. (B) X-gal staining (∼27 sp) reveals that expression of Ncx1 is restricted to the heart through E10. Importantly, there is no expression in any putative site of hematopoietic development including the YS and PSp region nor in the hematopoietic cells themselves (see blood vessels in Ncx1+/− YS). (C) Ncx1 RT-PCR was conducted on embryonic tissues from various ages to confirm the X-gal staining. At E8.0, Ncx1 is not yet detectable in either the embryo proper or YS. At E9.0 and E11.5, Ncx1 is detected exclusively in the heart cardiomyocytes. (D) Ten-micrometer sagittal sections of hematoxylin and eosin (H&E) stained E9.25 (19 sp) embryos. Panels Di,iii are 100× magnifications of sections that best profile the structure of the PSp region (*), which is clearly present in both WT and Ncx1−/− embryos. Image Di was cut at an oblique angle compared with image Diii, leaving only a small portion of the heart and upper body in view. Image Dv is an insert that transects the hypoplastic Ncx1−/− heart. Images Dii,iv are 200× magnifications of the PSp (*) regions. Endothelial cells can be seen lining the vessels (below yellow line) and circulating blood cells (arrows) are seen in the WT embryo but are notably absent in the Ncx1−/− embryo. The images in panels A and B were viewed on a Leica MZ9.5 Stereomicroscope (1.0× Planachromatic Lens/0.20 NA)(IL-3) with DFC320 CCD camera, captured with Leica application suite (LAS) software (Leica, Bannockburn, IL). Original magnification, ×60. The images in panel D were viewed on a Zeiss Axioskop Stereomicroscope (Zeiss Plan-Neofluor 10×/0.30 NA (top) and 20×/0.50 NA (bottom)) with SPOT RTKE cooled color CCD camera, and imported into the SPOT Advanced software (Diagnostic Instruments, Sterling Heights, NJ). Original magnification, ×100.
The Ncx1−/− mouse embryo provides an in vivo circulation-free environment.Ncx1 mutants were generated via insertion of LacZ reporter into exon 2 of the Ncx1 gene, thus all cells that normally express Ncx1 can be labeled with X-gal staining. (A) E9.5 embryos (WT(i), Ncx1+/−(ii), and Ncx1−/−(iii)) demonstrate that development continues well past the onset of circulation. (B) X-gal staining (∼27 sp) reveals that expression of Ncx1 is restricted to the heart through E10. Importantly, there is no expression in any putative site of hematopoietic development including the YS and PSp region nor in the hematopoietic cells themselves (see blood vessels in Ncx1+/− YS). (C) Ncx1 RT-PCR was conducted on embryonic tissues from various ages to confirm the X-gal staining. At E8.0, Ncx1 is not yet detectable in either the embryo proper or YS. At E9.0 and E11.5, Ncx1 is detected exclusively in the heart cardiomyocytes. (D) Ten-micrometer sagittal sections of hematoxylin and eosin (H&E) stained E9.25 (19 sp) embryos. Panels Di,iii are 100× magnifications of sections that best profile the structure of the PSp region (*), which is clearly present in both WT and Ncx1−/− embryos. Image Di was cut at an oblique angle compared with image Diii, leaving only a small portion of the heart and upper body in view. Image Dv is an insert that transects the hypoplastic Ncx1−/− heart. Images Dii,iv are 200× magnifications of the PSp (*) regions. Endothelial cells can be seen lining the vessels (below yellow line) and circulating blood cells (arrows) are seen in the WT embryo but are notably absent in the Ncx1−/− embryo. The images in panels A and B were viewed on a Leica MZ9.5 Stereomicroscope (1.0× Planachromatic Lens/0.20 NA)(IL-3) with DFC320 CCD camera, captured with Leica application suite (LAS) software (Leica, Bannockburn, IL). Original magnification, ×60. The images in panel D were viewed on a Zeiss Axioskop Stereomicroscope (Zeiss Plan-Neofluor 10×/0.30 NA (top) and 20×/0.50 NA (bottom)) with SPOT RTKE cooled color CCD camera, and imported into the SPOT Advanced software (Diagnostic Instruments, Sterling Heights, NJ). Original magnification, ×100.
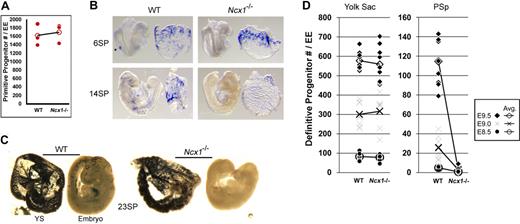
There was no significant difference in EryP numbers between WT, Ncx1+/−, and Ncx1−/− embryos (Figure 2A) before the onset of circulation (0-4 sp), and at this time, ζ-globin-expressing primitive erythroblasts were restricted to blood islands within the proximal YS of all embryos (Figure 2B). By 14 sp, ζ-globin staining was clearly evident in the WT and Ncx1+/− embryo proper but absent in the Ncx1−/− EP (Figure 2B). Through E9.5 (20-25 sp), the WT, Ncx1+/−, and Ncx1−/− YSs were all engorged with primitive erythroblasts. In contrast, while the WT and Ncx1+/− EP also contained primitive erythroblasts, the E9.5 Ncx1−/− EP was devoid of blood (Figure 2C). We have previously demonstrated that the YS is a site of preferential adhesion for hematopoietic progenitors.14 The restriction to the YS of otherwise freely circulating primitive erythroblasts in the absence of a circulation is consistent with the accepted YS origin of EryP. Assuming that the same effect applies to all hematopoietic progenitors, the Ncx1−/− embryo should permit the determination of the site of origin of the first definitive HPC.
