In this issue of Blood, Kondadasula and colleagues provide important clues for understanding successful cancer immunotherapy by demonstrating an intriguing mechanism through which CD16 synergizes with IL-12 to induce interferon-γ (IFN-γ) production by NK cells.
Mixed response rates to immunotherapies have focused attention on delineating the immunological parameters predictive of positive outcomes. The central role of IFN-γ in the eradication of experimental tumors by immunotherapy has prompted scrutiny of a prime IFN-γ producer, the NK cell. Recent work has demonstrated synergistic production of IFN-γ by NK cells stimulated with IL-12 and CD16 or other NK-cell receptors that signal via immunoreceptor tyrosine-based activation motifs (ITAMs).1,2 Trials of IL-12 and trastuzumab in patients with HER2-overexpressing tumors support a correlation between increased serum IFN-γ and clinical benefit, making mechanistic dissection of synergistic pathways of NK-cell IFN-γ production imperative.3
NK cells can receive a signal to produce IFN-γ through either cytokine receptors or receptors coupled to ITAM-containing signaling chains (ie, CD16 or NKp46). Cytokine signaling activates Janus kinases to phosphorylate STAT proteins, which translocate to the nucleus and affect transcription. In contrast, ITAMs are phosphorylated by Src-family kinases, and recruit either Syk or Zap70 to mediate downstream signaling. Given their distinct signaling properties, it is not surprising that ITAM-signaling receptors like CD16 synergize with IL-12 in the induction of IFN-γ. In contrast, the mechanism of this synergy, revealed in this issue of Blood by Kondadasula and colleagues, was unexpected.
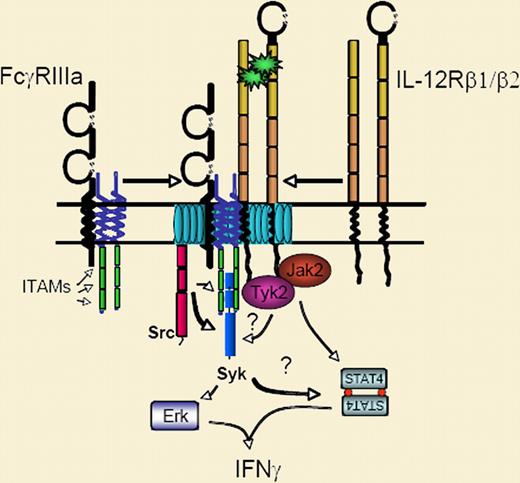
Cholesterol-rich membrane microdomains (also known as “rafts”) are known to be important in the signaling of ITAM-based receptors.4 Here, the authors build a compelling case for the involvement of these domains in IL-12 signaling as well. They show that IL-12 signaling requires lipid rafts and that coengagement of CD16 moves both receptors into these domains. Raft colocalization, in turn, leads to enhanced phosphoryation of Syk, STAT4, and ultimately, ERK (see figure). Although the evidence for the involvement of PI3K is a bit weak, the findings are still reminiscent of the Syk-Erk pathway defined in NK cells by Jiang et al, implying that this axis may be critical in a variety of NK-cell functions.5
Costimulation of natural killer (NK) cells with IL-12 and Fc receptor ligands causes both receptor complexes to partition into lipid rafts (aqua) within the membrane. The result is accentuated activation of STAT4, Syk, and Erk, and synergistic production of IFN-γ.
Costimulation of natural killer (NK) cells with IL-12 and Fc receptor ligands causes both receptor complexes to partition into lipid rafts (aqua) within the membrane. The result is accentuated activation of STAT4, Syk, and Erk, and synergistic production of IFN-γ.
So how does colocalization of IL-12 receptor and CD16 in lipid rafts translate into enhanced activation of Syk and STAT4? The authors suggest that Syk activated by CD16 might facilitate lipid-raft localization of the IL-12 receptor. An alternative explanation could be direct cross-phosphorylation of these receptor complexes. Colocalization in the rafts might drive Src-family kinases to phosphorylate the IL-12 receptor or STAT4. Conversely, perhaps Janus kinases within the IL-12 receptor complex directly phosphorylate the ITAMs associated with CD16. This latter possibility would be consistent with the IL-12–mediated phosphorylation of Syk reported here, as well as the growing evidence demonstrating crosstalk between ITAMs and receptor systems as diverse as integrins and TNF-receptor superfamily members. More work is clearly required to fully understand these phenomena, but as the intricacies of these processes become clearer, important clues for improving the immunotherapy of cancer will likely emerge.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal