Abstract
Natural killer (NK) cells express an activating receptor for the Fc portion of IgG (FcγRIIIa) that mediates interferon (IFN)–γ production in response to antibody (Ab)–coated targets. We have previously demonstrated that NK cells activated with interleukin-12 (IL-12) in the presence of immobilized IgG secrete 10-fold or more higher levels of IFN-γ as compared with stimulation with either agent alone. We examined the intracellular signaling pathways responsible for this synergistic IFN-γ production. NK cells costimulated via the FcR and the IL-12 receptor (IL-12R) exhibited enhanced levels of activated STAT4 and Syk as compared with NK cells stimulated through either receptor alone. Extracellular signal–regulated kinase (ERK) was also synergistically activated under these conditions. Studies with specific chemical inhibitors revealed that the activation of ERK was dependent on the activation of PI3-K, whose activation was dependent on Syk, and that sequential activation of these molecules was required for NK cell IFN-γ production in response to FcR and IL-12 stimulation. Retroviral transfection of ERK1 into primary human NK cells substantially increased IFN-γ production in response to immobilized IgG and IL-12, while transfection of human NK cells with a dominant-negative ERK1 abrogated IFN-γ production. Confocal microscopy and cellular fractionation experiments revealed that FcγRIIIa and the IL-12R colocalized to areas of lipid raft microdomains in response to costimulation with IgG and IL-12. Chemical disruption of lipid rafts inhibited ERK signaling in response to costimulation and significantly inhibited IFN-γ production. These data suggest that dual recruitment of FcγRIIIa and the IL-12R to lipid raft microdomains allows for enhanced activation of downstream signaling events that lead to IFN-γ production.
Introduction
Natural killer (NK) cells are large granular lymphocytes that participate in the innate immune response to virally infected and neoplastic cells.1 While most immune cells express both inhibitory and activating Fc receptors (FcRs), NK cells are unique in that they constitutively express only a low-affinity, activating FcR (FcγRIIIa, or CD16), which enables them to interact with antibody (Ab)–coated targets. In addition to their ability to mediate antibody-dependent cellular cytotoxicity (ADCC), FcR-activated NK cells have also been shown to secrete cytokines such as interferon (IFN)–γ, tumor necrosis factor (TNF)–α, and chemokines that inhibit tumor cell proliferation, enhance antigen presentation, and aid in the chemotaxis of T cells.1
We have previously demonstrated that NK cells secrete enhanced levels of IFN-γ in response to dual stimulation with IL-12 and an FcR stimulus, such as human breast cancer cells coated with an anti-HER2 mAb (trastuzumab).2 NK cells costimulated with Ab-coated tumor cells and IL-12 secreted 10-fold greater amounts of IFN-γ as compared with stimulation with either agent alone. Based on these findings, we initiated a phase 1 clinical trial in which IL-12 was administered with trastuzumab to patients with HER2-overexpressing malignancies.3 Increases in circulating levels of NK cell–derived IFN-γ were observed only in patients who derived a clinical benefit from therapy (defined as a clinical response or stabilization of disease lasting 6 months or more). These findings were confirmed in a subsequent phase 1 trial of trastuzumab plus IL-12 with paclitaxel, in which elevated levels of IFN-γ were detected within the serum of all 11 patients that exhibited clinical benefit, while no IFN-γ was detected within the serum of any patient with progressive disease.4 Based on the potential association between NK cell IFN-γ production and clinical benefit in patients receiving therapeutic mAbs, we investigated the intracellular signaling events that occurred within NK cells following costimulation with Ab-coated targets and IL-12.
Methods
Cytokines and antibodies
Recombinant human IL-12 was provided by Genetics Institute (Cambridge, MA), and was reconstituted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). Polyclonal human IgG (huIgG) was purchased from Sigma-Aldrich (St Louis, MO). The anti-FcγRIIIa mAb (clone 3G8) was obtained from Medarex (Annandale, NJ). Rabbit polyclonal phospho–extracellular signal–regulated kinase (p-ERK), total and p-p38 mitogen-activated protein kinase (MAPK), total and p-Akt, and total Syk Abs, total Lck antibody, and p-Lck (Tyr 505) Abs were purchased from Cell Signaling Technology (Beverly, MA). Goat polyclonal IL-12R anti-ERK mAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti–human p-STAT4 mAb was purchased from Zymed (Carlsbad, CA). Mouse monoclonal antiphosphotyrosine Ab and anti-STAT4 Ab were purchased from Upstate Biotechnology (Charlottesville, VA).
Isolation of human NK cells
NK cells were isolated directly from fresh leukopacks (American Red Cross, Columbus, OH) by 30-minute incubation with RossetteSep NK cell enrichment cocktail (Stem Cell Technologies, Vancouver, BC), followed by Ficoll Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Isolated NK cells (97% CD56+ or greater) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated pooled human AB serum (HAB; C-6 Diagnostics, Germantown, WI), 100 U/mL penicillin, 100 μg/mL streptomyocin, and 0.25 μg/mL amphotericin B (10% HAB medium).
Confocal microscopy
To label lipid raft microdomains, NK cells suspended in PBS plus 1% BSA were activated by cross-linking of FcγRIIIa via sequential treatment with 3G8 monoclonal antibody and AlexaFluor 488–labeled donkey anti–mouse IgG secondary antibody (both at 10 μg/mL; Invitrogen-Molecular Probes, Carlsbad, CA) in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C. Cells were then fixed in 2% paraformaldehyde for 20 minutes at room temperature. Fixed cells were then further stained with goat anti–IL-12R mAb and rabbit anti–p-Lck (Tyr 505) for 30 minutes at room temperature. A second staining step was performed with 1:250 dilutions of AlexaFluor 546–labeled donkey anti-goat and Alexa Fluor 647–labeled donkey anti–rabbit IgG secondary antibodies (Invitrogen-Molecular Probes) for 30 minutes at room temperature to label the respective proteins. For the corresponding control conditions (PBS, IL-12 alone, and FcR cross-linking alone), cells were fixed with 2% paraformaldehyde prior to staining for these 3 markers. Staining with isotype-matched control Abs was used to control for the fluorescent background signals of the 3 stains. Cells were mounted on poly-L-lysine–coated microscope slides and analyzed for lipid raft, FcγRIIIa, and IL-12R cellular distribution using sequential image acquisition (multitrack) and the Zeiss LSM 510 imaging system (Carl Zeiss, Jena, Germany). A total of 20 NK cells were evaluated per field at room temperature for each condition using 100× oil objective with 1.4 numeric aperture. For each label, digital images of the 4 treatment conditions were processed identically using Photoshop software (Adobe Systems, San Jose, CA).
Lipid raft microdomain isolation
NK cells (2 × 107/condition) suspended in serum-free RPMI media were left untreated or stimulated by FcR clustering in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C and then lysed for 10 minutes at 37°C in 0.94 mL of 0.5% Brij-98 lysis buffer (Sigma-Aldrich; 50 mM Tris [pH 8.0], 2 mM EDTA, 10 mM Na4P2O7, 10 mM NaF, 0.5% Brij-98, 150 mM NaCl, 100 mM Na3VO4, 0.5 μg/mL each aprotinin and leupeptin, 1 mM PMSF, and 10 μL/mL phosphatase inhibitors). A total of 0.94 mL of the lysate was mixed with 0.94 mL of 85% sucrose prepared in 0.5% Brij-98 buffer and loaded at the bottom of a 12-mL Beckman centrifuge tube (Beckman Coulter, Fullerton, CA), and carefully overlaid with 6.6 mL of 35% sucrose followed by 3.3 mL of 5% sucrose (both prepared in 0.5% Brij-98 buffer). The gradients were centrifuged at 38 000 rpm (174 000g) for 17 hours at 4°C in a Beckman SW41 rotor (Beckman Coulter). A total of 9 fractions (approximately 1.3 mL each) were collected from the top of the gradient and were used for immunoblotting. The wells of an 8% polyacrylamide gel were loaded with an equal volume of material collected from each fraction. Lipid raft fractions were visualized by probing the membrane with rabbit anti-human Lck antibody, and rabbit anti-human p-Lck (Tyr 505) antibody (“Results”). IL-12R and FcγRIIIa were visualized by immunoblotting with rabbit anti–IL-12R (Santa Cruz) and mouse monoclonal 3G8 antibodies, respectively. Lipid raft domains were recovered from the low-density fractions 2, 3, and 4.
In vitro costimulation assays
For immobilized IgG experiments, wells of a 96-well flat-bottom plate were coated with 100 μg/mL huIgG in cold PBS overnight at 4°C, washed with cold PBS, and then plated with immune cells (2 × 105 cells/well) and 10 ng/mL IL-12, as described previously.2 At the indicated time points, cell-free culture supernatants were harvested and analyzed for levels of IFN-γ by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). In signaling inhibition experiments, NK cells were pretreated overnight with DMSO (vehicle control) or specific chemical inhibitors of signal transduction molecules prior to addition onto IgG-coated wells. NK cells were checked for viability following the overnight pretreatment period and again at the conclusion of the experiment. Cell viability in all cases was greater than 90%. Chemical inhibitors were obtained from Calbiochem (La Jolla, CA), and included U0126 (ERK inhibitor), picetannol (Syk inhibitor), 1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (Akt inhibitor), anthra[1,9cd]pyrazol-6(2H)-1,9-pyrazolo anthrone (Jnk inhibitor), and wortmannin (PI3-K inhibitor).
Intracellular flow cytometry
Intracellular levels of IFN-γ within NK cells were detected using a FITC-conjugated mAb to human IFN-γ and a PE-conjugated mAb to the NK cell marker CD56 (both from BD Pharmingen, San Diego, CA) as previously described.2 The percentage of positively staining cells and mean fluorescence intensity (MFI) were calculated for the specified cell populations.
Immunoprecipitation and immunoblotting
FcγRIIIa on human NK cells were activated by sequential treatment with F(ab′)2 fragments of monoclonal antibody 3G8 and goat F(ab′)2 anti–mouse Ig secondary antibody (both at 10 μg/mL), in the presence of 50 ng/mL IL-12 (cell concentration = 107/mL). Resting and activated cells were lysed in TN1 buffer (50 mM-Tris (pm.8.0), 10 mM EDTA, 10 mM Na4P2O7, 10 mM NaF, 1% Triton-x-100, 125 mM NaCl, 10 mM Na3VO4, 10 μg/mL each aprotinin and Leupeptin), and postnuclear lysates were boiled in an equal volume of 2 × SDS sample buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 10% glycerol, 0.01% bromphenol blue, and 2% 2-mercaptoethanol) for 5 minutes prior to SDS–polyacrylamide gel electrophoresis (PAGE). For immunoprecipitation experiments, postnuclear lysates were incubated overnight with the primary antibody of interest or an isotype-matched control Ab and 35 μL protein G–agarose beads (Invitrogen-Molecular Probes). Immune complexes bound to beads were washed in TN1 and boiled in SDS sample buffer for 5 minutes. Proteins were separated by SDS-PAGE, transferred to nitrocellulose filters, probed with the primary Ab of interest, and developed by enhanced chemiluminescence (ECL). The ECL signal was quantitated by densitometry. To evaluate the phosphospecific signal in activated samples, signals were normalized to the amount of total target protein, and values were plotted as a fold increase over the levels in unstimulated samples.
Plasmid construction
Human ERK1 (GenBank5 accession no. NM_002746) was amplified by reverse-transcriptase polymerase chain reaction (PCR) from human peripheral blood mononuclear cell cDNA using the following primers: hERK1startBamH1, 5′-TAGGATCCATGGCGGCGGCGGCGGCTCA-3′; and hERK1stopEcoR1, 5′-TTGAATTCCTAGGGGGCCTCCAGCAC-TCCG-3′. The amplified 1.1-kb fragments were cloned into the PCR2.1 vector (Invitrogen). Site-directed mutagenesis was then applied to reverse-mutated nucleotides caused by PCR amplification. The BamH1-EcoR1 fragment excised from the PCR2.1 vectors were subcloned into the same restriction sites of the Epstein-Barr virus (EBV)/retroviral hybrid PINCO vector6 to create PINCO-ERK1. The dominant-negative (DN) ERK1K71R mutant has been described.7 ERK1K71R was amplified from a MigR1 backbone (a kind gift from Dr Danilo Perrotti, Columbus, OH) using the same primer pair and cloned directly into the PINCO vector. The ERK1 and ERK1K71R cDNA sequences were confirmed by DNA sequencing in both the PCR2.1 vector and the PINCO vector. The PINCO vector was kindly provided by Dr Martin Sattler (Harvard University, Cambridge, MA).
Retroviral transfection of human NK cells
ERK1-containing vectors were transiently transfected into the Phoenix-Ampho viral packaging cell line by the calcium phosphate cloroquine method, as previously described.8 Culture supernatants containing the viral particles were collected after 24 hours. Primary human NK cells were then infected with PINCO-ERK1, PINCO-ERK1K71R, or with the empty PINCO vector by culture with viral supernatants supplemented with 4 μg/mL polybrene (Sigma-Aldrich) and 900 IU/mL IL-2 (Roche Pharmaceuticals, Nutley, NJ), as described.8 Cells were then evaluated by flow cytometry for green fluorescent protein (GFP) fluorescence. Although the efficacy of infection was routinely less than 5%, a more than 60% pure population of infected cells was acquired by cell sorting on the basis of GFP expression. The viability of infected cells was greater than 95%. Culture of NK cells with IL-2 during the infection process up-regulated the NK cell surface expression of CD56, but did not affect the expression of FcγRIIIa (data not shown). ERK expression in the infected cell populations was confirmed by real-time PCR with ERK-specific primers. Equal numbers of GFP+ NK cells were plated on immobilized IgG in the presence of 10 ng/mL IL-12, and culture supernatants were harvested at 72 hours for quantification of IFN-γ release.
Statistics
Statistical analysis of ELISA cytokine levels were performed using a paired t test, with P levels less than .05 considered significant. In immunoblotting experiments, statistical analysis of fold increases in phosphoprotein were performed using the Student t test, with P levels less than .05 considered significant.
Results
Both FcγRIIIa and IL-12R localize to lipid raft domains on NK cells in response to FcR activation in the presence of IL-12
Lipid raft microdomains have been shown to function as specialized signaling platforms within activated immune cells. We therefore examined the spatial localization of FcγRIIIa and IL-12R with respect to lipid raft microdomains in both resting NK cells and in NK cells that had been activated by FcR cross-linking in the presence of IL-12. Previous studies indicated that Src family protein tyrosine kinases such as Lck are anchored in the cytoplasmic leaflet of raft domains and are thus enriched in the detergent-insoluble lipid rafts.9,10 A report by Chow et al showed that Lck is constitutively phosphorylated in its carboxy terminus at Tyr 505.11 Taking into account these studies, we used anti–human p-Lck (Tyr 505) in combination with a fluorescent label to identify the detergent insoluble lipid raft microdomains. Following fixation, cells were labeled with antibodies to p-Lck, FcγRIIIa, and IL-12R and examined by confocal microscopy. FcγRIIIa and IL-12R both stained diffusely around the cell membrane of unstimulated NK cells (Figure 1Ai), whereas lipid raft microdomains (as indicated by the presence of p-Lck) had a characteristic patchy staining pattern. The FcγRIIIa in non–cross-linked cells was only barely detectable when the microscope settings were calibrated for the detection of FcγRIIIa in stimulated cells (Figure 1Ai,iii). FcR cross-linking resulted in redistribution of the FcγRIIIa in discrete patches at the membrane, although the distribution of IL-12R was unaffected. In addition, p-Lck was seen to localize to the cytoplasmic aspect of the cell membrane in regions of FcR accumulation (Figure 1Aii merge). These results are consistent with previous reports documenting FcγRIIIa migration into lipid rafts following receptor activation.12 In contrast, NK cells stimulated with IL-12 alone did not exhibit any significant relocalization of the IL-12R, FcR, or lipid raft microdomains (Figure 1Aiii). Interestingly, upon costimulation of NK cells with IL-12 and FcR cross-linking, IL-12R was found in large patches which colocalized to FcR and p-Lck (Figure 1Aiv). These data reveal a possible spatial mechanism for the cross-talk between FcR and cytokine signaling pathways in primary human NK cells. In order to confirm the colocalization of FcγRIIIa and IL-12R observed in the confocal experiments, sucrose density gradient centrifugation was used to isolate membrane components from both resting and costimulated NK cells. Sequential fractions were separated by gel electrophoresis and probed for Lck, p-Lck, FcγRIIIa, and the IL-12R. As shown in Figure 1B, lipid rafts were detected primarily in fractions 2, 3, and 4 in both resting and costimulated NK cells (Figure 1B first and second rows). FcγRIIIa became associated with lipid rafts following costimulation of NK cells (Figure 1B third row). Likewise, IL-12R translocated to the lipid fractions following coactivation of FcγRIIIa and the IL-12R (Figure 1B fourth row). These data further support our observation that FcγRIIIa and the IL-12R redistribute to areas of lipid raft microdomains following dual stimulation.
Both FcγRIIIa and IL-12R localize to lipid raft domains on NK cells in response to FcR activation in the presence of IL-12. (A) Purified human NK cells were activated via FcγRIIIa ligation in the presence of huIL-12 for 5 minutes at 37°C (column iv). Control conditions consisted of untreated NK cells (column i), NK cells activated via FcR ligation alone (column ii), or NK cells activated with IL-12 alone (column iii). The green channel shows the location of FcγRIIIa and the red channel shows the distribution of IL-12R, while the blue channels represents p-Lck (a component of lipid raft microdomains11 ). Single confocal sections of the cells were captured in multitrack. Each set of frames in a given column is a representative individual NK cell selected from 1 of 20 analyzed cells. Superimposed white-yellow patches signify areas of colocalization of FcγRIIIa IL-12R and p-Lck. (B) Detergent-insoluble cholesterol-enriched lipid rafts were prepared by Brij-98 extraction of purified human NK cells that were left unstimulated (left column) or activated via FcγRIIIa ligation in the presence of IL-12 for 5 minutes (right column) and separated on a sucrose density gradient. A total of 9 sequential fractions collected from the gradient were subjected to immunoblot analysis for p-Lck, FcγRIIIa, and IL-12R. Fractions 2, 3, and 4 correspond to detergent-insoluble lipid fractions; fractions 7, 8, and 9 correspond to detergent-soluble fractions. All results shown are representative of 3 independent experiments.
Both FcγRIIIa and IL-12R localize to lipid raft domains on NK cells in response to FcR activation in the presence of IL-12. (A) Purified human NK cells were activated via FcγRIIIa ligation in the presence of huIL-12 for 5 minutes at 37°C (column iv). Control conditions consisted of untreated NK cells (column i), NK cells activated via FcR ligation alone (column ii), or NK cells activated with IL-12 alone (column iii). The green channel shows the location of FcγRIIIa and the red channel shows the distribution of IL-12R, while the blue channels represents p-Lck (a component of lipid raft microdomains11 ). Single confocal sections of the cells were captured in multitrack. Each set of frames in a given column is a representative individual NK cell selected from 1 of 20 analyzed cells. Superimposed white-yellow patches signify areas of colocalization of FcγRIIIa IL-12R and p-Lck. (B) Detergent-insoluble cholesterol-enriched lipid rafts were prepared by Brij-98 extraction of purified human NK cells that were left unstimulated (left column) or activated via FcγRIIIa ligation in the presence of IL-12 for 5 minutes (right column) and separated on a sucrose density gradient. A total of 9 sequential fractions collected from the gradient were subjected to immunoblot analysis for p-Lck, FcγRIIIa, and IL-12R. Fractions 2, 3, and 4 correspond to detergent-insoluble lipid fractions; fractions 7, 8, and 9 correspond to detergent-soluble fractions. All results shown are representative of 3 independent experiments.
Disruption of lipid raft microdomains severely attenuates NK cell IFN-γ production
We next wanted to determine whether lipid raft microdomains were required for the synergistic IFN-γ production that was observed following costimulation of NK cells with IL-12 and immobilized IgG. Primary human NK cells were pretreated with methyl-β-cyclodextrin (MβCD), a synthetic molecule that disrupts lipid rafts by sequestering cholesterol moieties. Following MβCD pretreatment, NK cells were cultured on plates that had been precoated with immobilized IgG in medium containing IL-12. After 12 hours of culture, NK cells were analyzed for levels of IFN-γ by intracellular flow cytometry. As shown in the top row of Figure 2A, the combination of immobilized IgG and IL-12 resulted in a significantly increased number of NK cells secreting IFN-γ versus either condition alone. Disruption of lipid raft domains by MβCD pretreatment led to a significant reduction in IFN-γ production by FcR and IL-12 costimulated NK cells (Figure 2A middle row). Reconstitution of cholesterol moieties to NK cells following MβCD pretreatment rescued the ability of NK cells to produce IFN-γ following costimulation (Figure 2A bottom row). In order to confirm the effect of MβCD on IFN-γ production, MβCD-treated NK cells were stimulated with immobilized IgG and IL-12, and IFN-γ production was measured after 24 hours of culture. As shown in Figure 2B, MβCD pretreatment severely attenuated IFN-γ production from NK cells costimulated with immobilized IgG and IL-12, suggesting a functional role for lipid raft microdomains in mediating IFN-γ production in human NK cells.
Disruption of lipid raft microdomains inhibits NK cell IFN-γ production in response to immobilized IgG and IL-12. (A) Purified human NK cells from a healthy donor were pretreated with MβCD (a cholesterol chelator) and then plated onto flat-bottom wells that were precoated overnight with 100 μg/mL human IgG (ie, immobilized Ab) in medium containing 10 ng/mL IL-12. Control conditions consisted of NK cells cultured with medium alone (Medium), immobilized IgG alone (IgG), or IL-12 alone (IL-12). NK cells were cultured with brefeldin-A for 12 hours and analyzed for IFN-γ production by intracellular flow cytometry. In another arm of the same experiment, NK cells that had been pretreated with MβCD were reconstituted with cholesterol prior to use in the immobilized IgG assay. The percentage of NK cells actively producing IFN-γ is shown within each dot plot. Results from a representative donor are shown (n = 4 donors tested). (B) NK cells pretreated with MβCD were used in an immobilized IgG assay with 10 ng/mL IL-12, as described in panel A. Cell culture supernatants were harvested after 24 hours and analyzed for IFN-γ content by ELISA. Results depict the mean plus or minus SEM of 4 donors tested. *P < .005 versus mock pretreatment (Mock Pre-Tx) control for the same stimulation condition.
Disruption of lipid raft microdomains inhibits NK cell IFN-γ production in response to immobilized IgG and IL-12. (A) Purified human NK cells from a healthy donor were pretreated with MβCD (a cholesterol chelator) and then plated onto flat-bottom wells that were precoated overnight with 100 μg/mL human IgG (ie, immobilized Ab) in medium containing 10 ng/mL IL-12. Control conditions consisted of NK cells cultured with medium alone (Medium), immobilized IgG alone (IgG), or IL-12 alone (IL-12). NK cells were cultured with brefeldin-A for 12 hours and analyzed for IFN-γ production by intracellular flow cytometry. In another arm of the same experiment, NK cells that had been pretreated with MβCD were reconstituted with cholesterol prior to use in the immobilized IgG assay. The percentage of NK cells actively producing IFN-γ is shown within each dot plot. Results from a representative donor are shown (n = 4 donors tested). (B) NK cells pretreated with MβCD were used in an immobilized IgG assay with 10 ng/mL IL-12, as described in panel A. Cell culture supernatants were harvested after 24 hours and analyzed for IFN-γ content by ELISA. Results depict the mean plus or minus SEM of 4 donors tested. *P < .005 versus mock pretreatment (Mock Pre-Tx) control for the same stimulation condition.
FcR stimulation of NK cells in the presence of IL-12 leads to enhanced activation of Syk and Erk, but not p38 MAPK, Jnk MAPK, or Akt
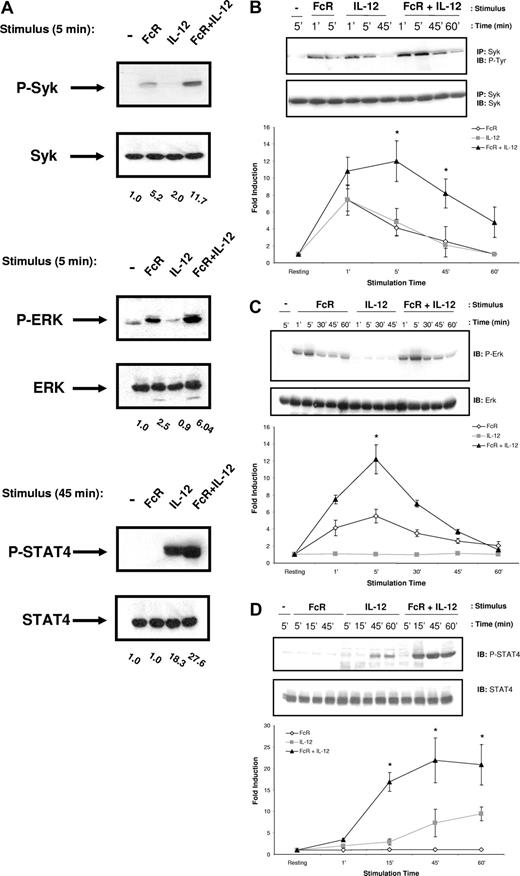
Lipid raft microdomains have been shown to be important in the amplification of activating signals downstream of several immunoreceptors, including the T-cell receptor (TCR).13 We therefore hypothesized that colocalization of FcγRIIIa and IL-12 to lipid raft microdomains following costimulation might allow for enhanced activation of signaling molecules associated with the receptors. In order to identify the signaling intermediates within human NK cells that are up-regulated in response to FcR and IL-12 stimulation, NK cells were activated by FcR cross-linking in the presence of IL-12, lysed at various time points, and subjected to immunoblot analysis. Control conditions consisted of resting NK cells and NK cells that were activated by FcR cross-linking or IL-12 treatment alone. Lysates were probed for activated proteins known to be involved in either FcR or IL-12R signaling, most notably STAT4, the Syk tyrosine kinase, members of the PI3-K/Akt family of signaling proteins, and MAPK family members. Enhanced activation of Syk and ERK was observed at early time points following FcR and IL-12 costimulation (Figure 3A). Levels of p-STAT4 were elevated at later time points in response to costimulation (Figure 3A). In contrast, Akt and p38 MAPK were activated in response to IL-12 alone, but levels were not significantly enhanced by the addition of FcR stimulation (data not shown). Levels of activated Jnk protein kinase were not detected under any of the stimulation conditions examined (data not shown). We next conducted a series of time-course experiments in order to examine the duration of activation of these signaling intermediates. Syk activation was detected as early as 1 minute after stimulation, peaked at 5 minutes after stimulation, and returned to baseline by approximately 1 hour (Figure 3B). A similar pattern of activation was detected for ERK phosphorylation (Figure 3C). In contrast, STAT4 activation was not detected until 30 minutes after stimulation (Figure 3D). p-STAT4 levels remained elevated at 1 hour after stimulation, and did not return to baseline until approximately 2 hours (data not shown).
Costimulation of NK cells leads to enhanced activation of Syk and ERK, but not p38, Jnk, or Akt. (A) Purified human NK cells (more than 97% CD56+/CD3−) underwent FcγRIIIa cross-linking by sequential treatment with an F(ab′)2 fragment of a mouseanti–human FcγRIIIa Ab (clone 3G8) and a secondary F(ab′)2 goat anti-mouse Ab. Recombinant human IL-12 was added at a concentration of 10 ng/mL at 37°C. Control conditions consisted of control Ab-treated NK cells (−), NK cells activated via FcR ligation alone (FcR), or NK cells activated with IL-12 alone (IL-12). Following stimulation, NK cells were lysed at the indicated time points for immunoblot analysis of p-Syk, p-ERK, p-STAT4, or p-Akt. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total protein and expressed in relative densitometric units. (B-D) Time course for the activation of Syk, ERK, and STAT4 in NK cells stimulated as described in panel A. Levels of each phosphoprotein were quantified via densitometry and plotted as fold increase in phosphoprotein normalized for total protein versus time for each treatment condition. Immunoblots depict results from one representative donor. Graphs below immunoblots represent the mean plus or minus SEM from all 5 donors tested. *P < .05 versus the fold induction with IL-12 or FcR cross-linking alone at the time point.
Costimulation of NK cells leads to enhanced activation of Syk and ERK, but not p38, Jnk, or Akt. (A) Purified human NK cells (more than 97% CD56+/CD3−) underwent FcγRIIIa cross-linking by sequential treatment with an F(ab′)2 fragment of a mouseanti–human FcγRIIIa Ab (clone 3G8) and a secondary F(ab′)2 goat anti-mouse Ab. Recombinant human IL-12 was added at a concentration of 10 ng/mL at 37°C. Control conditions consisted of control Ab-treated NK cells (−), NK cells activated via FcR ligation alone (FcR), or NK cells activated with IL-12 alone (IL-12). Following stimulation, NK cells were lysed at the indicated time points for immunoblot analysis of p-Syk, p-ERK, p-STAT4, or p-Akt. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total protein and expressed in relative densitometric units. (B-D) Time course for the activation of Syk, ERK, and STAT4 in NK cells stimulated as described in panel A. Levels of each phosphoprotein were quantified via densitometry and plotted as fold increase in phosphoprotein normalized for total protein versus time for each treatment condition. Immunoblots depict results from one representative donor. Graphs below immunoblots represent the mean plus or minus SEM from all 5 donors tested. *P < .05 versus the fold induction with IL-12 or FcR cross-linking alone at the time point.
Inhibition of ERK prevents IFN-γ production in response to stimulation with IgG and IL-12
In order to determine whether these signaling intermediates were involved in NK cell IFN-γ production following FcR and IL-12 stimulation, NK cells were pretreated overnight with specific chemical inhibitors prior to stimulation with IL-12, immobilized IgG, or the combination. NK cell IFN-γ production in response to IgG and IL-12 was found to be dependent on the activity of Syk (Figure 4A), PI3-K (Figure 4B), and ERK (Figure 4C), but not Akt, Jnk, or p38 MAPK (Figure 4D; data not shown). Notably, even partial inhibition of the ERK MAPK, achieved with low concentrations of the ERK inhibitor U0126, almost completely abrogated NK cell IFN-γ secretion. Similar results were obtained using a second ERK inhibitor, PD098059 (data not shown). Immunoblot analysis of activated protein levels was conducted in response to positive control stimuli in order to confirm the activity of the chemical inhibitors. As shown in Figure 4E, pretreatment of NK cells with the Akt, Jnk, and p38 MAPK inhibitors was successful in preventing activation of these signaling molecules in response to control stimuli, confirming that that the failure of these inhibitors to diminish NK cell IFN-γ production in response to IgG and IL-12 was not due to inactivity of the chemical inhibitors or diminished activity of these signaling pathways in NK cells.
IFN-γ production by FcR-stimulated NK cells in the presence of IL-12 is dependent upon activated Syk, PI-3K, and the ERK MAPK, but not p38, Jnk, or Akt. NK cells were pretreated with increasing concentrations of biochemical inhibitors of (A) Syk (piceatannol), (B) PI3-K (wortmannin), (C) ERK (U0126), or (D) Akt (Akt inhibitor; Calbiochem), and then plated in the immobilized IgG + IL-12 assay. Culture supernatants were harvested after 24 hours and analyzed for IFN-γ content by ELISA. Cells were counted via trypan blue exclusion following inhibitor pretreatment and following the IgG + IL-12 culture period to ensure equal viability of control-treated and inhibitor-treated NK cells. Pretreatment of NK cells with inhibitors of the p38 MAPK or Jnk MAPK had no effect on IFN-γ production in response to immobilized IgG and IL-12 (data not shown; similar results as with Akt inhibitor). (E) NK cells were pretreated with the indicated inhibitor and activated with various control stimuli (5 nM IL-2 or 50 ng/mL PMA plus 500 ng/mL ionomycin) for 10 minutes at 37°C. Cells were lysed, and levels of each phosphoprotein and total protein were measured by immmunoblot analysis. *P < .05 versus DMSO control for the IgG + IL-12 stimulation condition. Results represent the mean plus or minus SEM from n = 5 determinations.
IFN-γ production by FcR-stimulated NK cells in the presence of IL-12 is dependent upon activated Syk, PI-3K, and the ERK MAPK, but not p38, Jnk, or Akt. NK cells were pretreated with increasing concentrations of biochemical inhibitors of (A) Syk (piceatannol), (B) PI3-K (wortmannin), (C) ERK (U0126), or (D) Akt (Akt inhibitor; Calbiochem), and then plated in the immobilized IgG + IL-12 assay. Culture supernatants were harvested after 24 hours and analyzed for IFN-γ content by ELISA. Cells were counted via trypan blue exclusion following inhibitor pretreatment and following the IgG + IL-12 culture period to ensure equal viability of control-treated and inhibitor-treated NK cells. Pretreatment of NK cells with inhibitors of the p38 MAPK or Jnk MAPK had no effect on IFN-γ production in response to immobilized IgG and IL-12 (data not shown; similar results as with Akt inhibitor). (E) NK cells were pretreated with the indicated inhibitor and activated with various control stimuli (5 nM IL-2 or 50 ng/mL PMA plus 500 ng/mL ionomycin) for 10 minutes at 37°C. Cells were lysed, and levels of each phosphoprotein and total protein were measured by immmunoblot analysis. *P < .05 versus DMSO control for the IgG + IL-12 stimulation condition. Results represent the mean plus or minus SEM from n = 5 determinations.
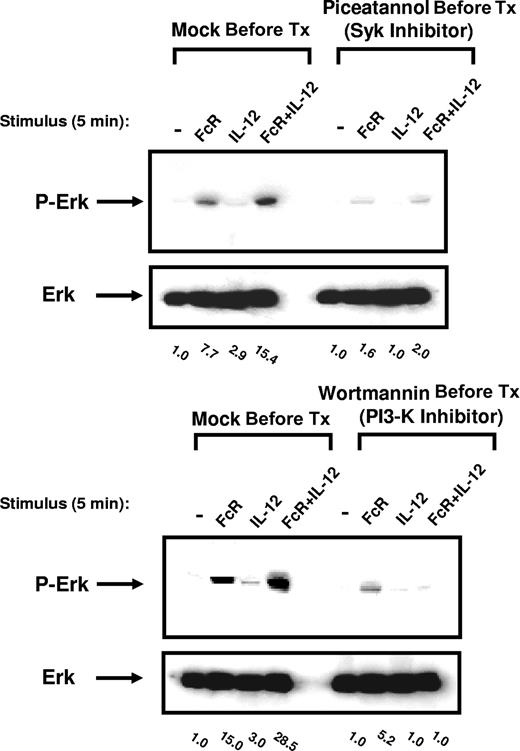
ERK activation in FcR- and IL-12–stimulated NK cells is dependent on Syk and PI3-K
Syk has recently been shown to regulate ERK activity in NK receptor (NKR)–stimulated NK cells through its effects on PI3-K.14 In order to determine the relationship between Syk, PI3-K, and ERK signal transduction following FcR and IL-12 costimulation, ERK activation was assessed in primary NK cells following pretreatment with the Syk inhibitor piceatannol or the PI3-K inhibitor wortmannin. Inhibition of either Syk or PI3-K significantly attenuated ERK activation following FcR and IL-12 costimulation, suggesting that activation of ERK following FcR and IL-12 costimulation is dependent on the activation of Syk and PI3-K (Figure 5). Conversely, inhibition of either PI3-K or ERK had no effect on Syk phosphorylation in response to FcR and IL-12 stimulation (data not shown). These results suggest that Syk lies upstream of PI3-K in this signaling cascade, and that PI3-K in turn lies upstream of ERK.
Inhibition of PI3-K or the Syk protein tyrosine kinase within NK cells prevents ERK activation following FcR and IL-12 stimulation. NK cells were pretreated overnight with 25 μM picetannol (Syk inhibitor) or wortmannin (PI3-K inhibitor) and then activated via FcγRIIIa cross-linking in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C. Whole-cell lysates were probed for levels of p-ERK and total ERK, as indicated. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total ERK and expressed in relative densitometric units. All results are representative of n = 5 healthy donors.
Inhibition of PI3-K or the Syk protein tyrosine kinase within NK cells prevents ERK activation following FcR and IL-12 stimulation. NK cells were pretreated overnight with 25 μM picetannol (Syk inhibitor) or wortmannin (PI3-K inhibitor) and then activated via FcγRIIIa cross-linking in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C. Whole-cell lysates were probed for levels of p-ERK and total ERK, as indicated. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total ERK and expressed in relative densitometric units. All results are representative of n = 5 healthy donors.
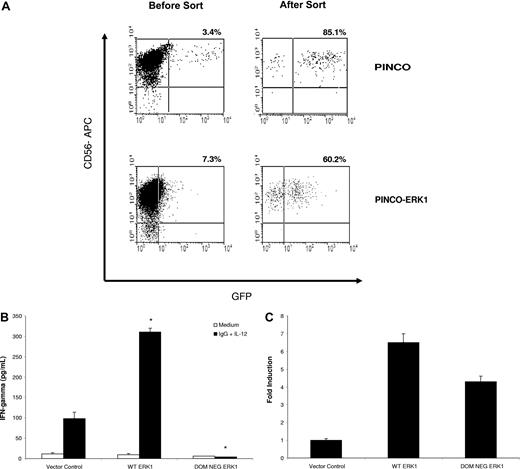
Overexpression of ERK within primary human NK cells enhances IFN-γ secretion in response to immobilized IgG and IL-12
In order to confirm the involvement of ERK in mediating NK cell IFN-γ secretion in response to immobilized IgG and IL-12, primary human NK cells were transduced with retroviral constructs expressing wild-type ERK1 or a DN form of ERK1. Overexpression of wild-type ERK1 via retroviral transduction of primary human NK cells (Figure 6A) significantly increased NK cell IFN-γ production in response to costimulation with immobilized IgG and IL-12, as compared with NK cells transduced with the empty vector (Figure 6B). In contrast, overexpression of DN ERK1 significantly reduced NK cell IFN-γ production in response to costimulation (Figure 6B). ERK expression in transfected cells was confirmed by real-time PCR using ERK-specific primers (Figure 6C). Due to the low numbers of NK cells obtained following cell sorting (20-25 000 GFP+ cells), we were unable to confirm ERK protein expression within transduced NK cells. However, recent work by our group has demonstrated that GFP fluorescence within infected NK cells serves as an adequate surrogate for protein expression under the conditions of the PINCO viral transfection system.15 Furthermore, the low numbers of NK cells obtained in these experiments precluded us from evaluating the effect of ERK overexpression on IFN-γ production in response to stimulation with IL-12 alone or immobilized IgG alone. However, in separate experiments, stimulation of ERK-overexpressing NK cells with IL-12 alone did lead to the expected enhancement of IFN-γ production, as compared with cells treated with the control vector (60 ± 5 pg/mL vs 20 ± 2 pg/mL). Likewise, ERK overexpression also increased IFN-γ production from NK cells stimulated with immobilized IgG alone (70 ± 16 pg/mL vs 15 ± 7 pg/mL in vector control cells). These data indicate that activation of ERK leads to IFN-γ production in NK cells costimulated through FcγRIIIa and IL-12R.
Overexpression of ERK1 within primary human NK cells increases IFN-γ production in response to immobilized IgG and IL-12. Primary human NK cells were transduced with wild-type ERK1 or a DN form of ERK1 using the PINCO retroviral transfection system. Following infection, NK cells were sorted on the basis of GFP expression. (A) Representative flow plots from NK cells transduced with the empty vector or with wild-type ERK1 prior to sorting and following sorting for GFP+ cells. (B) Equal numbers of sorted NK cells were cultured separately on immobilized IgG in the presence of 10 ng/mL IL-12. Culture supernatants were harvested at 24 hours for quantification of IFN-γ release by ELISA. (C) Real-time PCR of sorted NK cells for ERK mRNA expression. Results represent the mean plus or minus SEM from n = 3 independent experiments. *P < .01 versus vector control cells.
Overexpression of ERK1 within primary human NK cells increases IFN-γ production in response to immobilized IgG and IL-12. Primary human NK cells were transduced with wild-type ERK1 or a DN form of ERK1 using the PINCO retroviral transfection system. Following infection, NK cells were sorted on the basis of GFP expression. (A) Representative flow plots from NK cells transduced with the empty vector or with wild-type ERK1 prior to sorting and following sorting for GFP+ cells. (B) Equal numbers of sorted NK cells were cultured separately on immobilized IgG in the presence of 10 ng/mL IL-12. Culture supernatants were harvested at 24 hours for quantification of IFN-γ release by ELISA. (C) Real-time PCR of sorted NK cells for ERK mRNA expression. Results represent the mean plus or minus SEM from n = 3 independent experiments. *P < .01 versus vector control cells.
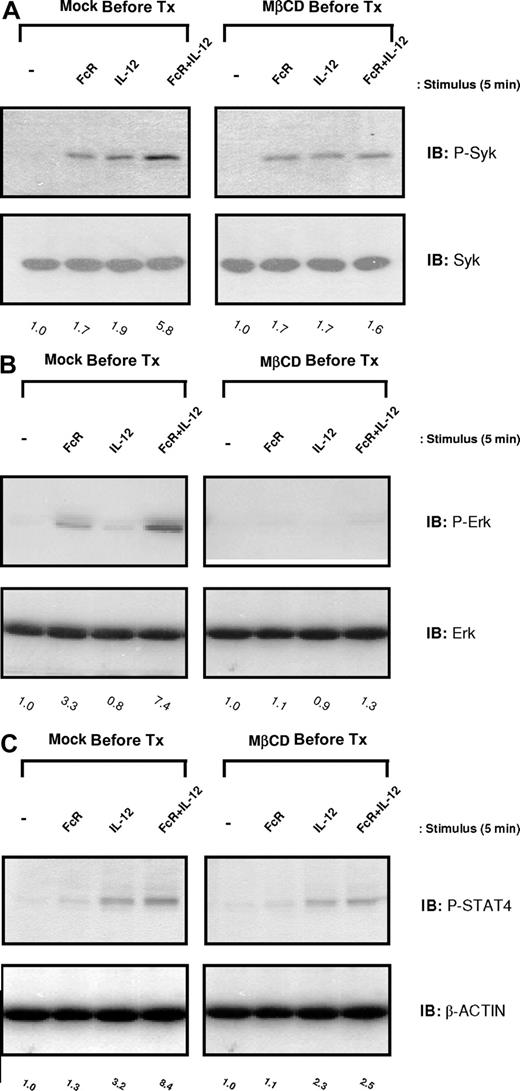
Inhibition of lipid raft microdomains by cholesterol depletion attenuates ERK activation and IFN-γ production within NK cells
In order to determine whether lipid raft domains were essential for the initiation of the NK cell signaling cascade that led to IFN-γ production, levels of activated Syk and ERK were examined in NK cells that had been pretreated with MβCD prior to FcR and IL-12 costimulation. NK cells pretreated with DMSO (vehicle control) exhibited enhanced levels of activated Syk, ERK, and STAT4 following FcR cross-linking in the presence of IL-12, consistent with previous results. In contrast, disruption of lipid raft domains by MβCD pretreatment severely attenuated the levels of Syk, ERK, and STAT4 activated in response to costimulation (Figure 7A-C). Collectively, these data demonstrate the importance of lipid raft microdomains as mediators of the enhanced signaling activity required for synergistic NK cell cytokine production.
Inhibition of lipid raft microdomains by cholesterol depletion severely attenuates activation of Syk, ERK, and STAT4 in NK cells following FcR + IL-12 stimulation. Purified human NK cells were pretreated with 5 nM MβCD (right column) or DMSO solvent control (Mock Pre-Tx; left column) for 1 hour and then activated via FcγRIIIa ligation in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C. Control conditions consisted of control Ab-treated NK cells (−), NK cells activated via FcR ligation alone (FcR), or NK cells activated with IL-12 alone (IL-12). Cells were lysed and analyzed for p-Syk (A), p-ERK (B), and p-STAT4 (C) by immunoblot analysis. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total protein and expressed in relative densitometric units. All results are representative of n = 3 determinations.
Inhibition of lipid raft microdomains by cholesterol depletion severely attenuates activation of Syk, ERK, and STAT4 in NK cells following FcR + IL-12 stimulation. Purified human NK cells were pretreated with 5 nM MβCD (right column) or DMSO solvent control (Mock Pre-Tx; left column) for 1 hour and then activated via FcγRIIIa ligation in the presence of 10 ng/mL IL-12 for 5 minutes at 37°C. Control conditions consisted of control Ab-treated NK cells (−), NK cells activated via FcR ligation alone (FcR), or NK cells activated with IL-12 alone (IL-12). Cells were lysed and analyzed for p-Syk (A), p-ERK (B), and p-STAT4 (C) by immunoblot analysis. Numbers below immunoblots represent the fold increase in phosphoprotein levels for each condition, normalized to total protein and expressed in relative densitometric units. All results are representative of n = 3 determinations.
Discussion
In the current report, we have provided data to support a model in which costimulation of NK cells via FcRγIIIa and IL-12R leads to the migration of these receptor elements to lipid raft microdomains and to enhanced activation of Syk. We propose that Syk in turn activates PI3-K, and that this leads to activation of the ERK MAPK.14 Inhibition of lipid rafts, Syk, PI3-K, or ERK abrogated downstream signaling events and the production of IFN-γ. Overexpression of ERK augmented the IFN-γ response to costimulation. These results provide a molecular mechanism for the enhancement of FcR-mediated effector functions by immune stimulatory cytokines.
Recent evidence has indicated that cell membranes contain specialized cholesterol-rich domains, termed lipid rafts, that function as signaling platforms for a number of different immune cell receptors and their downstream effectors. For example, upon engagement with antigen, the TCR localizes to lipid raft domains along with adaptor molecules SLP-76, ZAP-70, and protein kinase C (PKC), all of which mediate T-cell activation signals.13,16 With respect to FcRs, localization of FcγRIIa and FcγRIIb to lipid rafts has been shown to be critical for the initiation of cellular signaling in neutrophils and monocytes, respectively.17,18 Galandrini et al have shown that FcγRIIIa on the surface of NK cells redistributed to lipid raft domains upon its engagement by Ab-coated target cells along with SHIP-1, an inhibitory phosphatase with negative effects on PI-3K activity.12 We have demonstrated that activation of NK cells via FcR cross-linking in the presence of IL-12 resulted in a redistribution of both FcγRIIIa and IL-12R to areas of lipid raft microdomains within the cell membrane, an event that was required for NK cell IFN-γ production. To our knowledge, these findings are the first to demonstrate the enrichment of cytokine receptors into NK cell lipid raft microdomains. Of note, MβCD blocked IFN-γ production in response to IL-12 alone, although IL-12R did not migrate to lipid raft microdomains in the absence of FcR cross-linking. It is possible that IFN-γ production in response to IL-12 is mediated from low amounts of IL-12R molecules present within lipid rafts in resting NK cells. A similar scheme has been proposed in smooth muscle cells. Low amounts of TNF-R1 were present within lipid rafts of resting cells, and although treatment of the cells with TNF-α had no effect on the amount of lipid raft–associated TNF-R1, disruption of lipid rafts with MβCD inhibited TNF-α–induced activation of RhoA.19 Future studies examining the distribution of signaling proteins within resting and activated NK cells will be required in order to confirm this hypothesis. Importantly, inhibition of lipid raft formation also blocked the enhanced secretion of IFN-γ that is observed in response to stimulation of NK cells via the FcRγIIIa in the presence of IL-2 or IL-21 (data not shown), suggesting that the migration of cytokine receptors into lipid raft microdomains may be a common mechanism for enhanced NK cell IFN-γ production in response to cytokine stimulation.
Although the activation of Syk following FcR stimulation is well documented, our studies also revealed that Syk is activated following IL-12 stimulation, which has not been previously reported. The observation that inhibition of Syk severely diminished IFN-γ production from IL-12–stimulated NK cells reveals the potential importance of this molecule in IL-12–mediated signal transduction in NK cells. Our data suggest that that colocalization of FcγRIIIa and the IL-12R into lipid raft microdomains augments the activation of Syk, which promotes downstream signaling via PI3-K. Jiang et al have previously described a role for PI3-K in regulating ERK activity following engagement of NKR.20 The authors have demonstrated that PI3-K can control ERK activation through Rac1 and p21-activated kinase 1 (PAK1), and that this pathway is critical for NK cell cytotoxic activity. This group later went on to describe regulation of this pathway by Syk, which acted upstream of PI3-K.14 The present study strongly suggests that this pathway is also operative for FcRγIIIa-mediated cytokine production. In support of this model, our group has recently demonstrated that SHIP-1, through its enzymatic activity on PI3-K products, negatively regulates FcR-dependent IFN-γ production by inhibiting the downstream activation of ERK.21 In the current report, retroviral overexpression of ERK1 within human NK cells enhanced IFN-γ secretion in response to immobilized IgG and IL-12, confirming a critical role for ERK in mediating IFN-γ production within FcR- and IL-12–stimulated NK cells. Ortaldo et al have recently demonstrated that IFN-γ production from NK cells stimulated via NKG2D cross-linking in the presence of IL-12 was associated with enhanced activation of both p38 MAPK and ERK, and was abrogated in the presence of chemical inhibitors of either of these molecules.22 These results suggest that enhanced activation of ERK might be a common mechanism for increased IFN-γ production from NK cells stimulated via 2 activating receptors.
The exact mechanism by which activated ERK results in IFN-γ secretion remains to be elucidated. Traditionally, ERK is thought to induce IFN-γ secretion within NK and T cells by binding to the c-fos promoter and enhancing its transcription.23 The c-fos protein product can then dimerize with c-jun to form the transcription factor AP-1, which binds to the IFN-γ gene promoter.23 Of note, stimulation through FcγRIIIa has been shown to induce ERK-dependent AP-1 activity in NK cells.23,24 ERK can also control gene expression within immune cells by regulating the activity of numerous transcription factors, including Elk-1, c-myb, and TAL-1.25,26 In contrast to this indirect method of gene regulation by ERK, recent reports have implicated ERK in the direct enhancement of gene transcription via epigenetic mechanisms. Zhong et al have shown that ERK can phosphorylate histone H3 in vitro.27 Of note, macrophage secretion of IL-10 in response to FcγR stimulation was associated with ERK activation and histone H3 modifications at the IL-10 locus, and inhibition of ERK prevented both these histone modifications as well as the resultant IL-10 secretion.28 It is therefore possible that ERK enhances IFN-γ production in costimulated NK cells by regulating chromatin structure.
Activation of the transcription factor STAT4 was also enhanced in FcR- and IL-12–stimulated NK cells. Inhibition of PI3-K or ERK had no effect on IL-12–induced STAT4 activation (data not shown), which is consistent with previous studies showing that STAT4 activation in NK cells in response to IL-12 was not dependent on MAPK activity.29 This suggests that the activation of STAT4 and the activation of ERK following FcγRIIIa and IL-12 stimulation result from distinct and largely noninteracting signaling cascades. However, STAT4 activation in response to costimulation was abrogated following inhibition of Syk (data not shown). Lou et al have demonstrated that lipid raft reorganization in response to NKR engagement depended on Syk activity.30 It is currently unclear whether Syk signaling modulates STAT4 activation in a manner that is independent of PI3-K or whether Syk inhibition prevents STAT4 activity by preventing the formation of lipid rafts.30 The enhanced IFN-γ production that is observed when CD4+ T cells are costimulated with IL-12 (which activates STAT4) and IL-18 (which, like ERK, induces AP-1) has been attributed to the formation of STAT4/AP-1 heterodimers, which exhibit enhanced binding to the AP-1 binding sequence of the IFN-γ promoter.31 It is tempting to speculate that the synergy observed in the present report might also rely on this mechanism.
Taken together, our studies suggest a model in which dual stimulation of NK cells via FcγRIIIa and IL-12R initiates a signaling cascade that results in the activation of ERK and STAT4. Activation of these transcription factors in turn leads to potent IFN-γ secretion from costimulated NK cells. Our studies also suggest that redistribution of FcγRIIIa and IL-12 into areas of lipid raft microdomains following dual stimulation might allow for the amplification of ERK signaling by enabling enhanced activation of Syk. The relative importance of these signaling molecules as biomarkers of NK cell activation in response to Ab-coated target cells and IL-12 is currently being investigated. The elucidation of specific pathways for NK cell activation may provide targets for pharmacologic intervention.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank The Ohio State University Campus Microscopy and Imaging Facility (CMIF) for help with confocal imaging.
This work was supported in part by the Susan G. Komen Breast Cancer Foundation Dissertation Research Award (J.M.R. and R.P.). We would also like to acknowledge the support of National Institutes of Health (NIH) grant P01 CA95426 (S.T., M.A.C., and W.E.C.).
National Institutes of Health
Authorship
Contribution: J.M.R., R.P., J.Y., M.A.C., S.T., and W.E.C designed research; J.M.R., S.V.K., and R.P. performed research; S.T. and R.W.B. provided technical and intellectual expertise; A.L performed the statistical analysis of the data, S.V.K, J.M.R. and W.E.C. cowrote the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William E. Carson III, N924 Doan Hall, 410 West 10th Ave, Columbus, OH 43210; e-mail: william.carson@osumc.edu.
References
Author notes
*S.V.K. and J.M.R. contributed equally to this work.