Circulation is required to distribute hematopoietic progenitors and differentiated cells to the embryo proper. (A) Primitive progenitor colony number is not altered by the lack of flow in Ncx1−/− embryos. WT embryos produced an average of 1617 (± 255; mean ± SEM) colonies while Ncx1−/− embryos produced 1697 (± 227) colonies. (B) ζ-globin in situ hybridization specifically labels the primitive erythroblast lineage. At 6 sp, just after the first heart beats, no difference in blood distribution is detectable in Ncx1−/− or WT embryos. Note the cluster of cells in the embryo proper at the site of connection of the vitelline vasculature (*). At 14 sp, a significant amount of ζ-globin staining is detected in the embryo proper of the WT while the Ncx1−/− embryo remains devoid of erythroblasts. (C) Embryos (23 sp) were stained with benzidine, which stains all hemoglobin-containing cells dark blue/black. At this stage of development, all hemoglobin-containing erythroblasts are derived from primitive progenitors from the yolk sac (YS) and the vast majority of staining occurs in the YS. Note Ncx1−/− EP has no stained erythroblasts compared with age-matched WT littermates. (D) (Representative definitive progenitor numbers) There is an expansion of definitive HPCs in both the YS and embryo proper from E8.5 of development through E9.5 in the WT embryos (Table S1). Definitive HPCs in the YS expand from 82 to 578 (colonies/YS) and in the PSp from 5 to 115 (colonies/PSp) during this time period. In the absence of a circulation in the Ncx1−/− embryo, definitive HPC numbers in the YS range from 79 to 559 and are not significantly different from WT littermates. The Ncx1−/− PSp ranges from 0 to 3 definitive HPCs, which is a significant decrease (P < .05 by paired Student t test) from WT at all timepoints examined. Images in panels B,C acquired as for Figures 1A and B. Original magnification, ×60.
Circulation is required to distribute hematopoietic progenitors and differentiated cells to the embryo proper. (A) Primitive progenitor colony number is not altered by the lack of flow in Ncx1−/− embryos. WT embryos produced an average of 1617 (± 255; mean ± SEM) colonies while Ncx1−/− embryos produced 1697 (± 227) colonies. (B) ζ-globin in situ hybridization specifically labels the primitive erythroblast lineage. At 6 sp, just after the first heart beats, no difference in blood distribution is detectable in Ncx1−/− or WT embryos. Note the cluster of cells in the embryo proper at the site of connection of the vitelline vasculature (*). At 14 sp, a significant amount of ζ-globin staining is detected in the embryo proper of the WT while the Ncx1−/− embryo remains devoid of erythroblasts. (C) Embryos (23 sp) were stained with benzidine, which stains all hemoglobin-containing cells dark blue/black. At this stage of development, all hemoglobin-containing erythroblasts are derived from primitive progenitors from the yolk sac (YS) and the vast majority of staining occurs in the YS. Note Ncx1−/− EP has no stained erythroblasts compared with age-matched WT littermates. (D) (Representative definitive progenitor numbers) There is an expansion of definitive HPCs in both the YS and embryo proper from E8.5 of development through E9.5 in the WT embryos (Table S1). Definitive HPCs in the YS expand from 82 to 578 (colonies/YS) and in the PSp from 5 to 115 (colonies/PSp) during this time period. In the absence of a circulation in the Ncx1−/− embryo, definitive HPC numbers in the YS range from 79 to 559 and are not significantly different from WT littermates. The Ncx1−/− PSp ranges from 0 to 3 definitive HPCs, which is a significant decrease (P < .05 by paired Student t test) from WT at all timepoints examined. Images in panels B,C acquired as for Figures 1A and B. Original magnification, ×60.
From E8.25 (3 sp) through E9.5 (25 sp), definitive HPC number was assessed in more than 84 embryos by colony forming assays (Figure 2D). During this developmental time, HPCs in the WT and Ncx1+/− YS dramatically expanded in number from approximately 83 to nearly 600 colonies/YS, and to a lesser extent in the PSp from 3 to around 70 colonies/PSp. HPC expansion in the Ncx1−/− YS was equivalent to and even exceeded that detected in the WT and Ncx1+/− YS. Strikingly, no expansion of definitive HPCs was measured in the Ncx1−/− PSp (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In fact, less than 0.4% of definitive HPCs were found in the Ncx1−/− PSp or the allantois/placenta as late as E10.25 (32 sp) (data not shown). As this time point (25-32 sp) is concomitant with the seeding of the fetal liver (28 sp), we conclude that all primitive and definitive HPCs emerging before 32 sp are products of the YS and that YS-definitive HPCs seed the fetal liver via redistribution through the embryonic circulation.
Early pioneers of hematopoietic ontogeny hypothesized that the extra-embryonic YS was the site of origin of all hematopoietic cells.6,23 Cell tracing studies have recently demonstrated that cells of YS origin contribute to all hematopoietic lineages in the adult, but have not provided a direct assessment of the presence of hematopoietic stem cells (HSC) within the early YS.24 The Ncx1−/− embryo provides an in vivo circulation-free environment for the study of developmental hematopoiesis through 32 sp, well past the normal onset of the first heart beat at 4 to 6 sp. The availability of this novel mouse model now allows us to investigate the origin of HSCs in a way not previously possible.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant HL63169 and American Heart Association (AHA) grant 0515433Z.
National Institutes of Health
Authorship
Contribution: C.T.L. designed and conducted the research, analyzed data, and wrote the paper; M.Y. analyzed data; K.M. conducted research. S.J.C. contributed vital new reagents. J.P. analyzed data and and edited the paper. M.C.Y. designed research, analyzed data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mervin C. Yoder, Department of Pediatrics, Indiana University, Cancer Research Institute, 1044 W Walnut St, Room 402E, Indianapolis, IN 46202-5254; e-mail: myoder@iupui.edu.