Abstract
Acute inflammation is traditionally described as the influx of polymorphonuclear leukocytes (PMNs) followed by monocyte-derived macrophages, leading to resolution. This is a classic view, and despite subpopulations of lymphocytes possessing innate immune-regulatory properties, seldom is their role in acute inflammation and its resolution discussed. To redress this we show, using lymphocyte-deficient RAG1−/− mice, that peritoneal T/B lymphocytes control PMN trafficking by regulating cytokine synthesis. Once inflammation ensues in normal mice, lymphocytes disappear in response to DP1 receptor activation by prostaglandin D2. However, upon resolution, lymphocytes repopulate the cavity comprising B1, natural killer (NK), γ/δ T, CD4+/CD25+, and B2 cells. Repopulating lymphocytes are dispensable for resolution, as inflammation in RAG1−/− and wild-type mice resolve uniformly. However, repopulating lymphocytes are critical for modulating responses to superinfection. Thus, in chronic granulomatous disease using gp91phox−/− mice, not only is resolution delayed compared with wild-type, but there is a failure of lymphocyte re-appearance predisposing to exaggerated immune responses upon secondary challenge that is rescued by resolution-phase lymphocytes. In conclusion, as lymphocyte repopulation is also evident in human peritonitis, we hereby describe a transition in T/B cells from acute inflammation to resolution, with a central role in modulating the severity of early onset and orchestrating responses to secondary infection.
Introduction
Inflammation is controlled by a balance of proinflammatory and antiinflammatory signals, resulting in the development of an immune response followed by temporally released proresolution factors that lead to inflammation switching off and injured tissues returning to normal physiology.1 In this setting, attention is traditionally placed on phagocytes such as polymorphonuclear leukocytes (PMNs) and monocyte-derived macrophages, with comparatively less weight place on the importance of lymphocytes in acute inflammation and its resolution. Given their roles in early defense to bacteria and viruses, innate-type lymphocytes including B1 cells merit further exploration for their potential roles in host defense and restorative physiology, as it is becoming clear that diminished innate lymphocyte function or enhanced lymphocyte death by apoptosis, for instance, has being postulated to play a central role in the pathogenesis of burn injury2,3 and sepsis,4,5 respectively
One of the major impetuses for this current investigation stemmed from our previous observations6 and those made by others7-10 showing lymphocytes repopulating sites of tissue injury as inflammation abates, suggesting that lymphocytes might help to switch off acute inflammation. Investigating this possibility in experimental peritonitis, T and B lymphocytes, normal residents of the naive peritoneum, were found to regulate the severity of the early onset phase of acute inflammation by elaborating anti-inflammatory cytokines and dampening PMN influx. However, once PMNs begin to accumulate, resident lymphocytes disappear in response to PGD2 working through its DP1 receptor. As inflammation resolves, a unique profile of lymphocytes begin to repopulate the cavity. Repopulating lymphocytes, however, do not bring about resolution but replenish resolving tissues with the necessary cellular players (CD3, B1 cells, natural killer [NK] and γ/δ T cells as well as CD4+/CD25+ T cells) to control future innate immune-mediated responses. We provide relevance of these findings to human health by showing the absence of repopulating lymphocytes in nonresolving inflammation, which predisposes to secondary infection, resulting in severe inflammatory responses. Although lymphocytes in adaptive immunity are well understood, their role in innate immunity and resolution is highlighted here, as is their functional control by lipid mediators, of which there is a growing body of evidence. For instance, in addition to PGD2,11 lipoxins and aspirin-triggered epi-lipoxins inhibit human T-cell TNFα secretion,12 while docosahexanoic acid–derived protectin D1 blocks T-cell migration and TNFα and IFNγ secretion and promotes apoptosis in human T cells.13 Thus, from these current studies and those published by others,12,13 we highlight the regulatory role played by n-3 and n-6 polyunsaturated fatty acid metabolites on lymphocyte function during acute inflammation and its resolution.
Methods
Animal maintenance, induction of inflammation human peritonitis sampling
Hematopoietic prostaglandin D2 (PGD2) synthase (hPGD2S) knockout mice were generated as previously described.14 All other animals were bred under standard conditions and maintained in a 12-hour/12-hour light/dark cycle at 22 (±1)°C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations. Peritonitis was induced by the intraperitoneal injection of either type A zymosan (1 mg for all experiments unless otherwise stated), group B streptococcus (GBS), or LPS (1 mg/kg; Sigma-Aldrich, St Louis, MO), and cells were enumerated by haemocytometer at time points stated in “Results” by sterile phosphate-buffered saline (PBS) washout. Ethical approval (P/03/136A) for collection of human peritonitis samples was obtained from St Bartholomew's and the Royal London Hospitals from patients with end-stage renal failure undergoing peritoneal dialysis. For pharmacologic rescue experiments, BW245C (DP1 agonist15,16 ) or 15(R)-15-methyl PGD2 (DP2 agonist17 ) in 100 μL of PBS (pH 7.2)/BSA 0.1% was injected at equal doses at 30 minutes prior to and after the zymosan.
Trypsinization of peritoneal cavity
To determining the fate of peritoneal T and B cells, cavities of mice bearing a 4-hour zymosan-induced peritonitis was lavaged with sterile PBS to remove accumulated inflammatory cells and edema. A total of 5 mL prewarmed 5% trypsin was then added to the peritoneal cavity for 10 minutes, followed by an equal volume complete medium to acquire cells adhered to the peritoneal lining/greater omental lymphoid organ. Cells were then analyzed for composition by fluorescence-activated cell sorter (FACS).
FACS analysis and cytokine/chemokine analysis
Cytokines were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (eBioscience, San Diego, CA). FACS was carried out on a Becton Dickinson Facscalibur with data analyzed by CellQuest (BD, Franklin Lakes, NJ). Leukocytes were incubated with antibodies for 30 minutes to either CD3/CD19 (Serotec, Oxford, United Kingdom), B cells (Ly220; Serotec), CD5 (BD PharMingen, San Diego, CA), MAC-1/CD11b (BD PharMingen), NK and γ/δ cells (gift from Dr T. Hussell, Kennedy Institute, London, United Kingdom), GR1 (BD PharMingen), or F4/80 (Caltag Laboratories, Burlingame, CA) using respective isotype antibodies as controls (Serotec) and compensated as appropriate for dual labeling. For apoptosis, cells were incubated with annexin V/propidium iodide (BD PharMingen) and analyzed on Becton Dickinson Facscalibur with data analyzed by CellQuest.
Leukocyte separation and analysis
Contents of resolving-phase peritoneal cavities of wild-type animals were isolated, and macrophages were separated from remaining lymphocytes by adherence to the bottom of 6-well tissue-culture plates. Nonadherent cells were removed and used to isolate T and B cells as well as NK and γ/δ T cells for transfer back in to gp91phox knockout mice using FACS and relevant antibodies to confirm that their composition and ratios reflects that present in situ at resolution. Resolving-phase lymphocytes were enriched at a concentration of 106/mL and 0.5 mL injected into gp91phox knockout mice. In addition, one of the problems with identifying discrete populations of cells such as those found at sites of inflammation by FACS from a larger mixed cell population is that the fluorescence of one cell type after labeling with a fluorescent antibody may be masked by the natural fluorescence of others. In order to confirm the cell types identified using cell-surface antigen markers, peritoneal lymphocytes and macrophages were also isolated and put back into the FACS with their forward- and side-scatter signatures compared against specifically labeled cells. Thus, after lymphocytes were removed from 6-well plates, adherent cells, mainly macrophages, were eluted with Versene, washed with 2% fetal calf serum (FCS) in Hanks balanced salt solution (HBSS) and resuspended in Dulbecco modified Eagle medium (DMEM). These cells were further depleted of contaminant T and B cells using magnetic beads coated with rat monoclonal antibodies to mouse CD3 or B220 (Dynal Biotech, Paisley, United Kingdom). T and B lymphocytes were isolated using the Dynal mouse B-cell (or T-cell) negative isolation kit according to the manufacturer's instructions (Dynal Biotech). In brief, a mixture of rat monoclonal antibodies with specificity to all mouse non-B cells (or non-T cells when isolating T cells) cells were added to cell suspensions and incubated for 20 minutes at 4°C. Cells coated with the added monoclonal antibodies were then removed with magnetic beads coated with sheep polyclonal antibody to rat Ig. Purity of the cells were regularly greater than 95%.
Bacterial culturing
The clinical GBS isolate, NCTC10/84 (serotype V) was grown in Todd Hewitt Broth (THB) without agitation at 37°C to an OD600 of 0.4, equivalent to 108 cfu/mL. Bacteria collected by centrifugation were washed with sterile PBS. Mice were inoculated intraperitoneally with 3 × 107 cfu NCTC in 30 μL PBS. For survival experiments, mice were inoculated by intraperitoneal injection with 5 × 107 cfu NCTC in 0.3 mL PBS.
Results
Biphasic trafficking of lymphocytes during acute inflammation
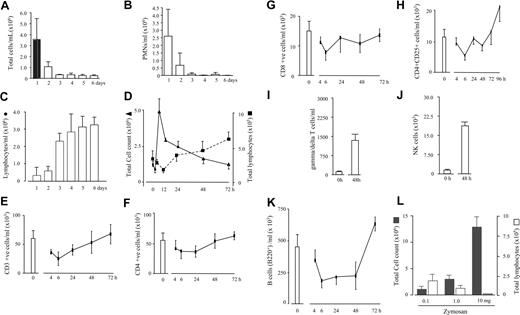
We6 and others7-10 have previously observed lymphocytes repopulating sites of injury as inflammation resolves, but without attributing a functional relevance to their reappearance. Trafficking of lymphocytes during resolution is apparent in inflammation associated with human chronic ambulatory peritoneal dialysis where, as inflammation decreases (Figure 1A,B), lymphocyte numbers increase (Figure 1C). Clinical assessment of these patients was based on patients presenting with abdominal pain, cloudy dialysate, and leukocyte count of more than 100/mm3. In all cases, peritonitis resolved by day 5 as determined by the appearance of a clear dialysate and abatement of abdominal symptoms. Similar results were evident in resolving experimental murine peritonitis (Figure 1D). Experiments were therefore carried out to establish the role these cells play in innate immune-mediated inflammation by characterizing, in the first instance, the profile of lymphocyte populations throughout the time course of an acute zymosan-induced peritonitis. In the naive murine peritoneum (0 hours), lymphocytes constitute about 50% of the total cell population, with the remaining being resident macrophages. Of the CD3 cells, CD4+ and CD8+ cells were found as well as lower numbers of CD4+/CD25+, γ/δ T cells, and NK cells (Figure 1E-J). However, the majority of lymphocytes in the naive cavity are B cells constituting about 70% to 80% of the total lymphocyte population labeling positively for CD19 as well as B220 (Figure 1K). Of these B cells, about 80% are B220low/CD5+/MAC-1low, indicative of a B1 phenotype, with the remainder being B220high/MAC-1− B2 cells. As inflammation initiates (1-4 hours), T and B cells disappear but repopulate the peritoneum again between 12 and 24 hours (Figure 1D). Notably, there were more CD4+/CD25+, γ/δ T cells, and NK cells found during resolution (Figure 1H-J) as well as B1 cells expressing higher levels of MAC-1 than in the naive state (Figure 1K), collectively referred to hereafter as resolution-phase lymphocytes. Figure S6 (available on the Blood website; see the Supplemental Materials link at the top of the online article) shows FACS analysis of lymphocyte cell-surface labeling. As inflammation peaks between 6 and 12 hours in this model and subsequently resolves (Figure 1D), it is argued that lymphocytes repopulate the peritoneum during or just after resolution occurs. Indeed, experimentally enhancing the severity of the inflammatory response within the peritoneum by injecting 3 separate doses of zymosan (0.1, 1.0, and 10 mg), therefore prolonging resolution, is associated with reduced lymphocyte repopulation (Figure 1L). Whether this is a delay or suppression is unclear at this stage. However, from the data presented here, we suggest that lymphocytes repopulate sites of inflammation once resolution occurs. Therefore, this questions what controls lymphocyte influx, which we suggest may be factors released by stromal and/or hematopoietic cells once restitution processes are under way. If so, lymphocyte repopulation is an active process and, until resolution occurs, lymphocyte repopulation is suppressed. Thus, from these experiments we show a shift in lymphocyte populations from the naive to a resolving state constituting more innate-type lymphocytes as well as a different phenotype of B1 cells.
Biphasic trafficking of lymphocytes during acute inflammation. (A-C) Patients undergoing chronic ambulatory peritoneal dialysis and who developed peritonitis that resolved were found to have lymphocytes at the time of recovery of clinical symptoms. Pursuing this observation in murine zymosan-induced peritonitis (D), T and B cells native to the naive peritoneal cavity were found to disappear within hours of stimulus injection. Once inflammation resolves, lymphocytes repopulate the peritoneum but comprise more (E-J) CD4+/CD25 and γ/δ T cells as well as NK cells, than is present in the naive cavity (0 hours) as well as (K) MAC-1+ B cells. (L) Experimentally enhancing the severity of the inflammatory response within the peritoneum by injecting 3 separate doses of zymosan (0.1, 1.0, and 10 mg) prolonged resolution and delayed lymphocyte repopulation (72 hours), suggesting that lymphocytes repopulate only after resolution occurs. n = 6 to 8 animals per group; *P ≤ .05; **P ≤ .01, as determined by analysis of variance (ANOVA), followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Biphasic trafficking of lymphocytes during acute inflammation. (A-C) Patients undergoing chronic ambulatory peritoneal dialysis and who developed peritonitis that resolved were found to have lymphocytes at the time of recovery of clinical symptoms. Pursuing this observation in murine zymosan-induced peritonitis (D), T and B cells native to the naive peritoneal cavity were found to disappear within hours of stimulus injection. Once inflammation resolves, lymphocytes repopulate the peritoneum but comprise more (E-J) CD4+/CD25 and γ/δ T cells as well as NK cells, than is present in the naive cavity (0 hours) as well as (K) MAC-1+ B cells. (L) Experimentally enhancing the severity of the inflammatory response within the peritoneum by injecting 3 separate doses of zymosan (0.1, 1.0, and 10 mg) prolonged resolution and delayed lymphocyte repopulation (72 hours), suggesting that lymphocytes repopulate only after resolution occurs. n = 6 to 8 animals per group; *P ≤ .05; **P ≤ .01, as determined by analysis of variance (ANOVA), followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Lymphocytes are dispensable for resolution but mediate responses to superinfection
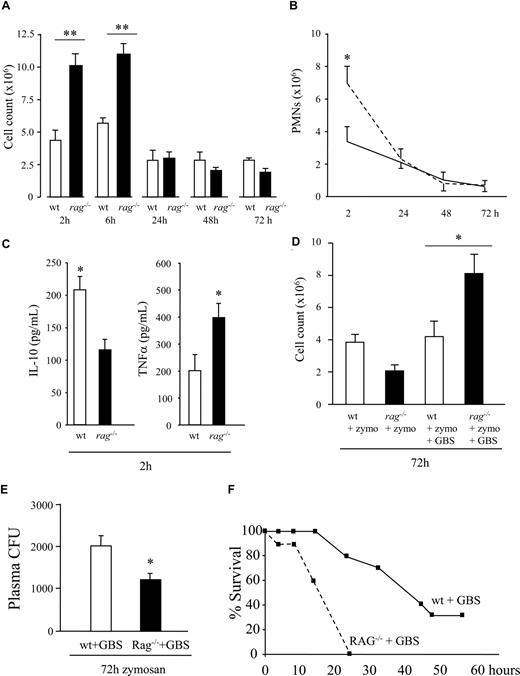
To examine the role of lymphocytes in acute inflammation, zymosan was injected into the peritoneal cavity of lymphocyte-deficient RAG1−/− mice. Inflammation at onset was greatly exaggerated in these animals, twice that in wild-type (Figure 2A), with the principal cell type being PMNs (Figure 2B). This exaggerated response in RAG1 knockout mice was associated with decreased exudate IL-10 and elevated TNFα levels (Figure 2C). Surprisingly, inflammation in wild-type and RAG1−/− mice resolved uniformly from 24 hours onward (Figure 2A,B), suggesting that lymphocytes have no role in switching off acute inflammation and that regardless of how many PMNs traffic to sites of tissue injury, resolution pathways are sufficiently adept at dealing with their disposal. Pursuing the idea that lymphocytes may protect against secondary infection, in a second experiment, RAG1−/− and wild-type mice were injected with a sublethal dose of GBS 48 hours after zymosan injection. Thus, live bacteria were introduced into the inflamed cavity as inflammation resolved, and its effects determined 24 hours later (Figure 2D). Wild-type mice that got zymosan followed by bacteria displayed fewer signs of illness compared with those that received GBS alone, which exhibited 50% mortality by 24 hours, with the remaining animals dying by 48 hours. However, injecting GBS into RAG1−/− mice 48 hours after receiving zymosan showed an approximate doubling of inflammatory cell accumulation compared with wild-type mice treated in the same way (Figure 2D). This resulted in a lower bacterial load in the plasma of GBS-treated RAG1−/− (Figure 2E) but substantially accelerated mortality due to the concomitant hyperinflammatory response (Figure 2F). Results from these studies suggest that inflammation in the resolving phase exerts greater defense against bacterial infection and lethality than do naive tissues. In addition, in terms of controlling initial leukocyte trafficking in response to nonspecific stimuli, protection is conferred by resident lymphocytes, with resistance to secondary infection exerted by repopulating, resolution-phase lymphocytes. Therefore, lymphocytes modulate host responses to injury/infection but are not required to bring about resolution (ie, clear PMNs and macrophages from inflamed sites). The different profile and proportion of lymphocyte repopulating during resolution is necessary to combat secondary infection.
Lymphocytes control early onset of innate inflammation but are dispensable for its resolution. (A,B) Although inflammation doubled in RAG1−/− during the onset phase in response to zymosan, coincident with (C) an imbalance of IL-10 versus TNFα, inflammation normalized with that of wild-type mice from 24 hours onward, suggesting no role for lymphocytes in actively bringing about resolution (ie, clearing PMNs or macrophages). (D) However, RAG1−/− and wild-type mice were injected with GBS during resolution (48 hours after zymosan injection), resulting in enhanced leukocyte accumulation in RAG1−/− but not wild-type mice 24 hours later. (E) This was associated with reduced bacterial colonization in plasma but (F) increased mortality in RAG1−/− mice as a result of the concomitant hyperinflammatory response. We therefore argue that lymphocytes are not required for bringing about resolution and propose that their reappearance hails the end of the inflammatory event and an attempt at restorative physiology. Their role in this setting is in protecting against secondary infection or injury with B cells, CD4+/CD25+ cells, and γ/δ T cells as well as NK cells playing a likely role in this setting. n = 6 to 8 animals per group, with experiments repeated on 2 separate occasions to confirm original findings. *P ≤ .05; **P ≤ .01, as determined by ANOVA, followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Lymphocytes control early onset of innate inflammation but are dispensable for its resolution. (A,B) Although inflammation doubled in RAG1−/− during the onset phase in response to zymosan, coincident with (C) an imbalance of IL-10 versus TNFα, inflammation normalized with that of wild-type mice from 24 hours onward, suggesting no role for lymphocytes in actively bringing about resolution (ie, clearing PMNs or macrophages). (D) However, RAG1−/− and wild-type mice were injected with GBS during resolution (48 hours after zymosan injection), resulting in enhanced leukocyte accumulation in RAG1−/− but not wild-type mice 24 hours later. (E) This was associated with reduced bacterial colonization in plasma but (F) increased mortality in RAG1−/− mice as a result of the concomitant hyperinflammatory response. We therefore argue that lymphocytes are not required for bringing about resolution and propose that their reappearance hails the end of the inflammatory event and an attempt at restorative physiology. Their role in this setting is in protecting against secondary infection or injury with B cells, CD4+/CD25+ cells, and γ/δ T cells as well as NK cells playing a likely role in this setting. n = 6 to 8 animals per group, with experiments repeated on 2 separate occasions to confirm original findings. *P ≤ .05; **P ≤ .01, as determined by ANOVA, followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Peritoneal lymphocytes disappear in response to PGD2 STOPPED
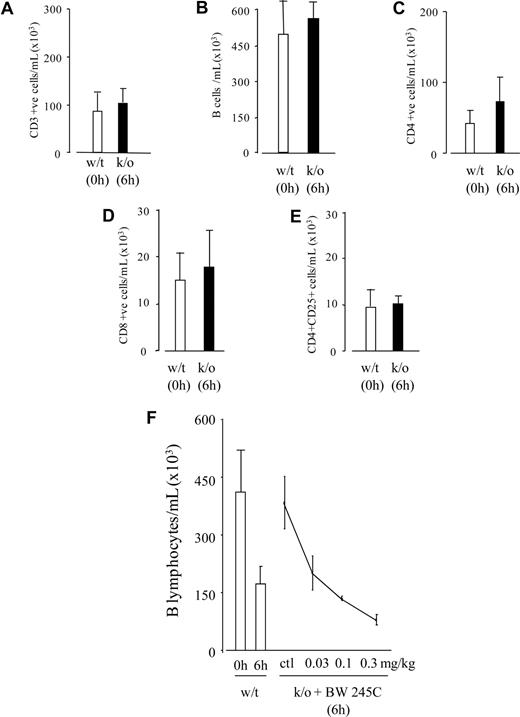
Investigating how lymphocytes disappear, we recorded equivalent numbers of T and B lymphocytes in the peritoneal cavity of hPGD2S knockout mice at 6 hours as in the naive cavity (0 hours) of wild-type (Figure 3A-E). hPGD2S metabolizes cyclooxygenase-derived PGH2 to PGD2,18 which activates 2 G-protein–coupled receptors, DP1 and DP2 (CRTH2).19,20 PGD2 is further dehydrated to the cyclopentenone PG, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2),21,22 with levels of PGD2 and 15d-PGJ2 peaking within the first few hours of acute peritonitis coincident with lymphocyte disappearance.11 Incidentally, the existence of 15d-PGJ2 in mammalian systems has been hotly debated over the years, but in these experiments, we used liquid chromatography–mass spectrometry–mass spectrometry (LC-MS-MS) to definitively confirm its presence in resolving inflammation.11 Adding BW245C (DP1 agonist15,16 ) to hPGD2S knockout mice reduced lymphocytes at 6 hours, with the clearance effects of DP1 on B cells (Figure 3F). 15(R)-15-methyl PGD2 (DP2 agonist17 ) had no effect on lymphocyte numbers in knockout mice, indicating that DP1 receptor activation is responsible for B-cell disappearance early in acute inflammation. The fate of CD3+ cells remains less clear. For instance, CD3 cells were examined for their adherence to the greater omental lymphoid organ, the so-called leukocyte disappearance reaction typical of peritoneal macrophages during acute peritonitis.23 But, addition of 5% trypsin to a 4-hour inflamed cavity for 10 minutes recovered displaced macrophages but not lymphocytes (Figure S7). Another possibility was that CD3 cells underwent programmed cell death in response to 15d-PGJ2, a highly reactive electrophile and, in our experience, a potent inducer of lymphocyte apoptosis. Although CD3 cells from hPGD2S−/− mice remained low for annexin V/propidium iodide labeling up to 6 hours, on a percentage basis there was a trend toward an increase in annexin V labeling within the equivalent population in wild-type mice. However, this did not reach significance due to the diminished numbers of peritoneal CD3 cells in wild-type mice available for analysis (data not included). Therefore, we can suggest that within a few hours of inducing an inflammatory response, peritoneal resident B cells disappear in a PGD2/DP1-dependent manner, but that the fate of CD3+ cells remains unclear.
PGD2 controls the clearance of peritoneal resident lymphocytes. In response to inflammatory stimuli, lymphocytes in the peritoneum disappear between 6 and 24 hours. (A-E) However, lymphocyte numbers in hPGD2S knockout mice at 6 hours (■) were found to be equivalent to that present in the naive cavity of wild-type mice (□), suggesting a role for either PGD2 and/or 15d-PGJ2 in the initial clearance of lymphocytes. (F) Adding back BW245C (DP1 receptor agonist) to hPGD2S knockout mice caused a reduction in B cells. Attempts made to identify the fate of CD3 cells generated inconclusive results, with data suggesting that they may die locally by programmed cell death (data not included). n = 8 animals per group. *P ≤ .05 as determined by Bonferroni t test, with data expressed as means plus or minus SEM.
PGD2 controls the clearance of peritoneal resident lymphocytes. In response to inflammatory stimuli, lymphocytes in the peritoneum disappear between 6 and 24 hours. (A-E) However, lymphocyte numbers in hPGD2S knockout mice at 6 hours (■) were found to be equivalent to that present in the naive cavity of wild-type mice (□), suggesting a role for either PGD2 and/or 15d-PGJ2 in the initial clearance of lymphocytes. (F) Adding back BW245C (DP1 receptor agonist) to hPGD2S knockout mice caused a reduction in B cells. Attempts made to identify the fate of CD3 cells generated inconclusive results, with data suggesting that they may die locally by programmed cell death (data not included). n = 8 animals per group. *P ≤ .05 as determined by Bonferroni t test, with data expressed as means plus or minus SEM.
Deficiency of repopulating lymphocytes in nonresolving chronic granulomatous disease
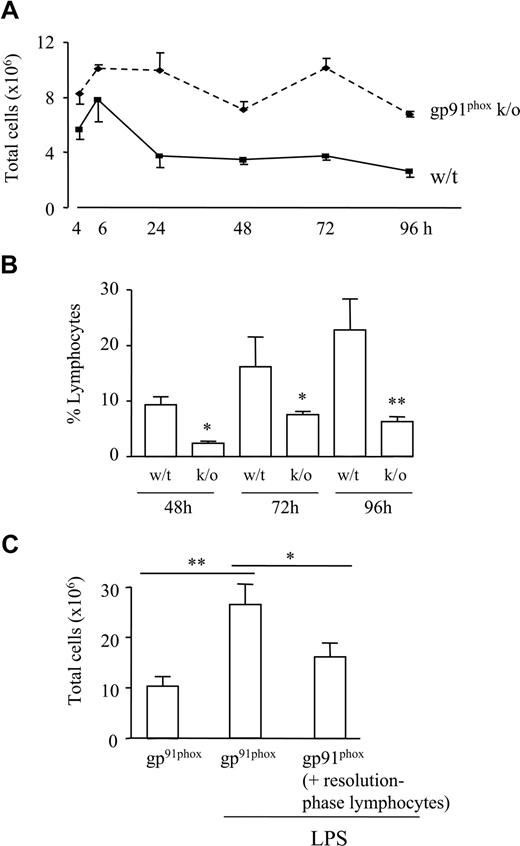
Finally, the relevance of these findings to human inflammatory diseases was determined by examining the role of lymphocytes in gp91phox knockout mice, an experimental model of human chronic granulomatous disease caused by defects in the phagocyte respiratory burst oxidase, which generates microbicidal superoxide.24,25 Hence, patients with chronic granulomatous disease lack antimicrobial capacity and the ability to combat bacterial and fungal infections. Moreover, there is the associated occurrence of inflammatory granulomas in lung, liver, and skin, which in some instances may arise from sterile stimuli, suggesting that their formation may be due to incomplete degradation of inflammatory debris and/or impaired resolution of inflammation.26,27 gp91phox knockout mice were injected intraperitoneally with sterile zymosan and found to have elevated leukocyte numbers compared with controls, with inflammation failing to resolve (Figure 4A). See also Figure S8 for comparison of cell types in both animals at resolution. Fewer lymphocytes were found at 48 to 96 hours in gp91phox knockout mice, the time frame of resolution and lymphocyte repopulation in wild-type mice (Figure 4B). Lymphocytes obtained from the resolution phase (72 hours) of normal strain-matched wild-type controls which were therefore composed of B1, NK, and γ/δ T cells as well as CD4+/CD25+ cells, were transferred back to gp91phox knockout mice (72 hours) and challenged with LPS. Inflammation was reduced in knockout mice that received resolution-phase lymphocytes compared with gp91phox mice alone (Figure 4C). These results suggest that during ongoing, nonresolving inflammation, the absence of lymphocytes may account for susceptibility to superinfection and the associated hyperinflammatory response.
Absence of repopulating lymphocytes during nonresolving inflammation. (A) Zymosan was injected into the peritoneal cavity of pg91phox knockout mice, which, when compared with controls, showed a more aggressive inflammatory response that failed to resolve. (B) FACS analysis of cell types present during resolution revealed a progressive repopulation of lymphocytes during resolution that was lower in pg91phox knockout mice. (C) Lymphocytes obtained from the resolution phase (72 hours) of normal strain-matched wild-type controls and comprising B1 cells, NK cells, and γ/δ T cells, as well as CD4+/CD25+ cells, were transferred back into the peritoneal cavity of gp91phox knockout mice (72 hours) and subsequently challenged, intraperitoneally, with LPS. Inflammation was reduced in gp91phox knockout mice that received resolution-phase lymphocytes compared with gp91phox mice alone. *P ≤ .05; **P ≤ .01 as determined by ANOVA, followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Absence of repopulating lymphocytes during nonresolving inflammation. (A) Zymosan was injected into the peritoneal cavity of pg91phox knockout mice, which, when compared with controls, showed a more aggressive inflammatory response that failed to resolve. (B) FACS analysis of cell types present during resolution revealed a progressive repopulation of lymphocytes during resolution that was lower in pg91phox knockout mice. (C) Lymphocytes obtained from the resolution phase (72 hours) of normal strain-matched wild-type controls and comprising B1 cells, NK cells, and γ/δ T cells, as well as CD4+/CD25+ cells, were transferred back into the peritoneal cavity of gp91phox knockout mice (72 hours) and subsequently challenged, intraperitoneally, with LPS. Inflammation was reduced in gp91phox knockout mice that received resolution-phase lymphocytes compared with gp91phox mice alone. *P ≤ .05; **P ≤ .01 as determined by ANOVA, followed by Bonferroni t test, with data expressed as means plus or minus SEM.
Discussion
Here, evidence is presented that lymphocytes play a pivotal role in controlling the onset of innate immune-mediated inflammation by regulating cytokine synthesis as well as host susceptibility to secondary infection. Analysis of lymphocyte subsets in the naive peritoneal cavity of mice revealed that B cells constitute about 70% to 80% of the total lymphocyte population, of which the majority have a B1 phenotype, with the remainder being B2 cells. CD3+ cells as well as NK and γ/δ cells make up the remaining 20% of lymphocytes. This profile differs to that found at resolution, which comprises more innate-type lymphocytes and B1 cells expressing MAC-1. Although we did not discern which lymphocyte or combination of lymphocytes bestowed protection at onset or at resolution, in a separate study, peritoneal CD3 T cells and in particular B220+ B cells were found to elaborate high levels of IL-10 in a DP1-dependent manner,11 most likely explaining the reduction in IL-10 in lymphocyte-deficient RAG1−/− mice and subsequent increase in PMN influx in these animals. Thus, given the relative proportion of T cells versus B1 cells in the inflamed cavity and the ability of B cells to synthesis comparatively high levels of IL-10, we suspect that B lymphocytes may one of the predominant cell types modulating acute inflammatory responses to nonspecific stimuli.
As PMNs begin to accumulate in response to zymosan, B cells disappeared in a PGD2-dependent manner, as there was equivalent numbers of B cells in the inflamed cavity of hPGD2S−/− at 6 hours as there was in the naive cavity of wild-type mice. This accumulation of B cells in hPGD2S knockout mice was reversed by BW245C, a DP1 receptor agonist, with DP2(CRTH2) playing no role in this setting. While the mechanism of PGD2-dependent B-cell clearance is unknown, a Toll-like receptor (TLR)–mediated transient down-regulation of integrins and CD9 on B1 cells was shown to be required for detachment of these cells from the local peritoneal matrix and their subsequent efflux from the inflamed cavity.28 Whether BW245C alters CD9 expression needs further investigation, but given that B cells disappear concomitantly with PGD2 synthesis, it is possible that DP1 activation may play a role in regulating this pathway of B1 cell detachment and efflux. The fate of CD3 cells, on the other hand, is less clear. While their attachment to the peritoneal lining can certainly be excluded, there is the possibility that these cells may die locally by programmed cell death in response to 15d-PGJ2, which is synthesized concomitant with their disappearance,11 and in our experience, a potent inducer of lymphocyte apoptosis. However, future detailed work is required to definitively identify whether they die locally or clear via draining lymphatics. Thus, PGD2 exerts a dual role on resident B cells at least—regulating their inflammatory cytokine release as well as their efflux from the inflamed peritoneal cavity.
The mechanism by which lymphocytes exert their protective effects in these experiments is unclear at this stage. Certainly, there is a cytokine imbalance favoring proinflammatory TNFα but reduced IL-10 in the inflamed peritoneum of RAG mice, thereby potentially explaining the enhanced influx of PMNs in these mice compared with wild-type. Indeed, we have shown that both T and B lymphocytes are capable of elaborating inflammatory cytokines, which, on balance, in the inflamed peritoneum at least, serve to limit PMN influx.11 However, in addition to cytokines/chemokines, cell adhesion molecules are also central to facilitating PMN adhesion and accumulation at sites of inflammation and, contrary to data presented here, there is evidence showing that lymphocytes trigger cell adhesion molecule expression. For instance, intracellular adhesion molecule 1 expression in Plasmodium-infected mice is reduced in the brain but not the lung of RAG1−/− mice, while P-selectin expression is attenuated in both organs in these animals.29 Equally, T cells were shown to enhance the expression of TNFα-triggered endothelial cell adhesion molecule expression, with these effects varying between vascular beds.30 On this basis, it is difficult to reconcile the role circulating lymphocytes play in up-regulating cell adhesion molecule expression on the leukocyte/endothelial cell interface to that of the resident peritoneal T/B cells other than to highlight the different profile of lymphocytes present in the peritoneum (B1, B2, and small numbers of CD4/CD25 cells) that exert a predominantly protective effect in both the naive and postresolution state.
The trigger for lymphocyte repopulation is unclear, but critical determinants of resolution such as PMN apoptosis or signals released by macrophages during phagocytosis of apoptotic leukocytes may play a central role. However, we have shown previously that inducible cyclo-oxygenase is expressed during and is essential for the resolution of acute inflammation,6,31,32 while others have reported that lipoxygenase-lipoxygenase interaction products of arachidonic as well as eicosapentaenoic and docosahexanoic acids dampen the severity of inflammatory onset and trigger resolution.33-35 Taking a closer look at whether cyclo-oxygenase or lipoxygenase play a role in lymphocyte repopulation, we found that not only is COX-2 expressed during the resolution phase of zymosan-induced peritonitis, but that its inhibition with either NS-398 or the nonselective COX inhibitor indomethacin impairs lymphocyte repopulation, in particular CD3+ cells. However, inhibition of lipoxygenase isoforms with baicalein was without effect. These data implicate a COX-2 metabolite in the recruitment of postresolution lymphocytes and therefore protection against host susceptibility to superinfection (J. Newsom, M. Stables, P.C.-N., G.B., and D.W.G., manuscript in preparation).
Comparing repopulating lymphocytes with those in the naive cavity revealed more NK cells, γ/δ T cells, and CD4+/CD25+ cells, in addition to B1 cells with higher Mac-1 labeling than that found at onset. The functional relevance of increased MAC-1 expression on resolving B1 cells is not apparent at this stage, but may reflect a state of differentiation/activation or a specific requirement for migration back to the resolved peritoneum. However, repopulating lymphocytes have no role in actively bringing about resolution, but protect against superinfection. In these experiments, GBS was given into the resolving peritoneal cavities of wild-type as well as RAG1−/− mice. Interestingly, the degree of inflammation in wild-type mice that received zymosan followed by GBS was not significantly greater than the level of inflammation in resolving wild-type mice not given GBS. In contrast, inflammation in RAG1−/− mice that received zymosan followed by GBS was almost twice that of inoculated wild-type mice. This suggests that resolution-phase lymphocytes confer protection against secondary infection. This was certainly confirmed by injecting live bacteria to RAG−/− mice undergoing resolution, which subsequently died substantially faster than wild-type mice treated in the same manner (Figure 2F). It is unknown why the proportion and profile of repopulating lymphocytes is different to that at onset. Perhaps as resolving tissues are physiologically altered as a consequence of the inflammatory event they underwent, host defense mechanisms need to be fundamentally different to guard against superinfection by recruiting more protective lymphocytes. On this theme, it is not really understood when acutely inflamed tissues revert back to their original state. Certainly, a population of macrophages (about 1 × 106) were found to linger for at least 3 weeks after a zymosan peritonitis apparently resolved, supporting the idea that although the original response was acute and transitory in terms of PMN influx and efflux, its effects may be longer-lasting than originally believed, thereby requiring a different profile and greater proportion of lymphocytes to modulate future inflammatory events. This may explain why inflammation in the resolving phase conferred greater protection against GBS lethality than uninflamed or naive mice (Figure 2D,F).
Taking these findings to a more clinically relevant setting, it became clear that unlike wild-type mice, there was a deficit of repopulating lymphocytes in nonresolving gp91phox knockout mice bearing zymosan-induced peritonitis. Replenishing gp91phox knockout mice with resolution-phase lymphocytes taken from strain-matched controls and then challenging animals with LPS conferred protection compared with sham-operated gp91phox knockout mice. Data from this study confirm that not only does lymphocyte repopulation fail to occur in nonresolving inflammation, but that resolving-phase lymphocytes protect against exaggerated inflammatory responses to superinfection (Figures 2D,4C). On this note, defects in innate lymphocyte functioning have been suggested to lead to secondary infections associated with burn injury,36 while increased lymphocyte apoptosis contributes to the pathogenesis of sepsis,4,5 underlining the crucial role lymphocytes play in host defense against nonspecific injury. Moreover, as NK cells were originally described for their ability to lyse tumor cells,37 and γ/δ T cells have well-known tumor surveillance properties,38,39 their absence from sites of nonresolving inflammation may be one of the predisposing factors to the development of inflammation-related cancer.40 Thus, one of the hazards of ongoing acute inflammation and consequently failed lymphocyte repopulation may be increased susceptibility to superinfection and even cancer as a result of lymphocyte apoptosis, lymphocyte immunoparalysis, or, as presented here, a failure of protective lymphocytes to repopulate after resolution.
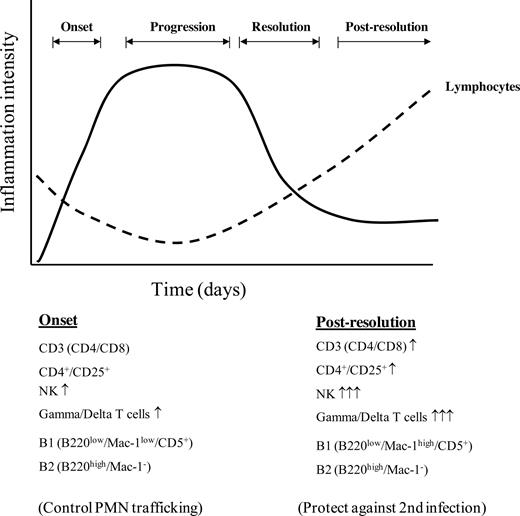
In summary, we report a biphasic role for lymphocytes during innate immune-mediated inflammation, summarized in Figure 5. The first phase controls PMN trafficking with lymphocytes then vacating the peritoneal cavity in response to PGD2 activating its DP1 receptor. The second phase is characterized by lymphocyte repopulation occurring after inflammation begins to resolve in a different proportion and profile to that of the naive state. Importantly, repopulating lymphocytes have no role in bringing about resolution, but protect against secondary infection.
A summary of the scheme of events that occurs in acute inflammation with reference to lymphocyte trafficking. As inflammation ensues resident lymphocytes begin to disappear, with B1 cells clearing via draining lymphatics and the fate of CD3 cells remaining unclear at this stage. Once inflammation begins to resolve, lymphocytes repopulate the site of injury in a profile different to that in the naive state.
A summary of the scheme of events that occurs in acute inflammation with reference to lymphocyte trafficking. As inflammation ensues resident lymphocytes begin to disappear, with B1 cells clearing via draining lymphatics and the fate of CD3 cells remaining unclear at this stage. Once inflammation begins to resolve, lymphocytes repopulate the site of injury in a profile different to that in the naive state.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge Dr Tracy Hussell (Kennedy Institute for Rheumatology, London, United Kingdom) for supplying antibodies to NK and γ/δ T cells and Dr Y. Urade of the Osaka Bioscience Institute (Japan) for supplying hPGD2S knockout mice.
D.W.G. is a Wellcome Trust funded Career Development Fellow and R.R. is a Kidney Research UK Funded Clinical Research Fellow.
Wellcome Trust
Authorship
Contribution: D.W.G. designed research and wrote the paper along with R.R., who carried out the research. T.L., G.B., J.B., and P.C.N. carried out experiments and provided essential experimental material. M.H., D.F., and M.M.Y. supplied essential experimental tools. D.W.G. and P.C.-N. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derek W. Gilroy, Centre for Clinical Pharmacology and Therapeutics, Division of Medicine, 5 University Street, University College London, London WC1E 6JJ, United Kingdom; e-mail: d.gilroy@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal