Abstract
In human monocytes, tumor necrosis factor (TNF) induces a proinflammatory response. In NF-κB–inhibited monocytes, TNF stimulates cell death/apoptosis. In the present study, we analyzed the response of acute myeloid leukemia (AML) cells to TNF stimulation in conjunction with NF-κB inhibition. In all AML-derived cells tested, NF-κB–inhibited cells were resistant to TNF-induced apoptosis. Further investigation revealed that the cytoprotective gene heme oxygenase-1 (HO-1) was induced in NF-κB–inhibited AML cells in response to TNF stimulation, and HO-1 was responsible for the resistance of AML cells to the cytotoxic actions of TNF. Moreover, after transfection with HO-1 siRNA, the resistance to TNF-induced cell death signals of AML cells was removed. The HO-1 promoter region contains antioxidant-response elements that can bind the transcription factor NF-E2–related factor 2 (Nrf2). We further demonstrated that Nrf2 was activated by TNF under NF-κB–inhibited conditions, to play the major role in up-regulating HO-1 expression and ultimately the fate of AML cells. These results demonstrate a novel mechanism by which TNF-induced cell death is inhibited in AML cells through the induction of HO-1, via Nrf2 activation.
Introduction
Understanding the mechanisms that control cell proliferation or apoptosis is essential to understanding and controlling diseases, such as acute myeloid leukemia. Monocytes and macrophages, which are derived from myeloid cells, have the ability to proliferate or die dependent on their environment and the controlling signals they encounter.1,2 One signal that has the capacity to cause either cellular proliferation or cell death in these cells is tumor necrosis factor (TNF). TNF binds 2 cell-surface receptors, TNFR1 and TNFR2, to regulate multiple cellular actions.3 Understanding the means by which TNF can control a cell's response has been extensively researched. However, it is still ambiguous as to the mechanism underlying the cell's decision to respond to a stimulus by undergoing cell death, survival, or proliferation.3
TNF has been recognized as an activator of nuclear factor-κB (NF-κB), a transcription factor implicated in the protection of many cell types, including monocytes and macrophages from apoptosis.4,5 Under physiologic conditions, signaling via TNFR does not lead to monocyte or macrophage cell death because TNF triggers the expression of antiapoptotic genes through the transcription factor NF-κB.6 Such genes include Fas-associated protein with death domain–like interleukin-1 (IL-1)–converting enzyme (FLICE/caspase-8)–inhibitory protein (FLIP) and inhibitor of apoptosis-1. The same cells, however, may become sensitive to cell death signals after the inhibition of NF-κB.7,8 Furthermore, multiple cell types deficient in mediators of TNF-induced NF-κB activation, including p65, inhibitor of NF-κB (IκB) kinase (IKK)–β, and IKK-γ, are highly sensitive to TNF-induced apoptosis.9-14 Therefore, NF-κB activation mediates inhibition of death inducing signals after treatment with TNF and is now considered a target for cancer therapy.15,16
TNF and other inflammatory mediators have been shown to regulate a variety of additional antiapoptotic genes that are regulated independently of NF-κB.17,18 These include heme oxygenase 1 (HO-1), the rate-limiting enzyme of heme catabolism. In cancer, HO-1 has been described as a protumoral molecule because of its antiapoptotic effects in colon cancer and liver cancer in murine models, and its pro-angiogeneic effects in human pancreatic cancer.19-21 In contrast, in human tongue cancer, low HO-1 expression has been associated with an increased risk of developing lymph node metastasis.22 Furthermore, HO-1 has been shown to inhibit rat and human breast cancer cell proliferation.23 Hence, the role of HO-1 in cancer biology is far from completely understood. No studies have been devoted to ascertaining the role of HO-1 in acute myeloid leukemia (AML).
The 5′-flanking region of the HO-1 gene contains binding sites for the transcription factors that regulate inflammation and apoptosis, including NF-κB and activator protein 1, AP-1.24,25 HO-1 also contains binding sites for the transcription factor Nrf2, which belongs to the Cap'n'Collar family of basic leucine zipper transcription factors, which also include Nrf1, Nrf3, Bach1, and Bach2.26 These transcription factors heterodimerize with other proteins, including members of the Jun and the small Maf families.26 Nrf2 binds to the cis-acting transcription regulatory elements, termed the antioxidant response element (ARE), with the core sequence nG/ATGACnnnGCn.27 The ARE is present in the promoters of a variety of cytoprotective and detoxification genes, including ferritin and NAD(P)H quinine oxidative reductase, both of which have been shown to have antiapoptotic properties.18,28 Nrf2 has been shown to induce HO-1 gene expression in various cell types by a diverse range of stimuli, including electrophiles, heme, heavy metals, antioxidants, and proinflammatory mediators.29,30 A cytoplasmic actin-binding protein, Keap1, is an inhibitor of Nrf2 that sequesters it in the cytoplasm and to the enhancement of Nrf2 degradation by proteasomes conferring tight regulation on the response.31 Electrophiles act to counteract sequestration of Nrf2 by Keap1 and provoke Nrf2 activation.32 Moreover, Nrf2 has been shown to be a regulator of the innate immune response in experimental sepsis,33 as well protecting against severe airway inflammation and asthma in mice.34 These studies not only suggest that HO-1 up-regulation is dependent on the expression and activation of Nrf2 but also that Nrf2 can regulate immune function.
In this study, we compared the levels of cell death between human AML cells to noncancerous primary human monocytes in response to TNF-induced cell death signals. We investigated the expression of the antiapoptotic gene HO-1 and tested its role in protecting AML-derived cells from TNF-induced cell death. The role of the transcription factor Nrf2 on HO-1-induced expression and in protecting AML-derived cells from death signals was also examined. We show that AML-derived cells possess enhanced protection against TNF-induced cell death signals compared with noncancerous primary human monocytes. Furthermore, in contrast to primary monocytes, TNF death-resistant cancerous cells induced the expression of cytoprotective HO-1 in response to TNF. This enhanced HO-1 expression protects AML cells from TNF-induced cell death. These findings provide important new insights into the apoptotic-resistant nature of AML cells and the regulation of death signals in cancer cells by HO-1.
Methods
Materials
The AML-derived cell lines THP-1, HL60, U937, and AML 193 cells were obtained from the European Collection of Cell Cultures. Recombinant human TNF was purchased from R&D Systems (Minneapolis, MN). Antihuman HO-1 antibody was purchased from Assay Designs (Ann Arbor, MI). Antihuman FLIP antibody was purchased from Abcam (Cambridge, MA). All other antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). BAY 11-7082 was procured from Calbiochem (San Diego, CA). zVAD-fmk was purchased from R&D Systems. RelA/p65 siRNA was purchased from Dharmacon RNA Technologies (Lafayette, CO), whereas Nrf2, FLIP, and HO-1 siRNAs were purchased from Ambion (Austin, TX). All other reagents were obtained from Sigma-Aldrich (St Louis, MO), unless indicated.
Cell culture
Primary monocytes, THP-1, HL60, and U937 cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine (Invitrogen, Carlsbad, CA), and 2-mercaptoethanol. AML 193 cells were cultured in Dulbecco modified Eagle medium with 5% fetal calf serum and 1 ng/mL granulocyte-macrophage colony stimulating factor. Cells were maintained in a humidified atmosphere at 37°C and 5% CO2. For monocyte and hematopoietic stem cell isolation, heparinized blood was collected from healthy volunteers and human peripheral blood mononuclear cells (PBMCs) isolated by Percoll (GE Healthcare, Little Chalfont, United Kingdom) density gradient centrifugation.35 PBMCs (4 × 106/mL) were incubated in complete medium for 2 hours at 37°C to allow adherence of monocytes.36 Positive selection of human hematopoietic stem cells were isolated from PBMCs using a CD34 positive selection kit (Miltenyi Biotec, Auburn, CA). Cell type was confirmed by microscopy and flow cytometry.
Proliferation/death and apoptotic assays
Cells were treated with 10 μM BAY 11-7082 (30-minute pretreatment) or transfected 24 hours previously with 30 nM of p65 siRNA before stimulation with various concentrations of TNF for a further 24 hours. After treatment, cell number was measured by incubation with MTS one-solution assay reagent (Promega, Madison, WI) at 37°C for 1 hour before reading absorbance in quadruplicate at 490 nm. To determine cells undergoing apoptosis, we examined cell cytospin preparations that were stained with Hoechst 33342 (Invitrogen) for 5 minutes at room temperature. The percentage of apoptotic cells was determined by epifluorescence examining characteristic nuclear condensation and chromosomal compaction as previously shown.37
RNA extraction and real-time PCR
Cells were untreated or treated with 10 μM of BAY 11-7082 or transfected with 30 nM siRNA before stimulation with TNF for various times at 37°C. In kinase experiments, cells were pretreated with kinase inhibitors for 30 minutes before treatment with BAY 11-7082. Total RNA was extracted from 2 × 105 cells using the Nucleic acid PrepStation from Applied Biosystems, according to the manufacturer's instructions. Reverse transcription was performed using the RNA polymerase chain reaction (PCR) core kit (Applied Biosystems, Foster City, CA). Real-time PCR primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), HO-1, and IL-1β were purchased from Invitrogen. Sequences of primers were as follows: GAPDH forward, 5′-ACCAGCCTCAAGATCATCAGCA-3′; GAPDH reverse, 5′-TGCTAAGCAGTTGGT GGTGC-3′; HO-1 forward, 5′-ATGGCCTCCCTGTACCACATC-3′; HO-1 reverse, 5′-TGTTGCGCTCAATCTCCTCCT-3′; IL-1β forward 5′-CTGGACCTCTGCCCTCTGG-3′; IL-1β reverse 5′-TCCATGGCCACAACAACTGA-3′; FLIPL forward 5′-GTTCAAGGAGCAGGGACAAG-3′; FLIPL reverse, 5′-TCCCATTATGGAGCCTGAAG-3′. Relative quantitative real-time PCR used SYBR green technology (Sigma-Aldrich) on cDNA generated from the reverse transcription of purified RNA. After preamplification (95°C for 2 minutes), the PCRs were amplified for 40 cycles (95°C for 15 seconds and 60°C for 1 minute) on a IQ5 Real-time PCR Detection System (Bio-Rad, Hercules, CA). Each mRNA expression was normalized against GAPDH mRNA expression using the comparative cycle threshold method.38
Western immunoblotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western analyses were performed as described previously.35 Briefly, whole cell lysates were extracted using radioimmunoprecipitation assay (RIPA) buffer method and sodium dodecyl sulfate-polyacrylamide gel electrophoresis separation performed. Nuclear extracts were prepared as described.35 Protein was transferred to nitrocellulose and Western blot analysis performed with the indicated antisera according to their manufacturer's guidelines. Detection was performed by electrochemical luminescence (ECL).
Electrophoretic mobility shift assay
Oligonucleotide probe containing the human HO-1 ARE site (underlined), 5′-GCATTTCTGCTGCGTCATGTTTGGGAGG-3′ was manufactured and biotinylated by Sigma-Genosys (The Woodlands, TX). For competition binding, the same sequence was manufactured without the biotin label. Nuclear extracts were prepared from 5 × 106 THP-1 cells or HL60 cells as previously described35 and incubated with the biotin-labeled probes using the LightShift Chemiluminescent electrophoretic mobility shift assay (EMSA) kit (Pierce Chemical, Rockford, IL), following the manufacturer's instructions. For supershift analysis, nuclear extracts from treated THP-1 and HL60 cells were preincubated with 1 μg of either antihuman Nrf2 or anti human p65 supershift antibodies (Santa Cruz Biotechnology) for 20 minutes before gel shift analysis.
Transfections
Primary monocytes, THP-1 cells, and HL60 cells (106/well) were transfected using Amaxa Nucleofector Technology, using equivalent molar concentrations of the siRNA (to yield a final concentrations of 30 nM), or with 0.5 mg of IκBa-dominant negative (IκBα-DN) construct. Transfected cells were incubated for 24 hours before the indicated treatments described above.
Measurement of reactive oxygen species
A dichlorofluorescin (DCF) assay was used to determine cellular reactive oxygen species (ROS) generation in THP-1 and HL60 cells.39 Briefly, 2 × 106 cells/well cultured in a 6-well plate were incubated with 10 μM of 6-carboxy-2′,7′-dicholorfluorescin diacetate (DCFH-DA) (Invitrogen) for 1 hour in the dark. The cells were then treated with appropriate stimuli and measured for the oxidation of DCFH-DA using a flow cytometer at the excitation and emission wavelengths of 485 nm and 530 nm, respectively. The fluorescent intensity measuring the oxidation of DCFH-DA by ROS represents the relative steady state of ROS generation in cells.
Statistical analyses
Student t test was performed to assess statistical significance from controls. Results with P less than .01 were considered statistically significant. Results represent the mean plus or minus SEM of 3 independent experiments. For Western blotting and EMSA experiments, data are representative of 3 independent experiments.
Results
Human AML-derived cell lines are resistant to TNF-induced cell death
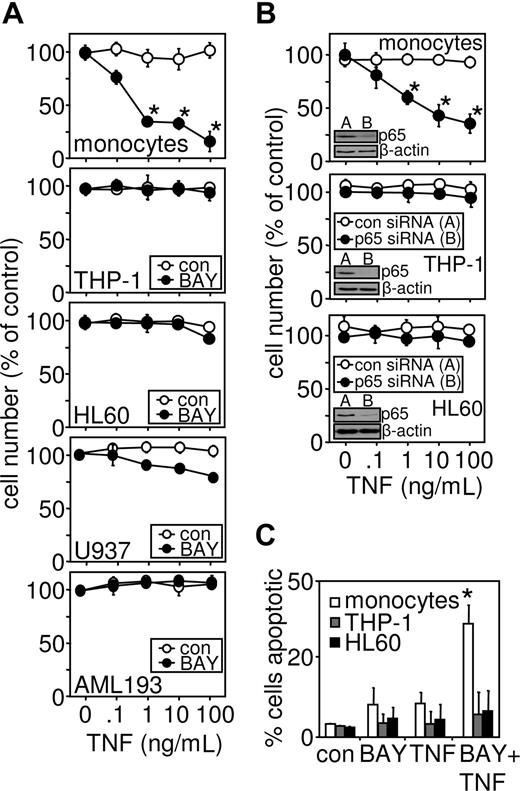
To determine the role of TNF in mediating cell death in human leukemic cells, an inhibitor of NF-κB activation (BAY 11-7082) was used.40 Compared with inhibitor alone, increasing concentrations of TNF greatly enhanced the loss of primary monocyte viability (Figure 1A). However NF-κB inhibition by BAY 11-7082 had little or no effect on the AML-derived cell lines, THP-1, HL60, U937, and AML 193 cells (Figure 1A). To confirm this response was specific to leukemia cells, we also treated CD34+ hematopoietic stem cells with TNF in combination with NF-κB inhibition (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To establish whether the NF-κB activation levels in freshly isolated monocytes were equivalent to AML-derived cell lines, we concentration-dependently inhibited the AML cells with various concentrations of BAY 11-7082 with and without TNF treatment. Figure S2A shows that BAY 11-7082 did not sensitize AML cells to TNF-induced cell death, even at the highest concentrations achievable. To confirm that these pharmacologic effects observed were because of the inhibition of NF-κB, we alternatively inhibited NF-κB activation by specifically knocking down p65/RelA protein levels, through transfection with p65-targeted siRNA (Figure 1B). The reduction of p65 protein levels by siRNA means was specific and matched the findings seen with BAY 11-7082. The mode of TNF-induced cell death of primary cells was apoptotic, as determined by Hoechst 33342 staining, which shows characteristic chromosomal compaction and nuclear karyorrhexis37 (Figure 1C).
Myeloid leukemia cells, but not noncancerous monocytes, are resistant to TNF-induced cell death. (A) Primary monocytes, THP-1, HL60, U937, and AML 193 cells were treated with 10 μM BAY 11-7082 30 minutes before treatment with the indicated concentrations of TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” (B) Primary monocytes, THP-1 cells, and HL60 cells were transfected with p65 siRNA 24 hours before stimulation with the indicated concentrations of TNF for 24 hours (inset, p65 protein knockdown 24 hours after transfection). Cell number was then assessed by MTS assay. (C) Apoptotic cells morphology detected by epifluorescence after staining with Hoechst 33342. Values indicate the mean plus or minus SEM from 3 independent experiments (* statistical significance, P < .01, between the different treatment groups).
Myeloid leukemia cells, but not noncancerous monocytes, are resistant to TNF-induced cell death. (A) Primary monocytes, THP-1, HL60, U937, and AML 193 cells were treated with 10 μM BAY 11-7082 30 minutes before treatment with the indicated concentrations of TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” (B) Primary monocytes, THP-1 cells, and HL60 cells were transfected with p65 siRNA 24 hours before stimulation with the indicated concentrations of TNF for 24 hours (inset, p65 protein knockdown 24 hours after transfection). Cell number was then assessed by MTS assay. (C) Apoptotic cells morphology detected by epifluorescence after staining with Hoechst 33342. Values indicate the mean plus or minus SEM from 3 independent experiments (* statistical significance, P < .01, between the different treatment groups).
TNF treatment in conjunction with NF-κB inhibition results in rapid HO-1 induction in AML-derived cell lines but not in primary monocytes
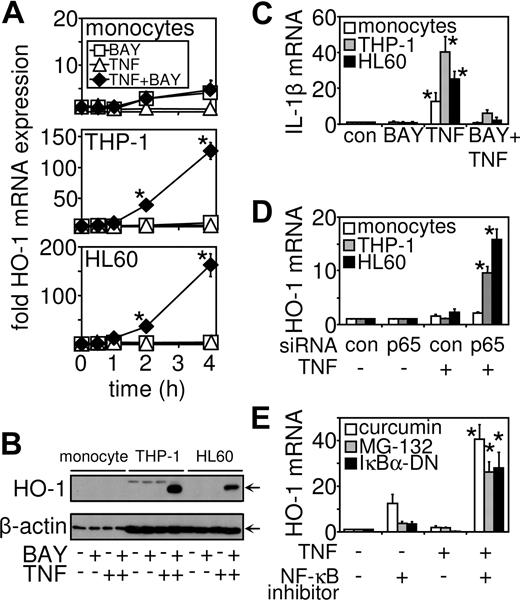
Because AML-derived cells (THP-1, HL60, U937, and AML 193) are resistant to TNF-induced cell death, we hypothesized that an antiapoptotic gene not regulated by NF-κB may be responsible for this resistance of these cancerous cells to cell death signals. A strong candidate was HO-1, which has been shown in tumors to protect cancerous tissue against apoptosis.19,20 Furthermore, we have previously shown that HO-1 is up-regulated in response to pro-inflammatory mediators and NF-κB inhibitors.30,41 We therefore examined the expression profile of HO-1 mRNA in NF-κB-inhibited TNF-treated THP-1 cells, HL60 cells, and primary monocytes. After treatment with TNF in NF-κB–inhibited cells, we found that HO-1 mRNA was induced in THP-1 and HL60 cells in a time-dependent manner; however, no induction was observed in primary monocytes (Figure 2A). To confirm this response was specific to leukemia cells, we also treated CD34+ hematopoietic stem cells with TNF in combination with NF-κB inhibition and showed that HO-1 was not induced in these cells either (Figure S1B). In contrast, HO-1 mRNA was not induced by stimulation with BAY 11-7082 or TNF alone (Figure 2A) in both AML-derived cell lines and primary cells. HO-1 protein induction was then confirmed by Western analysis. HO-1 was undetected in cells treated with BAY 11-7082 and TNF alone. However, these treatments in combination caused HO-1 protein expression in both THP-1 and HL60 cells, but not in noncancerous primary cells (Figure 2B). Immunoblots were reprobed with a mouse antihuman β-actin antibody to confirm equal loading between samples. As a control, we examined in all 3 cell types the expression of IL-1β mRNA in response to either BAY 11-7082 or TNF alone, or in combination. IL-1β mRNA has been shown to be induced by TNF in a highly NF-κB-dependent manner.30 Figure 2C shows that TNF induced IL-1β mRNA in primary monocytes, THP-1, and HL60 cells. This induction in IL-1b mRNA was inhibited by the addition of BAY 11-7082 in all 3 cell types. To confirm that the effects observed were the result of the inhibition of NF-κB, we used p65 siRNA to inhibit NF-κB activation (Figure 2D). Moreover, we also tested several known NF-κB inhibitors in combination with TNF stimulation to determine the effects on HO-1 expression. In THP-1 cells, expression of an IκBα-DN mutant or MG132 or curcumin pharmacologic agents was shown to allow induction of HO-1 mRNA expression when treated in combination with TNF (Figure 2E), thus proving the specificity of the BAY 11-7082 effects seen in these systems.
TNF in conjunction with NF-κB inhibition induces HO-1 gene expression in myeloid leukemia cells but not primary cells. (A) Primary monocytes, THP-1 cells, and HL60 cells were treated with either BAY 11-7082 (10 μM or transfected before treatment with TNF (10 ng/mL) for the indicated time. RNA was extracted and after reverse transcription, HO-1 mRNA expression was measured by real-time PCR. (B) HO-1 protein expression was measured by Western blot analysis. Cells were treated with 10 μM BAY 11-7082 30 minutes before 8 hours of treatment with 10 ng/mL TNF as indicated. (C) Primary monocytes, THP-1 cells, and HL60 cells were pretreated with 10 μM BAY 11-7082 30 minutes before treatment with TNF (10 ng/mL) for 4 hours. After reverse transcription, IL-1β mRNA expression was measured by real-time PCR. (D). Primary monocytes, THP-1 cells, and HL60 cells were transfected with p65 siRNA 24 hours before stimulation with TNF (10 ng/mL) for 4 hours. HO-1 mRNA expression was measured by real-time PCR. (E) THP-1 cells were pretreated for 30 minutes with either curcumin (1 μM) or MG132 (5 μM) or transfected 24 hours previously with 0.5 mg of a Iκ-Bα dominant negative mutant (IκBα-DN) construct before stimulation with TNF (10 ng/mL) for 4 hours. HO-1 mRNA expression was measured by real-time PCR. Values indicate mean plus or minus SEM from 3 independent experiments (*significance, P < .01, between the different treatment groups).
TNF in conjunction with NF-κB inhibition induces HO-1 gene expression in myeloid leukemia cells but not primary cells. (A) Primary monocytes, THP-1 cells, and HL60 cells were treated with either BAY 11-7082 (10 μM or transfected before treatment with TNF (10 ng/mL) for the indicated time. RNA was extracted and after reverse transcription, HO-1 mRNA expression was measured by real-time PCR. (B) HO-1 protein expression was measured by Western blot analysis. Cells were treated with 10 μM BAY 11-7082 30 minutes before 8 hours of treatment with 10 ng/mL TNF as indicated. (C) Primary monocytes, THP-1 cells, and HL60 cells were pretreated with 10 μM BAY 11-7082 30 minutes before treatment with TNF (10 ng/mL) for 4 hours. After reverse transcription, IL-1β mRNA expression was measured by real-time PCR. (D). Primary monocytes, THP-1 cells, and HL60 cells were transfected with p65 siRNA 24 hours before stimulation with TNF (10 ng/mL) for 4 hours. HO-1 mRNA expression was measured by real-time PCR. (E) THP-1 cells were pretreated for 30 minutes with either curcumin (1 μM) or MG132 (5 μM) or transfected 24 hours previously with 0.5 mg of a Iκ-Bα dominant negative mutant (IκBα-DN) construct before stimulation with TNF (10 ng/mL) for 4 hours. HO-1 mRNA expression was measured by real-time PCR. Values indicate mean plus or minus SEM from 3 independent experiments (*significance, P < .01, between the different treatment groups).
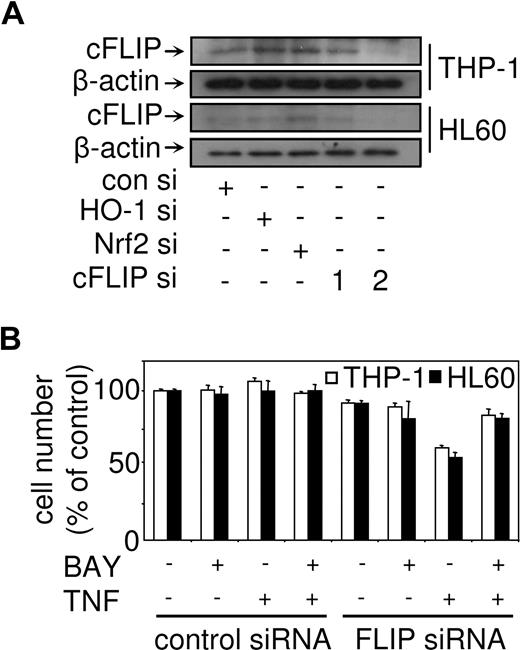
cFLIP is not regulated in response to TNF in conjunction with NF-κB inhibition
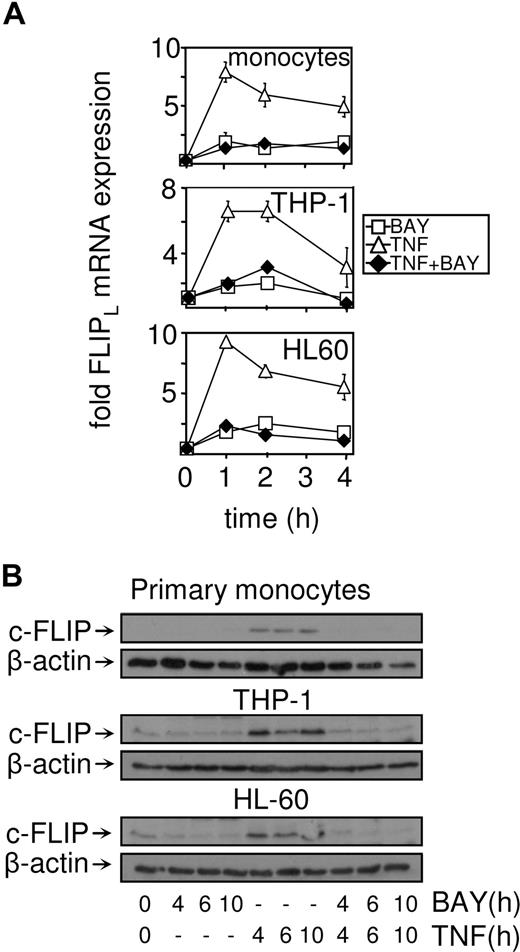
Because cFLIP has been shown to protect cells from TNF-induced apoptotic signals in models of leukemia research,5,42 we wanted to establish the expression profile of this protein in response to TNF in conjunction with NF-κB inhibition. We examined the expression of FLIPL mRNA in NF-κB–inhibited TNF-treated THP-1 cells, HL60 cells, and primary monocytes. After treatment with TNF alone, we found that cFLIP was induced in THP-1, HL60, and primary monocytes in a time-dependent manner. TNF-induced FLIPL mRNA was inhibited by the addition of BAY 11-7082 in nonleukemia and leukemia cells (Figure 3A). cFLIP protein expression was then confirmed by Western blot analysis. cFLIP was low in cells treated with BAY 11-7082 alone and in combination with TNF. However, in response to TNF, cFLIP protein expression in THP-1, HL60, and primary monocytes was induced (Figure 3B). Immunoblots were reprobed with a mouse antihuman β-actin antibody to confirm equal loading between samples.
cFLIP mRNA and protein are not induced in response to TNF plus NF-κB inhibition. (A) Primary monocytes, THP-1 cells, and HL60 cells were treated with BAY 11-7082 (10 μM) before treatment with TNF (10 ng/mL) for the indicated time. RNA was extracted and after reverse transcription, FLIPL mRNA expression was measured by real-time PCR. (B) HO-1 protein expression was measured by Western blot analysis. Cells were treated with 10 μM BAY 11-7082 for 30 minutes before treatment with 10 ng/mL TNF for the indicated time.
cFLIP mRNA and protein are not induced in response to TNF plus NF-κB inhibition. (A) Primary monocytes, THP-1 cells, and HL60 cells were treated with BAY 11-7082 (10 μM) before treatment with TNF (10 ng/mL) for the indicated time. RNA was extracted and after reverse transcription, FLIPL mRNA expression was measured by real-time PCR. (B) HO-1 protein expression was measured by Western blot analysis. Cells were treated with 10 μM BAY 11-7082 for 30 minutes before treatment with 10 ng/mL TNF for the indicated time.
HO-1 protects AML cells from TNF-induced cell death
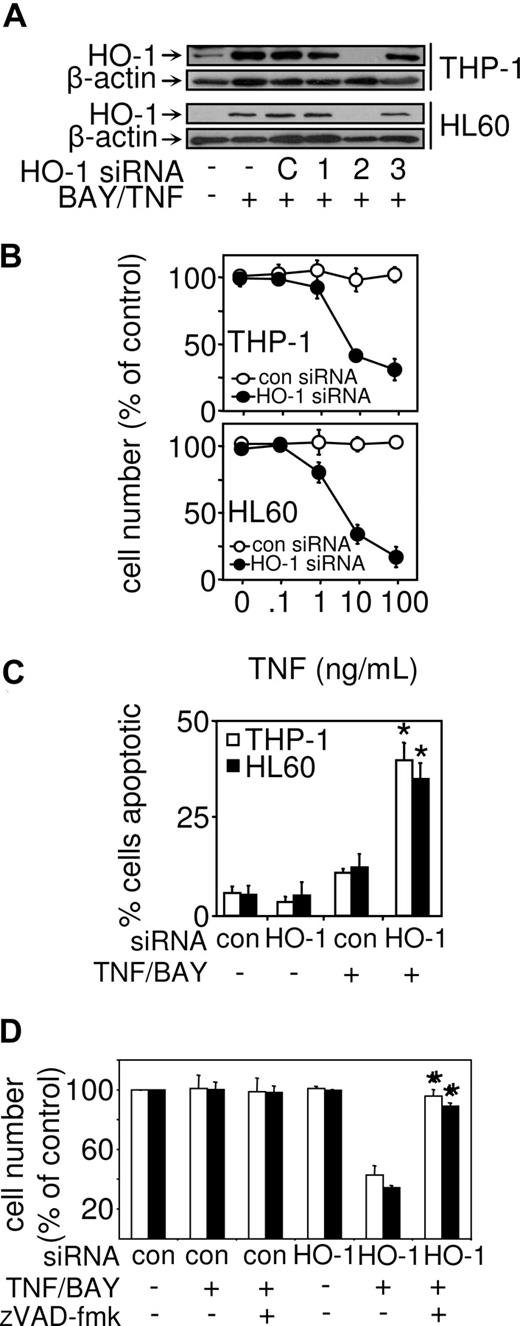
To determine whether HO-1 induction in NF-κB–inhibited TNF-treated cells protects AML-derived cells from cell death, we investigated the role of HO-1 using targeted siRNA. We screened 3 HO-1 siRNAs for their ability to inhibit HO-1 protein expression induced by TNF in conjunction with NF-κB inhibition. Figure 4A demonstrates that HO-1 siRNA-2 had the most significant effect on HO-1 protein expression in both THP-1 and HL60 cells. Transfection of THP-1 and HL60 cells with HO-1 siRNA-2 resulted in these cells becoming susceptible to TNF-induced cell death (Figure 4B), thus demonstrating that HO-1 plays an important role in protecting AML cells from death inducing signals. The mode of TNF-induced cell death of in THP-1 and HL60 cells transfected with HO-1 siRNA-2 was again apoptotic determined by Hoechst 33342 staining (Figure 4C). To establish that cell death observed in HO-1 deficient cells was the result of caspase activation, we used the pan caspase inhibitor zVAD-fmk. Figure 4D shows that, in response to zVAD-fmk, cell death in HO-1–deficient cells was inhibited. This demonstrates that induced HO-1 protects human leukemia cells from caspase-dependent cell death.
Cell death in response to TNF and NF-κB inhibition are restored in myeloid leukemia cells by HO-1 siRNA and is caspase-dependent. (A) Where indicated, THP-1 and HL60 were untransfected, transfected with control siRNA, or with 3 different HO-1 siRNAs for 24 hours and then treated with 10 μM BAY 11-7082 and 10 ng/mL TNF for 8 hours. Protein expression was measured for HO-1 by Western blot analysis. (B) THP-1 cells and HL60 cells were transfected with control siRNA or HO-1 siRNA-2 before treatment with 10 μM of BAY 11-7082 in conjunction with various concentrations of TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments. (C) Apoptotic cells detected by epifluorescence after staining with Hoechst 33342. (D) THP-1 cells and HL60 cells were transfected with control siRNA or HO-1 siRNA-2 before treatment with 50 μM of the caspase inhibitor of zVAD-fmk. Cells were then treated with BAY 11-7082 in conjunction with TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments (*statistical significance, P < .01, between the different treatment groups).
Cell death in response to TNF and NF-κB inhibition are restored in myeloid leukemia cells by HO-1 siRNA and is caspase-dependent. (A) Where indicated, THP-1 and HL60 were untransfected, transfected with control siRNA, or with 3 different HO-1 siRNAs for 24 hours and then treated with 10 μM BAY 11-7082 and 10 ng/mL TNF for 8 hours. Protein expression was measured for HO-1 by Western blot analysis. (B) THP-1 cells and HL60 cells were transfected with control siRNA or HO-1 siRNA-2 before treatment with 10 μM of BAY 11-7082 in conjunction with various concentrations of TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments. (C) Apoptotic cells detected by epifluorescence after staining with Hoechst 33342. (D) THP-1 cells and HL60 cells were transfected with control siRNA or HO-1 siRNA-2 before treatment with 50 μM of the caspase inhibitor of zVAD-fmk. Cells were then treated with BAY 11-7082 in conjunction with TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments (*statistical significance, P < .01, between the different treatment groups).
cFLIP regulates TNF-induced cell death but not when NF-κB is inhibited
To establish the role of cFLIP in regulating cell death in response to TNF in combination with NF-κB inhibition in AML-derived cells, we used cFLIP siRNA to knockdown cFLIP expression. Figure 5A shows that the FLIP siRNA construct 2 had the most significant knockdown in both THP-1 and HL60 cells. Interestingly, we also showed that the use of Nrf2 siRNA and HO-1 siRNA had no effect on cFLIP protein expression (Figure 5A). Transfection of THP-1 and HL60 cells with cFLIP siRNA-2 resulted in the cells becoming sensitive to TNF alone, but not in combination with BAY 11-7082 (Figure 5B). This suggests that cFLIP plays an important protective role in response to TNF, however, when NF-κB is inhibited cFLIP has little or no role to play in regulating cell death responses. Previously, we have shown that cycloheximide (CHX) can inhibit FLIP expression.5 Therefore, to confirm that FLIP has a limited role in regulating apoptosis in our leukemia cells treated with TNF in combination with NF-κB inhibition, we pretreated cells using CHX before stimulation with TNF and BAY 11-7082. Interestingly, treatment with CHX alone and in combination with TNF and BAY 11-7082 induced cell death in THP-1 and HL60 (Figure S2B). Western blot analysis revealed that CHX inhibited both FLIP and Nrf2 expression in leukemia cells (Figure 3C). Taken together, these results demonstrate that FLIP has a limited role in regulating cell death in response to TNF in combination with NF-κB.
TNF cell death responses in human myeloid leukemia cells with cFLIP targeted by siRNA. (A) THP-1 and HL60 were untransfected, transfected with control siRNA, or with HO-1 siRNA, Nrf2 siRNA, and 2 cFLIP siRNA for 24 hours Protein expression for cFLIP was measured by Western blot analysis. (B) THP-1 cells and HL60 cells were transfected with control siRNA or cFLIP siRNA-2 before treatment with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments.
TNF cell death responses in human myeloid leukemia cells with cFLIP targeted by siRNA. (A) THP-1 and HL60 were untransfected, transfected with control siRNA, or with HO-1 siRNA, Nrf2 siRNA, and 2 cFLIP siRNA for 24 hours Protein expression for cFLIP was measured by Western blot analysis. (B) THP-1 cells and HL60 cells were transfected with control siRNA or cFLIP siRNA-2 before treatment with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF for 24 hours. Cell number was then assessed by MTS assay as described in “Proliferation/death and apoptotic assays.” Values represent mean plus or minus SEM from 3 independent experiments.
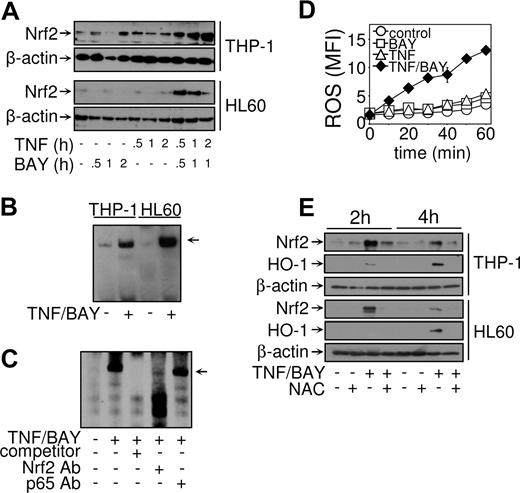
Nrf2 regulates HO-1 expression in NF-κB–inhibited TNF-treated AML cells
Nrf2 is a key transcription factor in the regulation of cytoprotective genes, including HO-1. Nuclear accumulation is an important mechanism for the activation of Nrf2. To examine whether treatment with TNF in conjunction with NF-κB inhibition induced Nrf2 accumulation, THP-1 cells and HL60 cells were treated for various times with TNF or BAY 11-7082 alone, or in combination. Nuclear extracts were prepared and analyzed for Nrf2 expression by Western blot analysis. Figure 6A demonstrates that Nrf2 appeared in the nucleus in response to combined TNF treatment and NF-κB inhibition in both THP-1 and HL60 cells. Furthermore, the kinetics of Nrf2 protein appearance in the nucleus coincided with binding of a complex to the HO-1 ARE (Figure 6B). The binding of this complex was inhibited by the addition of unlabeled oligonucleotide, demonstrating the specificity of the sequence (Figure 6C). The presence of Nrf2 in the complex was confirmed by supershift analysis. Anti-p65/RelA (NF-κB) supershift antibodies were used as a negative control. The complex bound to the HO-1 ARE was abolished with anti-Nrf2 antibodies but not with anti-p65 antibodies (Figure 6C), illustrating a complete role for Nrf2 transcription factor but not NF-κB transcription factor. Because it has been shown that Keap1 regulates the oxidative sensitive activation of Nrf226 and that ROS is known to be produced in response to TNF,3 we hypothesized that Nrf2 activation in this system was induced by ROS. We analyzed the production of ROS in HL60 and THP-1 cells using dichlorodihydrofluorescein diacetate. This showed that, in response to TNF in combination with NF-κB inhibition, both HL60 and THP-1 generated the production of ROS (Figure 6D). Furthermore, when we inhibited the effect of ROS using a N-acetylcysteine (NAC, a ROS quencher) we found that it inhibited the induction of Nrf2 protein accumulation in the nucleus in response to TNF in combination with BAY 11-7082 (Figure 6E). We next examined the effect of NAC on HO-1 expression. Western analysis revealed that HO-1 expression was also inhibited by the addition of NAC when treated with TNF in combination with BAY 11-7082 (Figure 6E). This demonstrates that the production of ROS is responsible for the activation of Nrf2 and subsequent expression of HO-1 in response to TNF-induced cell death signals in AML-derived cell lines.
TNF in conjunction with NF-κB inhibition induces Nrf2 activation. THP-1 cells and HL60 cells were untreated or treated with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF, for the indicated times and nuclear extracts prepared. (A) Western blot analysis was carried out using anti-Nrf2 and anti–β-actin antibodies. (B) Samples treated where indicated with 10 μM BAY 11-7082 30 minutes before treatment with TNF (10 ng/mL) for a further 1 hour. EMSA reactions were performed using biotinylated double-stranded oligonucleotides corresponding to the human HO-1 ARE. (C) An unbiotinylated HO-1 ARE probe was included as a cold competitor (as indicated). Nuclear extracts from THP-1 cells untreated or treated with TNF in conjunction with NF-κB inhibition were left alone or preincubated with 1 μg anti-Nrf2 or anti-p65 (NF-κB) supershift antibodies before EMSA. (D) THP-1 cells and HL60 cells were loaded with 5 μM of DCF-DA before treatment with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF. Cells were then assessed using flow cytometry as described in “Proliferation/death and apoptotic assays.” Mean fluorescence intensity (MFI, arbitrary units) of the generation of ROS is measured. (E) THP-1 cells and HL60 cells were pretreated with 10 mM NAC before treatment with BAY 11-7082 plus TNF. Western blot analysis was carried out using anti-Nrf2, anti HO-1, and anti-β-actin antibodies. Results are representative of similar findings from at least 3 separate experiments.
TNF in conjunction with NF-κB inhibition induces Nrf2 activation. THP-1 cells and HL60 cells were untreated or treated with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF, for the indicated times and nuclear extracts prepared. (A) Western blot analysis was carried out using anti-Nrf2 and anti–β-actin antibodies. (B) Samples treated where indicated with 10 μM BAY 11-7082 30 minutes before treatment with TNF (10 ng/mL) for a further 1 hour. EMSA reactions were performed using biotinylated double-stranded oligonucleotides corresponding to the human HO-1 ARE. (C) An unbiotinylated HO-1 ARE probe was included as a cold competitor (as indicated). Nuclear extracts from THP-1 cells untreated or treated with TNF in conjunction with NF-κB inhibition were left alone or preincubated with 1 μg anti-Nrf2 or anti-p65 (NF-κB) supershift antibodies before EMSA. (D) THP-1 cells and HL60 cells were loaded with 5 μM of DCF-DA before treatment with 10 μM BAY 11-7082, or 10 ng/mL TNF, or BAY 11-7082 plus TNF. Cells were then assessed using flow cytometry as described in “Proliferation/death and apoptotic assays.” Mean fluorescence intensity (MFI, arbitrary units) of the generation of ROS is measured. (E) THP-1 cells and HL60 cells were pretreated with 10 mM NAC before treatment with BAY 11-7082 plus TNF. Western blot analysis was carried out using anti-Nrf2, anti HO-1, and anti-β-actin antibodies. Results are representative of similar findings from at least 3 separate experiments.
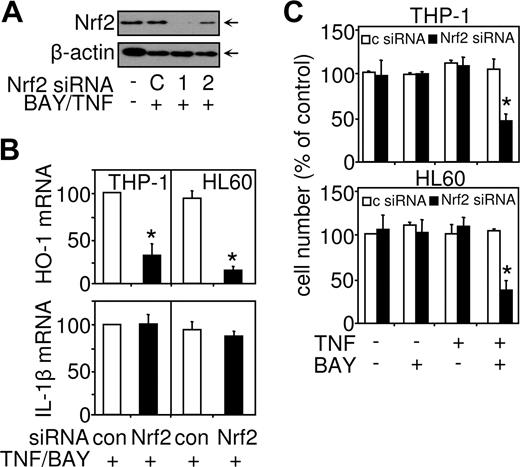
Knockdown of Nrf2 down-regulates HO-1 expression and sensitizes AML cell to TNF-induced cell death
To confirm that Nrf2 is responsible for the induced expression of HO-1 observed after combined TNF treatment plus NF-κB inhibition, we used siRNA constructs to knockdown Nrf2 gene expression (Figure 7). We screened 2 Nrf2 siRNAs for there ability to knock down Nrf2 nuclear protein expression induced by TNF in conjunction with NF-κB inhibition (Figure 5A). Nrf2 siRNA-1 had the most significant effect on Nrf2 nuclear protein expression; therefore, this siRNA was used in subsequent experiments to knock down Nrf2 function. Nrf2 siRNA-1 transfected into THP-1 and HL60 cells inhibited the induction of HO-1 mRNA expression compared with a control siRNA (Figure 5B). The Nrf2-targeted siRNA inhibited HO-1 expression in response to combined TNF treatment plus NF-κB inhibition by approximately 80% in both HL60 and THP-1 cells, compared with control siRNA experiments. These results demonstrate that, in response to TNF treatment in conjunction with NF-κB inhibition, Nrf2 controls the induction of HO-1 in AML-derived cells. Confirmation of the specificity of Nrf2 siRNA was examined by measuring the expression of IL-1b in response to TNF in Nrf2 siRNA transfected cells (Figure 5B). Nrf2 siRNA had no effect on TNF-induced IL-1β mRNA expression, further demonstrating the NF-κB (not Nrf2) dependence of the human IL-1β gene. Furthermore, we used Nrf2 siRNA to determine whether Nrf2 activation by TNF in conjunction with NF-κB inhibition protected AML cells from cell death. Transfection of THP-1 and HL60 cells with Nrf2 siRNA resulted in these cells becoming susceptible to TNF-induced cell death (Figure 5C), thus demonstrating that Nrf2 plays an important role in generating HO-1 protein and protecting AML cells from death-inducing signals.
siRNA-mediated knockdown of Nrf2 results in down-regulation of HO-1–mediated expression and sensitization to TNF-induced cell death. THP-1 cells and HL60 cells were transfected with 30 nM of either control siRNA or Nrf2 siRNA for 24 hours before treatment for 4 hours or 24 hours with TNF (10 ng/mL) alone or in conjunction with 30 minutes preincubated BAY 11-7082 (10 μM). (A) Protein was extracted and Nrf2 or β-actin levels measured by Western blot analysis. (B) mRNA extracts were prepared from the 4-hour-treated cells, reverse transcribed, and HO-1 or IL-1β mRNA expression detected by real-time PCR. (C) Cell number was assessed by MTS assay from 24-hour–TNF-treated cells. Values indicate mean plus or minus SEM from 3 independent experiments (*statistical significance, P < .01, between the different treatment groups).
siRNA-mediated knockdown of Nrf2 results in down-regulation of HO-1–mediated expression and sensitization to TNF-induced cell death. THP-1 cells and HL60 cells were transfected with 30 nM of either control siRNA or Nrf2 siRNA for 24 hours before treatment for 4 hours or 24 hours with TNF (10 ng/mL) alone or in conjunction with 30 minutes preincubated BAY 11-7082 (10 μM). (A) Protein was extracted and Nrf2 or β-actin levels measured by Western blot analysis. (B) mRNA extracts were prepared from the 4-hour-treated cells, reverse transcribed, and HO-1 or IL-1β mRNA expression detected by real-time PCR. (C) Cell number was assessed by MTS assay from 24-hour–TNF-treated cells. Values indicate mean plus or minus SEM from 3 independent experiments (*statistical significance, P < .01, between the different treatment groups).
Discussion
The present study demonstrates that AML-derived cell lines are resistant to TNF-induced cell death signals. The model for studying TNF-induced cell death in which NF-κB is inhibited in conjunction with TNF activation is well established as a system for looking at NF-κB–driven antiapoptotic mechanisms.9,10,43-46 In this study, we show that the cytoprotective gene HO-1 was induced in AML-derived cell lines but not primary white blood cells under NF-κB–inhibited TNF-treated conditions. Moreover, induction of HO-1 protected AML-derived cells from undergoing cell death. In contrast, primary cells underwent apoptosis and did not induce HO-1 expression when treated with TNF in conjunction with NF-κB inhibition. When we blocked HO-1 expression using HO-1 targeted siRNA, AML cells then became susceptible to TNF-induced cell death. Moreover, we showed that TNF, in combination with NF-κB inhibition, generated ROS production in apoptosis-resistant leukemia cells, and this induced activation of the transcription factor Nrf2 to up-regulate HO-1 expression, which subsequently provides cellular resistance to TNF-mediated apoptosis. These results provide novel cancer research insight into the mechanisms of TNF-induced apoptosis and their regulation by HO-1 and Nrf2.
NF-κB is a transcription factor that is known to be involved in the inflammatory and innate immune responses. Although the importance of NF-κB in immunity is undoubted, recent evidence indicates that NF-κB and its associated signaling pathways are also important for tumor development.16 This has led to the development of numerous inhibitors of NF-κB. Small molecules and viral vectors that inhibit IKK, or other aspects of the NF-κB activation pathways, have been shown to induce cell death and inhibit the proliferation of tumors or tumor-derived cell lines.16 At present, the most significant clinical data have been obtained with bortezomib, a proteasome inhibitor, for the treatment of multiple myeloma.47 Moreover, recent data showed that bortezomib and PR-171 have significant antiproliferative and proapoptotic effects on AML cells.48 This work by Stapnes et al showed that the level of cell death on AML cells induced by either bortezomib or PR-171 was between 5% and 20%, suggesting a limited role for these molecules in treating AML.48 In the present study, we showed that inhibiting NF-κB using small molecule inhibitor BAY 11-7082 alone or in conjunction with TNF activation induced at most approximately 25% cell death in 1 of 4 AML-derived cell lines tested; however, generally, these AML cells were dramatically resistant to the cytotoxic effects of TNF. Taken together, this suggests that some, but not all, AML-derived lymphoid tumors would be sensitive to NF-κB targeted inhibition. Indeed, various studies have shown that cancer cells are resistant to drugs, such as bortezomib.49,50 However, this is the first study to show that this cancer cell resistance is directly due to up-regulation of HO-1 and that blockade of this HO-1 pathway reverts cells from a TNF-resistant to a TNF-sensitive phenotype, now allowing near-complete deletion of AML cells to TNF stimuli (Figure 3).
Monocytes play a key role in regulating immune responses and, as such, require defense mechanisms that will protect them from oxidative damage. We have previously shown that proinflammatory mediators as well as NF-κB inhibitors can induce HO-1 in human monocytes.30,41 Induction of HO-1 expression in monocytes is thought to mediate potent anti-inflammatory effects, possibly by restraining them from initiating tissue injury and by modulating their role in inflammatory responses.51 Moreover, HO-1 has been shown to have potent antiapoptotic properties.52 Here we show that HO-1 is induced in AML-derived cell lines in response to NF-κB inhibition in conjunction with TNF activation. Furthermore, this induced expression of HO-1 protects AML-derived cell lines from cell death. HO-1 plays a key role in protecting AML-derived cells from TNF-induced cell death signals and cancer cell apoptosis-resistance and, therefore, would be an ideal target for drug intervention, possibly in combination with NF-κB inhibition.
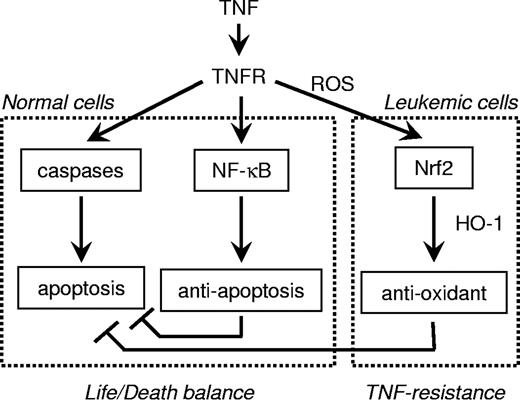
We have recently shown that FLIP can provide some leukemia cells with protection against apoptosis in cells treated with TNF alone.5 Here we demonstrated that FLIP had little or no effect in protecting these AML leukemia-derived cells from TNF-induced cell death signals. This demonstrates a new paradigm shift in the signaling mechanisms controlling the life/death balance coordinated by proapoptotic caspase signals and antiapoptotic NF-κB pathways (Figure 8). These results demonstrate for the first time that TNF resistance of a cancer cell does not involve NF-κB responsiveness but instead the antioxidant pathways. We envisage that apoptosis resistance in cancer cells (that does not involve NF-κB pathways) may arise because of excessive responsiveness of the ARE, which then overprotects the cell from normal oxidative damage and apoptosis signals. Thus, in certain types of neoplastic cells, the knockdown of the ARE pathways seen here (ROS, Nrf2, HO-1) could result in a favorable treatment strategy.
Overview of the mechanism by which human AML leukemia cells resist TNF-induced cell death.
Overview of the mechanism by which human AML leukemia cells resist TNF-induced cell death.
HO-1 belongs to a family of cytoprotective and detoxification genes that possess ARE in their regulatory regions. Regulation of the HO-1 gene is cell type–specific and differs among species.53 In human as well as other species, the Nrf family of transcription factors can bind to the ARE. We have recently shown that LPS can induce HO-1 in human monocytes via Nrf2 activation.30 In addition, others have shown that various proinflammatory mediators can induce HO-1 induction in various cell types through the activation of Nrf2.51 Moreover, several dietary antioxidants and NF-κB inhibitors, including MG132, PDTC, curcumin, and a-lipoic acid, have all been shown to induce HO-1 through Nrf2 activation.29,30,41 These studies here suggest that Nrf2 plays a key role in the induction of HO-1 expression. TNF stimulation or NF-κB inhibition alone had no effect on Nrf2 activation; however, in combination, Nrf2 appeared in the nucleus and bound to the HO-1 ARE by 30 minutes. Nrf2 siRNA not only quenched induced HO-1 mRNA expression but also made the cell susceptible to TNF-induced cell death. This work here suggests that Nrf2 may play an important role in regulating cell death responses in cancer cells.
In summary, we show in AML-derived cells, but not primary cells, that HO-1 is up-regulated in response to TNF stimulation in conjunction with NF-κB inhibition. Furthermore, this induction of HO-1 protects AML cells from cell death signals. The signaling machinery involved in regulating this response includes the transcription factor Nrf2. HO-1 and Nrf2 may protect against the detrimental effects of inflammation and oxidative stress but may also help protect cancerous AML cells from TNF-mediated cell death, resulting in clinically devastating levels of apoptosis resistance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Langa and Dr A. Taylor of the MacEwan laboratory for their suggestions, Dr S. Sexton (University of East Anglia) for help with blood collections and useful discussions, and to Dr J. K. Sethi (University of Cambridge) for the IκBα-DN construct.
This work was supported by a Leukemia Research Fund grant (D.J.M.).
Authorship
Contribution: S.A.R. performed the research; S.A.R. and D.J.M. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. MacEwan, School of Chemical Sciences and Pharmacy, University of East Anglia, Norwich, NR4 7TJ, United Kingdom; e-mail: d.macewan@uea.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal