Abstract
Although membrane phospholipid phosphatidylinositol-4,5bisphosphate (PIP2) plays a key role as signaling intermediate and coordinator of actin dynamics and vesicle trafficking, it remains completely unknown its involvement in the activation of cytolytic machinery. By live confocal imaging of primary human natural killer (NK) cells expressing the chimeric protein GFP-PH, we observed, during effector-target cell interaction, the consumption of a preexisting PIP2 pool, which is critically required for the activation of cytolytic machinery. We identified type I phosphatidylinositol-4-phosphate-5-kinase (PI5KI) α and γ isoforms as the enzymes responsible for PIP2 synthesis in NK cells. By hRNA-driven gene silencing, we observed that both enzymes are required for the proper activation of NK cytotoxicity and for inositol-1,4,5-trisphosphate (IP3) generation on receptor stimulation. In an attempt to elucidate the specific step controlled by PI5KIs, we found that lytic granule secretion but not polarization resulted in impaired PI5KIα- and PI5KIγ-silenced cells. Our findings delineate a novel mechanism implicating PI5KIα and PI5KIγ isoforms in the synthesis of PIP2 pools critically required for IP3-dependent Ca2+ response and lytic granule release.
Introduction
Natural killer (NK) cells and cytotoxic T lymphocytes are critical effectors in the defense against tumor and viral infections1 ; they exert cytotoxic function through the polarized secretion of granules containing proteolytic molecules, such as perforin and granzymes. This process involves several steps, including the formation of a cytolytic synapse between cytolytic effector and target cell, the rapid reorientation of the microtubule-organizing center along with lytic granules toward the target contact area followed by granule docking and fusion at specialized secretory domains within the cytolytic synapse.2,3
Several structurally distinct receptors have been implicated in the activation of NK-cell cytolytic machinery: when cross-linked by the corresponding ligands on target cell, they trigger multiple and intersecting signaling pathways responsible for functional activation.4 A vast array of activating NK receptors belonging to different families are coupled to the lipid modifying enzymes phosphatidylinositol3-kinase (PI3K) and phospholipase Cγ (PLCγ), which provide signals critically required for the activation of the cytolytic machinery5-8 ; notably, both enzymes use the membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) as common substrate.9 Besides its role as signaling intermediate, PIP2 also acts as critical regulator of various cellular processes, including actin remodeling, membrane and vesicle trafficking, adhesion, and ion transport.10,11 Surprisingly, the role of PIP2 and its regulatory mechanisms in lymphocyte-mediated cytotoxicity remain completely undefined.
The main cellular source of PIP2 are type I phosphatidylinositol-4-phosphate-5-kinase (PI5KI) family members which phosphorylate PI4P on the D5 position of the inositol ring. Three major PI5KI isoforms (α, β, and γ) have been identified.12-15 Increasing evidences indicate that PI5KIs, providing spatially and functionally distinct PIP2 pools, guarantee the regulation of specific cellular events: PI5KIγ, for instance, is required for focal adhesion and synaptic vesicle trafficking,16,17 PI5KIβ for constitutive receptor endocytosis,18 whereas PI5KIα acts in the regulation of processes dependent on local changes of actin dynamics, such as membrane ruffling and phagocytosis.19,20
In this report, we took advantage of the fusion protein consisting of GFP and the pleckstrin homology (PH) domain of PLCδ1, that is known to bind PIP2 with high affinity and specificity,21-23 to monitor the dynamics and function of PIP2 during the cytotoxic event. We present evidences of localized changes in the concentration of PIP2 at the cytolytic synapse area where it is required for granule exocytosis. The mechanisms underlying these changes and their functional consequences were studied by silencing individually each PI5KI isoform.
We report that both PI5KIα- and PI5KIγ-dependent PIP2 pools are major contributors of inositol-1,4,5-trisphosphate (IP3) production and lytic granule exocytosis induced by receptor stimulation; in contrast, they are redundant in the control of PI3K activity and granule polarization.
Methods
Antibodies and reagents
Anti-NKp46 (BAB218) mAb was provided by Dr A. Moretta (University of Genova, Genova, Italy); anti-CD16 (B73.1) and anti-major histocompatibility complex (MHC) class I (W6.32) monoclonal antibodies (mAbs) were kindly provided by Dr G. Trinchieri (Schering Plough, Dardilly, France). Anti-PI5KIγ mAb was provided from Dr P. De Camilli (Yale University School of Medicine, New Haven, CT). Anti-2B4 (CD244) mAb was from Immunotech (Marseille, France). Anti-PI5KIα, anti-PLCγ1, anti- PLCγ2 pAbs were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt and anti-pAkt pAbs were from Cell Signaling Technology (Danvers, MA). Anti-pTyr and anti-Vav mAbs were from Upstate Biotechnology (Charlottesville, VA). Anti-perforin mAb was from Ancell (Bayport, MN). Goat anti-mouse (GAM) F(ab′)2 was from Cappel (MP Biomedicals, Irvine, CA). Alexa Fluor 350-labeled GAM mAb was from Invitrogen (Carlsbad, CA). All chemicals were from Sigma-Aldrich (Milan, Italy).
Lentiviral vector cloning and production
The cDNA encoding the GFP-PH sequence (gift from Dr T. Meyer, Stanford University, CA)21 was cloned into pWPT-GFP transfer vector (gift from Dr T. Didier, University of Geneva, Geneva, Switzerland)24 to generate the recombinant plasmid pWPT-GFP-PH.25 shRNA-encoding DNA sequences targeting PI5KIα, PI5KIβ, and PI5KIγ18,26 were cloned into XhoI/HpaI site of lentiviral pLL3.7 vector.27
Cell systems and lentiviral infection
Primary cultured human NK-cell populations28 were infected as described.24 Cells were used for the experiments after 12 hours starving in cytokine-free medium. When indicated, high GFP-PH– and GFP–expressing cells were sorted by fluorescence-activated cell sorter (FACS) the day before the experiment.
The human NK-cell line, NK92, was infected as above. GFP-positive cells were FACS-sorted and expanded. Before the experiments, cells were cultured for 18 hours in cytokine-free medium.
Live imaging and fluorescence analysis
For live imaging, P815 target cells were seeded on 8-well chamber glass coverslip (Nalge Nunc International, Rochester, NY) in thermostated Leiden chamber holder of a laser confocal inverted microscope (TCS-SP2, Leica Microsystems, Deerfield, IL; 40× oil objective) at 37°C. GFP-PH-expressing anti-CD16-treated primary cultured NK cells were added to the chamber. Images were captured using Leica Confocal Software, converted to video file MOV (QuickTime) and edited with NHI-Image J 1.35s software (National Institutes of Health, Bethesda, MD). Video were recorded at the frequency of one image every 30 seconds up to 10 minutes. To quantify the variation of fluorescence intensity, a region of interest was assigned for each cytolytic interaction examined; fluorescence intensity was integrated above the cytosolic level. To allow comparison between experiments, the fluorescence was normalized to the maximum level recorded within the selected area that was assumed as 1. At least 30 cytolytic conjugates were quantitatively analyzed for each experiment.
To analyze granule polarization, anti-2B4-treated NK92 cells were resuspended in prewarmed medium, mixed to P815 cells, briefly pelleted, and incubated for 15 minutes at 37°C. Cell conjugates were gently resuspended and spun onto poly-L-lysine–coated glass slides. On fixing and permeabilization, cells were stained with anti-perforin mAb followed by Alexa Fluor 350-labeled GAM mAb and analyzed by fluorescence microscopy. Granule polarization was assessed in 100 NK92/P815 conjugates and expressed as percentage of conjugates containing polarized granules respect to total conjugates.
Cell labeling and phosphoinositide analysis
In vivo [32P]orthophosphate labeling of primary cultured NK cells and NK92 cell line was performed as described.28 When indicated, radiolabeled anti-CD16-treated NK cells were mixed to P815 target cells (E/T ratio = 2:1), briefly centrifuged at 1200 rpm and incubated at 37°C for the indicated times. Chloroform/methanol/HCl lipid extraction was followed by lipid drying under N2. An aliquot of each sample was recovered and counted in a β-counter for sample normalization; the remaining samples were spotted on Silica gel plates (Merck, Whitehouse Station, NJ), separated by TLC and visualized by autoradiography. The spot corresponding to PIP2 was quantified by densitometric analysis (National Institutes of Health Image software).
IP3 production was measured using an [3H]IP3 competitive radioreceptor assay kit (PerkinElmer Life and Analytical Sciences, Waltham, MA) on stimulation of NK92 (4 × 106 cells/sample) with anti-2B4 or anti-MHC I mAb-coated polystyrene beads (2.5-μm diameter; Interfacial Dynamic, Portland, OR).
Cytotoxic and degranulation assay
Primary cultured NK cells and NK92 cell line were assessed in a redirected killing 51Cr release assay toward P815 cell line in the presence of the indicated mAbs, as described.28
For N-benzyloxycarbonyl-L-lysine thiobenzyl (BLT) esterase activity, cells were stimulated by plastic-immobilized mAbs or by addition of phorbol-12-myristate-13-acetate (PMA; 50 ng/mL) plus ionomycin (0.5 μg/mL). After 4 hours, cell-free supernatants were assayed for BLT esterase (granzyme A) activity on incubation with 0.2 M Tris-HCl, pH 8.1 containing 10−4 M BLT and 2.2 × 10−4 M dithio-bis-nitrobenzoic acid. The percentage of specific release was calculated on the basis of the total enzymatic content.
RT-PCR analysis
A total of 1 μg total RNA was used for cDNA first-strand synthesis for polymerase chain reaction (PCR) reaction in the presence of FastStart Taq DNA polymerase (Roche Diagnostics, Mannheim, Germany). Forward and reverse primers for PCR amplification were: 5′-GAACGGTTCCAGCG-CTTCAT-3′ and 5′-GTCTCTCCAACTAGAGGTGA-3′ for PI5KIα; 5′-TGAAGGCTTCACCGTCTAAG-3′ and 5′-CAGAAGCATTGTCA-TCCTGC-3′ for PI5KIβ; 5′-GCAGTCCTACAGGTTCATCA-3′ and 5′-GCACTGTAATCTGCTGCAGA-3′ for PI5KIγ; PCR conditions were as follows: 94°C for 50 seconds, 58°C for 50 seconds, and 72°C for 50 seconds (35 cycles for PI5KIα and PI5KIγ, 40 cycles for PI5KIβ).
Antibody-mediated stimulation and immunoblot analysis
For the evaluation of PLCγ, Akt and Vav phosphorylation, NK92 cells were pretreated at 4°C with anti-2B4 mAb, washed, and stimulated with GAM F(ab′)2 at 37°C. After stimulation, cells were lysed. PLCγ1 and PLCγ2 immunoprecipitates were obtained. Immunoprecipitated samples or total lysates were separated on SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed by immunoblotting.
IFN-γ release
For determination of interferon-γ (IFN-γ) levels, NK92 cells were stimulated with plastic-immobilized anti-2B4 mAb or rIL-2 (200 U/mL) at 37°C for 18 hours. IFN-γ levels in cell supernatant was analyzed by DuoSet ELISA Development kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Statistics
Data were analyzed with Microsoft Excel software applying the 2-tailed Student t test. Differences assumed significant when P was less than .05.
Results
PIP2 dynamics during the activation of NK cytolytic machinery
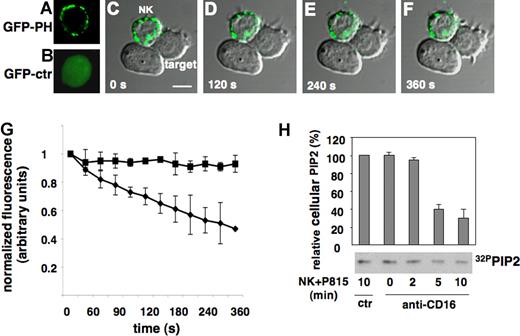
To investigate PIP2 distribution and dynamics at the cytolytic synapse, we expressed, in primary cultured NK cells, the chimeric construct GFP-PH that is known to bind PIP2 with high affinity and specificity.21-23 By means of lentiviral-driven expression, we obtained high transduction efficiency without affecting NK-cell phenotype and functional properties.25 As illustrated in Figure 1A, in isolated NK cells GFP-PH distributed throughout the plasma membrane where it localized in discrete microdomains; as expected, control GFP distributed homogeneously within the cell (Figure 1B). To monitor PIP2 dynamics in live cells during the cytolytic event, anti-CD16-treated NK cells were allowed to bind to Fc receptor-positive P815 target cells by reverse antibody-dependent cellular cytotoxicity (rADCC) and analyzed by time-lapse confocal microscopy. On interaction with target cells, the density of fluorescence signal undergoes a progressive reduction in the area of cytolytic synapse, thus supporting the consumption of a preexisting PIP2 pool. Figure 1C-F shows a single NK cell simultaneously interacting with 2 target cells: as reported by others3 only one cytolytic interaction is formed at a time; the utilization of PIP2 was only detected in cytolytic interactions as suggested by the occurrence of target cell membrane blebbing (at these times, we did not observe photo bleaching). The lack of detection of probe relocation in the cytosol may be attributable to the dilution effect. No GPF redistribution or disappearance was observed in control GFP expressing cells (R.G., unpublished data, October 11, 2005).
PIP2 dynamics at NK-cell cytolytic synapse. Primary cultured NK cells were infected with recombinant lentiviruses encoding GFP (GFP-ctr) or GFP-PH and used for time-lapse confocal microscopy. (A) Membrane distribution of GFP-PH and (B) GFP-ctr. Anti-CD16-treated GFP-PH-expressing cells were added to Fc receptor-positive targets. Images were obtained at 30-second intervals. (C-F) Representative images show dichroic phase contrast and fluorescence. The numbers indicate the time after NK cells have made contact with target cells. Bar represents 5 μm. (G) Levels of membrane GFP-PH was quantified and binned into 30-second intervals. The mean fluorescence intensity was calculated on NK cells forming synapses with target cells on randomly acquired fields in 3 independent experiments (mean ± SD; n = 100). Abscissa: time in seconds on NK/target cell contact. Ordinate: relative fluorescence. To allow comparison between experiments, fluorescence intensity of a defined area within cytolytic synapse (♦) or contact-free membrane area (■) was normalized to the maximum signal recorded for each individual area. (H) Biochemical quantification of PIP2 during the cytotoxic event. 32P-radiolabeled primary cultured NK cells were treated with anti-MHCI (ctr) or anti-CD16 mAb and allowed to interact with P815 target cells at 37°C for the indicated times. PIP2 value of control-mAb stimulated sample was assumed as 100%. Data are the mean plus or minus SD of 3 separate experiments.
PIP2 dynamics at NK-cell cytolytic synapse. Primary cultured NK cells were infected with recombinant lentiviruses encoding GFP (GFP-ctr) or GFP-PH and used for time-lapse confocal microscopy. (A) Membrane distribution of GFP-PH and (B) GFP-ctr. Anti-CD16-treated GFP-PH-expressing cells were added to Fc receptor-positive targets. Images were obtained at 30-second intervals. (C-F) Representative images show dichroic phase contrast and fluorescence. The numbers indicate the time after NK cells have made contact with target cells. Bar represents 5 μm. (G) Levels of membrane GFP-PH was quantified and binned into 30-second intervals. The mean fluorescence intensity was calculated on NK cells forming synapses with target cells on randomly acquired fields in 3 independent experiments (mean ± SD; n = 100). Abscissa: time in seconds on NK/target cell contact. Ordinate: relative fluorescence. To allow comparison between experiments, fluorescence intensity of a defined area within cytolytic synapse (♦) or contact-free membrane area (■) was normalized to the maximum signal recorded for each individual area. (H) Biochemical quantification of PIP2 during the cytotoxic event. 32P-radiolabeled primary cultured NK cells were treated with anti-MHCI (ctr) or anti-CD16 mAb and allowed to interact with P815 target cells at 37°C for the indicated times. PIP2 value of control-mAb stimulated sample was assumed as 100%. Data are the mean plus or minus SD of 3 separate experiments.
The quantitative estimation of PIP2 dynamics in Figure 1G shows the progressive reduction of PIP2 levels at the area of cytolytic synapse; its consumption reached maximum levels at 6 minutes on target cell binding. On the contrary, no significant variations were observed in contact free membrane area.
The use of PIP2 during the cytotoxic event was also validated by TLC analysis of phospholipids extracted from radiolabeled NK cells stimulated by rADCC. In accordance with time lapse data, we observed a progressive reduction of endogenous PIP2 levels on target cell interaction, reaching a plateau between 5 and 10 minutes after stimulation (Figure 1H). These observations suggest that PIP2 is used during the activation of cytolytic machinery.
The reduced availability of PIP2 is associated with impairment of NK-cell cytotoxicity
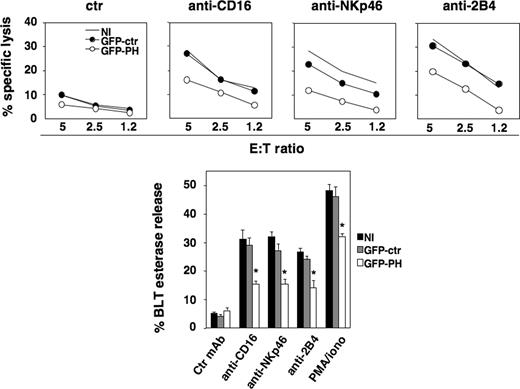
The expression of GFP-PH construct is a useful tool to competitively inhibit different PIP2-dependent processes.29,30 Primary cultured NK cells were sorted by FACS to isolate cells expressing GFP-PH at high levels. Cell viability was not affected by PIP2 masking (F.M., unpublished data, January 15, 2006). Uninfected, GFP- and GFP-PH-expressing cells were tested in a redirected killing assay toward P815 target cells in the presence of antibody directed against CD16, NKp46, or 2B4 activating NK receptors. GFP-PH–expressing cells exhibited a significant down-regulation of cytotoxic activity compared with uninfected population, whereas control GFP had no significant effect (Figure 2 top panels). We also investigated lytic granule secretion by measuring granzyme A activity in the supernatants of NK cells stimulated with the aforementioned Abs or with PMA plus ionomycin. Our findings show that PIP2 masking induced a significant reduction of receptor- or PMA/ionomycin-triggered granule release compared with control populations; total enzymatic activity was comparable between experimental groups.
GFP-PH–mediated PIP2 masking impairs NK-cell cytotoxic function. Primary cultured NK cells were left uninfected or were infected with recombinant lentiviruses encoding GFP or GFP-PH constructs. Three days after infection, cells were assessed in a 51Cr release assay against P815 target cells (top) in the presence of anti-MHC class I (ctr), anti-CD16, anti-NKp46, or anti-2B4 mAb. One representative experiment of 5 performed is shown. Differences between GFP-PH group and NI or GFP-ctr groups in 5 independent experiments at all E:T ratios were significant. (Bottom) The same cell populations were stimulated with plastic-immobilized mAbs, as indicated, or PMA plus ionomycin. After 4 hours, cell supernatants were collected and assessed for BLT esterase release. Data represent the percentage (mean ± SD) of specific release (sample/total release) from 3 independent experiments. Differences between GFP-PH group and NI or GFP-ctr groups were significant (*P < .003).
GFP-PH–mediated PIP2 masking impairs NK-cell cytotoxic function. Primary cultured NK cells were left uninfected or were infected with recombinant lentiviruses encoding GFP or GFP-PH constructs. Three days after infection, cells were assessed in a 51Cr release assay against P815 target cells (top) in the presence of anti-MHC class I (ctr), anti-CD16, anti-NKp46, or anti-2B4 mAb. One representative experiment of 5 performed is shown. Differences between GFP-PH group and NI or GFP-ctr groups in 5 independent experiments at all E:T ratios were significant. (Bottom) The same cell populations were stimulated with plastic-immobilized mAbs, as indicated, or PMA plus ionomycin. After 4 hours, cell supernatants were collected and assessed for BLT esterase release. Data represent the percentage (mean ± SD) of specific release (sample/total release) from 3 independent experiments. Differences between GFP-PH group and NI or GFP-ctr groups were significant (*P < .003).
These data demonstrate that a pool of preexisting PIP2 is required for the efficient granule-mediated cytotoxic function.
PI5KI silencing impairs the activation of NK-cell cytolytic machinery
Three distinct isoforms of the lipid kinase PI5KI (α, β, and γ) are known to provide spatially and functionally distinct PIP2 pools.15
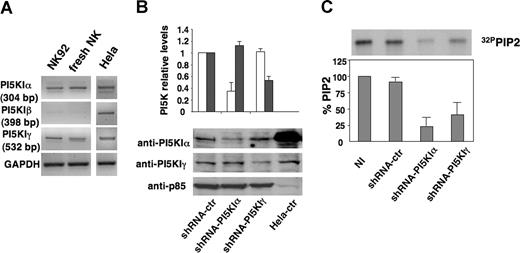
Because it was unknown which PI5KI isoforms were present in NK cells, we analyzed, by RT-PCR, the expression of the different isoforms in freshly isolated NK cells and in the NK-cell line, NK92. Figures 3A and S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) demonstrate that NK cells express PI5KIα and PI5KIγ isoforms, whereas β was almost undetectable.
shRNA-driven PI5KIα and PI5KIγ silencing in NK92 cells. (A) Analysis of PI5KI isoform expression in NK cells. Total RNA was extracted from NK92 cells, freshly isolated NK cells, and HeLa cells and subjected to RT-PCR with PI5KIα, PI5KIβ, and PI5KIγ; 35 (α and γ) or 40 (β) cycles of RT-PCR analysis is shown. GAPDH-specific PCR was used as loading control. (B) NK92 cells were infected with lentiviruses encoding shRNA sequences targeting PI5KIβ (shRNA-ctr), PI5KIα (shRNA-PI5KIα), or PI5KIγ (shRNA-PI5KIγ). Total cell lysates of infected populations and HeLa cells were analyzed by immunoblotting with the indicated Abs. (C) Total phospholipids were extracted from 32P-radiolabeled uninfected (NI) or silenced NK92 cells. Equal counts of lipids were resolved by TLC followed by autoradiography (top). The spot corresponding to PIP2 was quantified by densitometric analysis. PIP2 levels of uninfected population were assumed as 100%. Data represent mean plus or minus SD of 3 independent experiments (bottom).
shRNA-driven PI5KIα and PI5KIγ silencing in NK92 cells. (A) Analysis of PI5KI isoform expression in NK cells. Total RNA was extracted from NK92 cells, freshly isolated NK cells, and HeLa cells and subjected to RT-PCR with PI5KIα, PI5KIβ, and PI5KIγ; 35 (α and γ) or 40 (β) cycles of RT-PCR analysis is shown. GAPDH-specific PCR was used as loading control. (B) NK92 cells were infected with lentiviruses encoding shRNA sequences targeting PI5KIβ (shRNA-ctr), PI5KIα (shRNA-PI5KIα), or PI5KIγ (shRNA-PI5KIγ). Total cell lysates of infected populations and HeLa cells were analyzed by immunoblotting with the indicated Abs. (C) Total phospholipids were extracted from 32P-radiolabeled uninfected (NI) or silenced NK92 cells. Equal counts of lipids were resolved by TLC followed by autoradiography (top). The spot corresponding to PIP2 was quantified by densitometric analysis. PIP2 levels of uninfected population were assumed as 100%. Data represent mean plus or minus SD of 3 independent experiments (bottom).
PI5KIα and PI5KIγ were individually knocked down by lentiviral vector-driven short hairpin (sh)RNA. Alternatively to primary cultured NK cells, we used the NK92 cell line because it represents an excellent model for the study of NK-cell functions.5,31 NK cells were infected with PI5KI α, β, or γ-specific shRNA-encoding sequences keeping the PI5KI β sequence as negative control; GFP-positive cells were FACS-sorted. The effects of the specific shRNAs on the steady-state levels of endogenous PI5KIs were analyzed. Figure 3B shows the immunoblot (bottom panel) and quantitative analysis (top panel) of α and γ isoform levels in silenced and control virus-infected NK92 cells. Expression was reduced by 70% and 50%, on average, for PI5KIα and PI5KIγ enzymes, respectively. No major alterations in term of viability, growth rate, and surface phenotype were detected (R.G., unpublished data, June 19, 2006).
PI5KI silencing was further confirmed by evaluating endogenous PIP2 levels by TLC analysis of phospholipids extracted from radiolabeled cells (Figure 3C top panel). Quantitative analysis demonstrated that PIP2 levels in α- and γ-silenced cells were decreased, on average, up to 75% and 60%, respectively, whereas endogenous PIP2 levels of control virus-infected cells were superimposable to those of uninfected cells (Figure 3C).
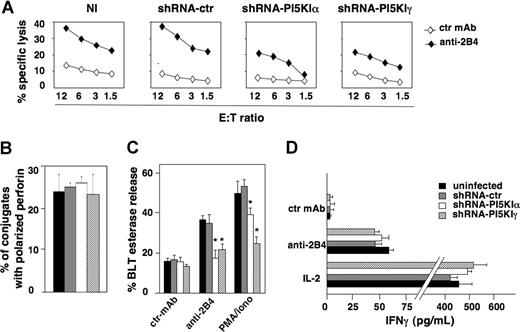
To investigate whether PI5KIα- and PI5KIγ-dependent PIP2 pools could have a specific role in NK-cell functions, we analyzed cytotoxic activity and cytokine production on 2B4 receptor stimulation, which is known to fully activate NK92 cell functional program.31 When we tested silenced and control populations in 2B4-induced redirected killing assay, we observed significant impairment of cytotoxicity in both PI5KIα- and PI5KIγ-silenced cells compared with control populations (Figure 4A). We also assessed the impact of PI5KI silencing on reverse ADCC in primary cultured NK cells, and we observed that PI5KIα and PI5KIγ greatly reduced CD16-induced cytotoxicity (Figure S1).
PI5KI isoform silencing impairs NK92 cell cytotoxic function but not IFN-γ release. (A) Uninfected (NI), shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ populations were assessed in a 51Cr release assay against P815 target cells in the presence of anti-MHC class I (ctr) or anti-2B4 mAb. One representative experiment of 5 performed is shown. Differences between shRNA-PI5KIα or shRNA-PI5KIγ populations and NI or shRNA-ctr populations were significant in 5 independent experiments at all E:T ratios. (B) shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 cells were stimulated by 2B4-induced redirected killing as in Figure 2 (top panels). Cell conjugates were fixed, stained with anti-perforin mAb, and analyzed by fluorescence microscopy. The percentage of NK92 cells conjugated with target cells containing polarized granules was calculated on randomly acquired fields of 3 independent experiments (mean ± SD, n = 100 conjugates). The difference obtained between the groups is not significant. (C) The same cell populations were stimulated with plastic-immobilized mAbs or PMA plus ionomycin as indicated. After 4 hours, cell supernatants were collected and assessed for BLT esterase release. Data represent the percentage (mean ± SD) of specific release (sample/total release) from 3 independent experiments. Differences between shRNA-PI5KIα or shRNA-PI5KIγ populations and NI or shRNA-ctr populations were significant (*P < .002). (D) The same cell populations were stimulated with plastic-immobilized anti-2B4 mAb or with rIL-2 (200 U/mL). After 18 hours, supernatants were collected and assessed for IFN-γ levels. Data are expressed as mean plus or minus SD from 3 independent experiments. The difference obtained between the groups is not significant.
PI5KI isoform silencing impairs NK92 cell cytotoxic function but not IFN-γ release. (A) Uninfected (NI), shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ populations were assessed in a 51Cr release assay against P815 target cells in the presence of anti-MHC class I (ctr) or anti-2B4 mAb. One representative experiment of 5 performed is shown. Differences between shRNA-PI5KIα or shRNA-PI5KIγ populations and NI or shRNA-ctr populations were significant in 5 independent experiments at all E:T ratios. (B) shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 cells were stimulated by 2B4-induced redirected killing as in Figure 2 (top panels). Cell conjugates were fixed, stained with anti-perforin mAb, and analyzed by fluorescence microscopy. The percentage of NK92 cells conjugated with target cells containing polarized granules was calculated on randomly acquired fields of 3 independent experiments (mean ± SD, n = 100 conjugates). The difference obtained between the groups is not significant. (C) The same cell populations were stimulated with plastic-immobilized mAbs or PMA plus ionomycin as indicated. After 4 hours, cell supernatants were collected and assessed for BLT esterase release. Data represent the percentage (mean ± SD) of specific release (sample/total release) from 3 independent experiments. Differences between shRNA-PI5KIα or shRNA-PI5KIγ populations and NI or shRNA-ctr populations were significant (*P < .002). (D) The same cell populations were stimulated with plastic-immobilized anti-2B4 mAb or with rIL-2 (200 U/mL). After 18 hours, supernatants were collected and assessed for IFN-γ levels. Data are expressed as mean plus or minus SD from 3 independent experiments. The difference obtained between the groups is not significant.
To understand which step of the cytolytic pathway was impaired, NK92 cells were allowed to bind to P815 target cells by 2B4-induced redirected killing; fluorescence microscopy analysis revealed that the percentage of cells forming conjugates with target cells was similar in control and in silenced populations (almost 25%; F.M., unpublished data, July 21, 2006); moreover, the ability to polarize lytic granules was normal in silenced cells with respect to control cells (Figure 4B). On the contrary, the secretory step was significantly impaired by the knockdown of PI5KI enzymes: when NK92 cells were stimulated with anti-2B4 mAb or PMA plus ionomycin, the levels of granzyme A activity in cell supernatants demonstrated a profound defect in silenced cells (Figure 4C). Interestingly, the impairment of secretion observed in PMA/ionomycin-stimulated samples indicates that γ- and, to a lesser extent, α-dependent pools are functionally required for granule exocytosis also in PLCγ-independent step(s).
It has been previously shown that distinct pathways regulate cytotoxicity and cytokine production downstream of specific NK- cell receptors.32 To assess whether PI5KIα and PI5KIγ isoforms are required for IFN-γ production, we measured IFN-γ levels in the supernatants of NK92 cells on 2B4 or rIL-2 stimulation. No major differences in cytokine levels were observed in silenced and control populations (Figure 4D).
Our findings indicate that both PI5KIα- and PI5KIγ-dependent PIP2 pools are required for the proper activation of the cytolytic secretory pathway but are dispensable or redundant in the control of microtubule-mediated granule movement and IFN-γ release.
Effect of PI5KI silencing on receptor-triggered PLCγ and PI3K activation
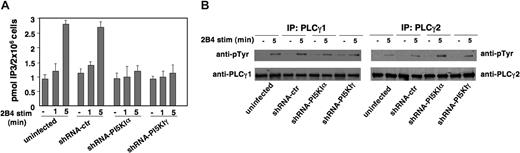
We then investigated whether PLCγ activity is regulated by PI5KIα- or PI5KIγ-dependent PIP2 pools. To this purpose, IP3 levels were determined in NK92 cells on 2B4 stimulation. The peak of IP3 response, which was observed at 5 minutes after stimulation in control populations, was dramatically reduced in PI5KIα- or PI5KIγ-silenced cells, whereas similar levels were detected in uninfected and control virus-infected populations (Figure 5A). Because PLCγ activity can be regulated by tyrosine phosphorylation,33 we investigated whether PIP2 is required for this event. As shown in Figure 5B, PI5KI silencing had no major impact on 2B4-stimulated PLCγ1 and 2 tyrosine phosphorylation compared with control NK92 cells; therefore, the weakened IP3 response in silenced cells is likely to result from the reduced substrate availability than to a defect in PLCγ activation.
PI5KI isoform silencing impairs receptor-triggered PLCγ activity. (A) Uninfected, shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 populations were left unstimulated or were stimulated with anti-2B4-coated polystyrene beads for the indicated times. Lipids were extracted and IP3 levels were measured. Data are expressed as mean plus or minus SD from 3 independent experiments. (B) The same cell populations were left unstimulated or were stimulated with anti-2B4 mAb. PLCγ1 and PLCγ2 immunoprecipitates were analyzed by immunoblotting with anti-pTyr mAb. The same membranes were reprobed with anti-PLCγ1 or PLCγ2 Ab for sample normalization. One representative experiment of 4 performed is shown.
PI5KI isoform silencing impairs receptor-triggered PLCγ activity. (A) Uninfected, shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 populations were left unstimulated or were stimulated with anti-2B4-coated polystyrene beads for the indicated times. Lipids were extracted and IP3 levels were measured. Data are expressed as mean plus or minus SD from 3 independent experiments. (B) The same cell populations were left unstimulated or were stimulated with anti-2B4 mAb. PLCγ1 and PLCγ2 immunoprecipitates were analyzed by immunoblotting with anti-pTyr mAb. The same membranes were reprobed with anti-PLCγ1 or PLCγ2 Ab for sample normalization. One representative experiment of 4 performed is shown.
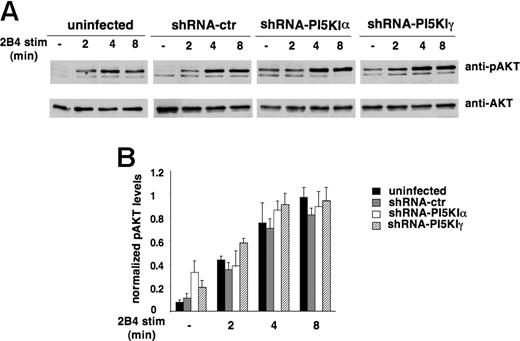
To investigate the effect of PI5KI silencing on PI3K activity, we assessed 2B4-induced Akt phosphorylation. As shown in Figure 6, Akt phosphorylation reached a plateau between 4 and 8 minutes after stimulation with a kinetics and length of response superimposable in silenced and control populations; interestingly, both α- and γ-silenced cells exhibited a reproducible increase in the basal levels of Akt phosphorylation. As a further read of PIP3 production, we assessed Vav tyrosine phosphorylation, a signaling event that is dependent on PI3K activation. No major impact on 2B4-induced Vav phosphorylation was observed in PI5KIα- and PI5KIγ-silenced cells with respect to control NK92 (Figure S2).
PI5KI isoform silencing does not impair receptor-triggered PI3K activity. (A) Uninfected, shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 populations were stimulated with anti-2B4 mAb for the indicated times. Akt phosphorylation status was evaluated by immunoblot analysis with anti-phospho Akt (anti-pAKT) Ab. The same membranes were reprobed with anti-total Akt (anti-AKT) Ab for sample normalization. One representative experiment is shown. (B) Data were quantified by densitometric analysis. Phospho-Akt was normalized for the respective total Akt levels. Data are expressed as arbitrary units (mean ± SD) of 5 separate experiments. The difference obtained between the groups is not significant.
PI5KI isoform silencing does not impair receptor-triggered PI3K activity. (A) Uninfected, shRNA-ctr, shRNA-PI5KIα, or shRNA-PI5KIγ NK92 populations were stimulated with anti-2B4 mAb for the indicated times. Akt phosphorylation status was evaluated by immunoblot analysis with anti-phospho Akt (anti-pAKT) Ab. The same membranes were reprobed with anti-total Akt (anti-AKT) Ab for sample normalization. One representative experiment is shown. (B) Data were quantified by densitometric analysis. Phospho-Akt was normalized for the respective total Akt levels. Data are expressed as arbitrary units (mean ± SD) of 5 separate experiments. The difference obtained between the groups is not significant.
Our results are consistent with a nonredundant role for PI5KIα- and PI5KIγ-dependent PIP2 pools in PLCγ-mediated IP3 generation and with kinase redundancy for proper PI3K activity.
Discussion
The results reported in this study provide evidence that PI5KI family members are integral components of the biochemical machinery regulating the activation of lymphocyte cytolytic secretory pathway.
To monitor the spatial distribution and dynamics of PIP2 we expressed, in primary human NK cells, the GFP-PH fusion protein, which has been proven to be a valuable tool to visualize PIP2 and to sequester it from functional interactions in live cells.21-23 In agreement with previous observations,34 we observed that such probe almost exclusively localizes at the plasma membrane where it distributes in discrete microdomains partially colocalizing with lipid rafts (R.G., unpublished data, December 2005). The interaction of NK cells with target cells was followed by a rapid consumption of PIP2 in the area of cytolytic synapse, whereas in contact-free membrane areas or in noncytolytic interactions we did not observe major PIP2 variations, thus ruling out rapid membrane turnover as the process responsible for the localized loss of membrane-associated GFP-PH probe. Accordingly, a progressive reduction of PIP2 levels was also observed in radiolabeled NK cells on target cell interaction. The consumption of a preexisting PIP2 pool was evident in conditions of rADCC (Figure 1) and natural killing toward 721.221 lymphoblastoid target cells (F.M., unpublished data, March 13, 2006), indicating that PIP2 use during the cytolytic event occurs irrespective of the receptor(s) involved. Interestingly, the reduction of PIP2 levels in the synapse area is reminiscent of PIP2 disappearance observed in forming phagosomes.23,30
The contribution of individual metabolic pathways to PIP2 use is likely attributable to its enzymatic conversion into critical signaling intermediates, such as IP3 and diacylglycerol or PIP3, by PLCγ, and PI3K, respectively. However, PIP2 interaction with ligands with higher affinity may also contribute to the displacement of the probe during the cytolytic event.
Because of GFP-PH-mediated PIP2 masking, which is known to interfere with PLCγ activity,21 we observed a functional impairment of cytotoxic activity and lytic granule exocytosis, in primary NK cells stimulated through activating receptors belonging to different families, including CD16, NKp46, and 2B4; such functional reduction was only observed in cell populations expressing the probe at high levels suggesting a dose-dependent PIP2 masking effect (F.M., unpublished data, February 11, 2006).
PIP2 represents less than 1% of plasma membrane phospholipids; it is responsible for a wide range of membrane-related phenomena, including vesicle trafficking, actin dynamics and ion channel regulation.10,11 By computational analysis, it was proven that, on receptor stimulation, PIP2 synthesis compensates its hydrolysis.35 Recently, the activation-induced release from sequestering proteins, named pipmodulins (ie, myristoylate alanine-rich C-kinase substrate), has been proposed as an additional layer of regulation.36 Although the relative importance of PIP2 sequestration and synthesis remains unresolved, the enzymes mainly responsible for PIP2 production are type I PI5K kinases, which include 3 isoforms (α, β, and γ), as well as several splice variants. Emerging evidences support the concept that PI5KI isoforms have functional diversity related to their ability to provide multiple PIP2 pools governing distinct functions.15 We found that PI5KIα and PI5KIγ represent the major PIP2 synthesizing enzymes in NK cells; we observed that primary NK cells as well as NK92 (Figure 3) and NKL (R.G., unpublished data, May 3, 2006) cell lines expressed the ubiquitous α and the neuronal γ isoforms at RNA and protein levels. Interestingly, among primary cells, platelets and mast cells have been found to express the γ isoform as well,37,38 probably indicating a role of such enzyme in the dynamics of secretory lysosomes.39
By shRNA-driven silencing of individual PI5KI isoforms, we addressed their specific involvement in the regulation of NK-cell cytotoxic function. At variance with a previous report,18 we did not observe that the silencing of an isoform results in a compensatory increased of the other. We report here that PI5KIα and PI5KIγ are both required for the efficient activation of cytolytic machinery in NK92 cells: a significant down-regulation of 2B4-induced redirected killing was indeed observed in silenced populations. In these experiments, we chose the natural cytotoxicity receptor 2B4 as a receptor model because, in NK92 cells, it can fully activate cell functional program5,31 ; it should be stressed that the same results were obtained assaying cytotoxic activity toward 721.221 target cells (F.M., unpublished data, June 2, 2006) and CD16-induced cytotoxicity in primary cultured NK cells.
How might PI5KI-dependent signals regulate NK-cell cytotoxicity?
PI3K and PLCγ, which share PIP2 as substrate, are critical signaling components of cytotoxicity induced by the majority of NK activating receptors, including 2B4.5,6,8,31 Increasing evidence highlights that distinct signals promote granule polarization and secretion.40 In human NK cells, PI3K plays a pivotal role in ADCC and natural cytotoxicity against certain tumor cells5,41 ; such kinase leads to the formation of the critical membrane-bound second messenger, PIP3, which acts as a docking site for several PH domain-containing proteins, such as Vav family proteins, in turn responsible for the activation of Rho family small G proteins.42,43 The well-characterized PI3K→Rac1→PAK1→MEK-ERK1/2 pathway critically controls the polarization of lytic granules.5 The lack of perturbation of 2B4-dependent PI3K activity that we observed in PI5KIα- and PI5KIγ-silenced cells suggests functional redundancy of α- and γ-dependent PIP2 pools in providing PI3K substrate; in this regard, recent data showed that the reduction of PIP2 levels in dominant negative RhoA-expressing cells does not affect BCR-induced Akt phosphorylation.44 Alternatively, the residual PIP2 levels in silenced cells may be sufficient to guarantee the proper receptor-induced PI3K activation. In addition, in silenced cells, we observed a reproducible increase in basal Akt phosphorylation levels that may be attributable to a defect of PIP2-mediated PTEN activation.45 Moreover, in line with the unaffected PI3K activity, PI5KIα and PI5KIγ silencing does not perturb the ability of lytic granules to move and polarize toward the contact area.
The PLCγ product IP3 triggers the mobilization of intracellular calcium ion (Ca2+) from internal stores resulting in transient intracellular flux followed by a sustained Ca2+ influx from the extracellular medium.46 IP3-induced Ca2+ response is an absolute requirement for lytic granule secretion. Recently, in both human and mouse, the secretory step, but not the polarization of lytic granules, has been shown to depend on Ca2+ flux.8,47 Accordingly, in fresh resting NK cells, the failure of a given receptor, such as NKG2D, to induce granule exocytosis correlates with the lack of Ca2+ response on receptor stimulation.4 We show here that both PI5KIα and PI5KIγ are required for the efficient PLCγ activity: in silenced cells, we observed almost complete inhibition of 2B4-induced IP3 generation; the decreased response is not associated with impaired PLCγ tyrosine phosphorylation, indicating that both α- and γ-dependent PIP2 pools are not redundantly required for PLCγ activity. Accordingly, in silenced cells, a significant impairment of receptor-induced cytolytic granule release was observed. These findings nicely correlate with our previous observations of the role of Arf6 small G protein on CD16-triggered PI5KIα activation and lytic granule release.28 In particular, the activation of PI5KI certainly contributes to Arf6-mediated control of granule exocytosis.28 Moreover, our data extend previous reports demonstrating a critical role for PI5KI in Ca2+ response induced by B-cell receptor and G protein–coupled receptor.26,48
Our findings indicate that, in contrast to the recurrent theme of functional redundancy among signaling molecules, PI5KIα and PI5KIγ isoforms play a nonredundant role in PIP2 refilling necessary for the proper PLCγ activity and granule secretion.
IFN-γ production downstream of selected activating receptors is known to depend on PLCγ activity7,49 ; in 2B4-stimulated silenced cells, however, we observed normal IFN-γ levels indicating that its production is independent from PLCγ; in this respect, a previous report revealed the selective requirement of p38 MAPK pathway for 2B4-induced IFN-γ production.50 We also report normal levels of IL-2–induced IFNγ production in PI5KI-silenced cells; such an observation is in line with the finding of the lack of PLCγ-dependent signals in IL-2–stimulated human NK cells.49 The lack of a defect in IFN-γ secretion in silenced cells does not conflict with the finding of an impaired release of lytic granules; it has been reported, indeed, that such processes used different secretory pathways.51
The IP3/Ca2+-dependent factors required for lytic granule exocytosis are largely unknown. The high-affinity Ca2+ binding protein synaptotagmin is an interesting candidate; recently, synaptotagmin VII has been implicated in exocytosis of lytic granules.52 Furthermore, PIP2 itself is required in the ATP-dependent fusion phase of exocytosis via a direct binding to the C2B domain of synapto1tagmin, further contributing to its localization and activation.53 Another layer of PLCγ-dependent control of exocytosis may be mediated by the diacylglycerol-downstream effector protein kinase C, which is known to regulate granule secretion during natural cytotoxicity.54
Finally, the impaired secretion induced by Ca2+ ionophore indicates that the neuronal γ- and, to a lesser extent, α-dependent signals, also control IP3/Ca2+-independent step(s). One interesting possibility is the PIP2-mediated regulation of Wiskott-Aldrich syndrome protein (WASP)-Arp2/3 pathway, which is required for actin nucleation55 and for the formation of an organized cytolytic synapse56,57 ; another possibility is the control of vesicle fusion step via PIP2 interaction with the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) factor syntaxin, as reported in other secretory systems.58 Notably, WASP and syntaxin 11 mutations are responsible for Wiskott-Aldrich syndrome56,57 and familiar hemophagocytic lymphohistiocytosis 4 immunodeficiencies,59 respectively, both characterized by a defective cytotoxic function. Experiments are ongoing to elucidate whether PIP2 pools are actually implicated in the regulation of actin- and SNARE-dependent events in cytotoxicity.
The identification of the different components of the exocytic pathway and the characterization of their function in the control of the cytotoxic granule-mediated immune response remain a main goal for future studies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank D. Milana, A. M. Bressan, and P. Birarelli for expert technical assistance, Raffaella Centicolella and Paola Di Russo for manuscript editing, Dr D. Bonci for very helpful suggestions with shRNA-encoding lentivirus generation, and Dr S. Morrone for cell sorting.
This work was supported by grants from Italian Association for Cancer Research, the Italian Ministry for University and Research, and the Center of Excellence.
Authorship
Contribution: F.M. and C.C. performed experiments, analyzed data, and helped draft the manuscript; E.M. performed fluorescence-based experiments; M.P. and L.F. supervised the laboratory activities; A.S. designed research and discussed data; R.G. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ricciarda Galandrini, Department of Experimental Medicine, Sapienza University, viale Regina Elena, 324, 00161 Rome, Italy; e-mail: ricciarda.galandrini@uniroma1.it; or Angela Santoni, Department of Experimental Medicine, Sapienza University, viale Regina Elena, 324, 00161 Rome, Italy; e-mail: angela.santoni@uniroma1.it.
References
Author notes
*F.M. and C.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal