Abstract
Sphingosine-1-phosphate (S1P) is now emerging as a potent lipid mediator produced by mast cells that contributes to inflammatory and allergic responses. In contrast to its weak effect on degranulation of murine mast cells, S1P potently induced degranulation of the human LAD2 mast-cell line and cord blood–derived human mast cells (hMCs). S1P also stimulated production and secretion of cytokines, TNF-α and IL-6, and markedly enhanced secretion of a chemokine, CCL2/MCP-1, important modulators of inflammation. S1P is produced in mast cells by the 2 sphingosine kinases, SphK1 and SphK2. SphK1 but not SphK2 plays a critical role in IgE/Ag-induced degranulation, migration toward antigen, and CCL2 secretion from hMCs, as determined by specifically down-regulating their expression. However, both isoenzymes were required for efficient TNF-α secretion. Taken together, our data suggest that differential formation of S1P by SphK1 and SphK2 has distinct and important actions in hMCs.
Introduction
Mast cells (MCs) play pivotal roles in immediate-type and inflammatory allergic reactions initiated by cross-linking with antigen (Ag) of the antigen-specific immunoglobulin E (IgE) on their cell-surface high-affinity receptors for IgE (FcϵRI). MC activation leads to release of preformed mediators, such as histamine, localized in specialized granules, and the de novo synthesis and secretion of a plethora of cytokines, chemokines, eicosanoids, and the bioactive sphingolipid metabolite, sphingosine-1-phosphate (S1P). The combined actions of these mediators trigger symptoms associated with allergy and propagate inflammatory responses.
S1P levels are elevated in the bronchoalveolar lavage (BAL) fluid of asthmatics after Ag challenge, suggesting that secretion of S1P by MCs is of relevance in inflammatory responses and asthma.1 S1P is a ligand for 5 G protein–coupled receptors (GPCRs), designated S1P1-5, through which it exerts many of its actions.2,3 Indeed, S1P is secreted by activated MCs,4,5 and studies with rodent MCs have shown that this S1P is able to rapidly bind and activate its receptors, S1P1 and S1P2, in an autocrine manner. S1P1 induces cytoskeletal rearrangements, leading to the movement of MCs toward an Ag gradient, whereas activation of S1P2 is critical for degranulation.4
In rodent MCs and human bone marrow–derived mast cells (BMMCs), aggregation of FcϵRI leads to activation of both isoforms of sphingosine kinase, SphK1 and SphK2, the enzymes that produce S1P from sphingosine.4,6-8 Down-regulation of SphK1 but not SphK2 in these MCs inhibits calcium mobilization, degranulation, and migration induced by Ag.4,6,7 In human BMMCs and murine MCs, SphK1 translocates to the plasma membrane within minutes of FcϵRI clustering.4,6 SphK1 interacts with the protein tyrosine kinases, Src kinase members Lyn and Fyn (FcϵRI proximal kinases that initiate the signaling events following cross-linking of this receptor), but not Src or other tyrosine kinases, such as Syk. Following interaction with Lyn, SphK1 is recruited to membrane rafts where conformational changes may occur in both kinases that favor their respective activities.8 Although activation of both SphK1 and SphK2 required Fyn kinase in murine BMMCs, only SphK1 activation was dependent on the adapter Grb2-associated binder 2 and phosphatidylinositol 3-kinase.9
Based on results with fetal liver–derived MCs from SphK1−/−, SphK2−/−, and SphK1−/−SphK2−/− mice, it was suggested that SphK2, but not SphK1, may modulate calcium influx and downstream signaling, particularly PKCα and β, leading to degranulation and the production of eicosanoids and cytokines.10 By contrast, passive systemic anaphylaxis was impaired in SphK1−/− mice, but not in SphK2−/− mice, and plasma histamine levels were correlated with SphK1 expression and circulating levels of S1P. Hence, it was suggested that SphK2 is a determinant of intrinsic MC function, whereas SphK1 plays an extrinsic role.10,11 Nonetheless, given the differences in the gene expression profiles and functional differences between human and mouse MCs (reviewed in Bischoff12 ), SphK1 and SphK2 may play different roles in human MCs.6 Not much is known of the functions of SphK1 and SphK2 in human MCs, and only few studies have been focused on the potential roles of S1P in human MCs.
Here we demonstrate that in contrast to murine MCs, S1P potently induces degranulation of human MCs. Moreover, SphK1 but not SphK2 plays a critical role in Ag-induced degranulation and migration of both developing and mature human MCs. Nevertheless, both SphK1 and SphK2 are required for efficient cytokine secretion from human MCs. Our data indicates that S1P and the kinases that produce it are important for human mast cell (hMC) functions.
Methods
Culture and transfection of human mast cells
LAD2 cells were provided by Drs Arnold Kirshenbaum and Dean Metcalfe (NIH, Bethesda, MD). The cell line was established from bone marrow aspirates of a patient diagnosed with MC sarcoma/leukemia.13 LAD2 cells are closely related to CD34+-derived hMCs and express functional FcϵRI receptors.13 Cells were cultured in serum-free medium (StemPro-34; Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine, and 100 ng/mL recombinant human stem-cell factor (generous gift from Amgen, Thousand Oaks, CA).
All experimental protocols involving human tissues were approved by the Human Studies Committee at Virginia Commonwealth University (Richmond, VA). Informed consent was obtained in accordance with the Declaration of Helsinki. Umbilical cord blood was obtained at the time of delivery and collected in heparin-coated tubes. Stem cell factor (SCF)–dependent cord blood–derived mast cells (CB-MCs) were obtained as previously described.14 Briefly, umbilical cord blood was diluted 1:1 in PBS and erythrocytes were eliminated by 2 consecutive density gradient centrifugations. Cells at the interphase, consisting of mononuclear progenitor cells, were cultured for 8 to 12 weeks in RPMI 1640 supplemented with 10% heat-inactivated controlled process serum replacement medium-3 (Sigma-Aldrich, St Louis, MO), 2 mM l-glutamine, 0.1 mM nonessential amino acids, 10 mM HEPES, 50 μM 2-ME, 200 U/mL penicillin, and 100 μg/mL streptomycin. Contaminating macrophages/monocytes were depleted by negative selection with antihuman CD14 mAb-coated immunomagnetic beads (Dynal Biotech, Oslo, Norway).14 Purified CB-MCs were used between 8 and 12 weeks when more than 95% stained positively with toluidine blue.
siRNA transfection
Expression of SphK1 and SphK2 was down-regulated with sequence-specific siRNA from Qiagen as previously described.15 In addition, ON-TARGETplus SMARTpool siRNA against SphK1, SphK2, and control siRNA from Dharmacon (Lafayette, CO) was used to confirm lack of off-target effects. LAD2 cells were transfected with 100 nM sequence-specific ON-TARGETplus SMARTpool siRNAs against SphK1, SphK2, and control siRNA in Stem-Pro-34 medium using Lipofectamine 2000 (Invitrogen). CB-MCs were transfected with 100 nM siRNAs in RPMI 1640 culture medium, using Oligofectamine (Invitrogen).
Reverse-transcriptase PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) and was reverse transcribed with Superscript II (Invitrogen). Quantitative polymerase chain reaction (PCR) was performed with premixed primer-probe sets using the ABI 7800 (Applied Biosystems, Foster City, CA).
Degranulation and chemotaxis
MCs were sensitized overnight with 1 μg/mL dinitrophenyl (DNP)–specific mouse IgE produced as described previously.16 MCs were washed and stimulated with 30 ng/mL DNP-HSA (Ag; Sigma-Aldrich) at 37°C unless indicated otherwise. Secretion of granules was determined by measuring the release of the granule marker β-hexosaminidase with a colorimetric assay in which the production of p-nitrophenol from p-nitrophenyl-N-acetyl-β-d-glucosaminide is measured.4 Values are expressed as the percentage of total cellular β-hexosaminidase released into the medium. Chemotaxis was measured in transwells (Costar, Cambridge, MA) with 8-μm pore size. Culture medium (600 μL) was added to the lower chambers, and cells (105/100 μL culture medium) were added to the upper chambers. After 30 minutes, chemoattractants were added to the lower chamber and cells were allowed to migrate for 24 hours. Cell numbers in the upper and lower chambers were determined with a Coulter counter model Z1 (Beckman Coulter, Fullerton, CA).
Cytokine ELISAs
Human IL-6, TNF-α, and CCL2/MCP-1 were measured by enzyme-linked immunosorbent assay (ELISA) with purified biotinylated mouse or rat mAbs specific for each cytokine. Standard curves were prepared with recombinant cytokines (BD Biosciences, San Diego, CA). Assays were performed following the manufacturer's protocols, using Maxisorb 96-well plates (Nunc, Rochester, NY). Briefly, wells were coated overnight at 4°C with capture mAbs, blocked with PBS containing 10% FBS, washed in PBS/0.05% Tween 20, and incubated for 2 hours at room temperature with standards or samples diluted in PBS/10% FBS. Wells were washed, incubated with biotin detection mAb and streptavidin-HRP conjugate for 1 hour at room temperature, washed, and incubated with peroxidase substrate. Absorbance was measured at 450 nm with an EL800 microplate reader (Biotek, Winooski, VT). The lower limit of detection was 5 to 8 pg/mL.
Western blotting
Down-regulation of SphK1 and SphK2 proteins was confirmed by Western blotting using SphK1- and SphK2- specific antibodies, respectively, essentially as described previously.15
Statistical analysis
Experiments were repeated at least 3 times with consistent results. For each experiment, data from triplicate samples were calculated and expressed as means plus or minus SD. Values of P less than .05, as determined by Student t test, were considered significant.
Results
S1P stimulates degranulation and cytokine and chemokines release by the human LAD2 mast cell line
S1P is a recent addition to the plethora of mediators released by activated MCs.4,5 Because there are numerous important functional differences between human and murine MCs (reviewed in Bischoff12 ), it was important to examine the functions of S1P and the kinases that produce it in human MCs. To this end, we used the LAD2 human mast cell line that is closely related to CD34+-derived hMCs and expresses functional FcϵRI receptors.13 In addition to CD117 (receptor for SCF), these cells also express CD88, the receptor for the anaphylatoxin C5a, on their cell surface and approximately 90% are double-positive for tryptase and chymase (MCTC; data not shown), the phenotype predominant in skin MCs.17
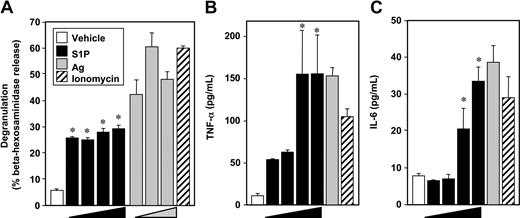
In contrast to the weak effect of S1P on degranulation of murine MCs,4,9,10,18 S1P potently induced degranulation of LAD2 human MCs (Figure 1A), as assessed by beta-hexosaminidase release, albeit to a lesser extent than Ag. Although there were no significant effects of S1P at concentrations of 0.1 nM and lower, maximal S1P-induced degranulation was observed at 1 nM (Figure 1A). In contrast, when sensitized LAD2 cells were treated with various concentrations of Ag, a typical bell-shaped dose response for degranulation was observed (Figure 1A).
S1P induces degranulation of human LAD2 mast cells and cytokine release. LAD2 cells were treated with vehicle (□), increasing concentrations of S1P (1 nM, 10 nM, 100 nM, 1 μM, ■), or ionomycin (1 μM, ▨) for 2 hours, or sensitized overnight with anti-DNP IgE (1 μg/mL), washed, and then stimulated for 2 hours with increasing concentrations of Ag (10, 30, 100 ng/mL,  ) or with 30 ng/mL Ag, as indicated. Degranulation was assessed by β-hexosaminidase release (A). Secretion of TNFα (B) and IL-6 (C) was measured by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P < .01.
) or with 30 ng/mL Ag, as indicated. Degranulation was assessed by β-hexosaminidase release (A). Secretion of TNFα (B) and IL-6 (C) was measured by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P < .01.
S1P induces degranulation of human LAD2 mast cells and cytokine release. LAD2 cells were treated with vehicle (□), increasing concentrations of S1P (1 nM, 10 nM, 100 nM, 1 μM, ■), or ionomycin (1 μM, ▨) for 2 hours, or sensitized overnight with anti-DNP IgE (1 μg/mL), washed, and then stimulated for 2 hours with increasing concentrations of Ag (10, 30, 100 ng/mL,  ) or with 30 ng/mL Ag, as indicated. Degranulation was assessed by β-hexosaminidase release (A). Secretion of TNFα (B) and IL-6 (C) was measured by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P < .01.
) or with 30 ng/mL Ag, as indicated. Degranulation was assessed by β-hexosaminidase release (A). Secretion of TNFα (B) and IL-6 (C) was measured by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P < .01.
These potent effects of S1P on degranulation suggest that LAD2 cells express functional S1P2 receptors at the cell surface, since we previously established that this S1P receptor is involved in degranulation of rodent MCs.4 In agreement, FTY720-phosphate, an agonist of all S1P receptors except S1P2, did not induce degranulation.19 Moreover, quantitative real-time PCR (QPCR) revealed that like rodent MCs, LAD2 cells as well as cord blood–derived human MCs and human skin MCs express similar levels of S1P1 and S1P2, but do not express S1P3-5 receptors (data not shown).
S1P at 1 μM was nearly as effective as Ag or ionomycin at stimulating secretion of TNF-α and IL-6 (Figure 1B,C), 2 very important pleiotropic cytokines that elicit a general inflammatory response and have been implicated in IgE-associated chronic allergic diseases.20 In contrast to its effect on degranulation, concentrations of S1P of 1 and 10 nM only slightly increased TNF-α and had no effects on IL-6 secretion.
Roles of SphK1 and SphK2 in activation of LAD2 cells by antigen
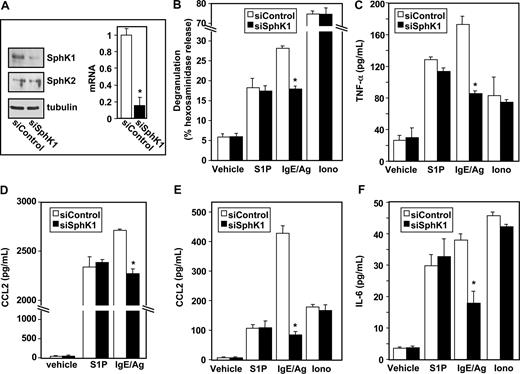
Having established that S1P can induce degranulation and cytokine release from LAD2 cells (Figure 1), and is secreted by them upon antigenic stimulation,5 we next examined the involvement of the SphKs that produce S1P in Ag-induced activation of human MCs by down-regulation of their expression. LAD2 cells express similar levels of SphK1 and SphK2 mRNA and siRNA targeted to SphK1 reduced expression of SphK1 but not SphK2 as determined by QPCR and Western blotting (Figure 2A, and data not shown). Down-regulation of SphK1 also markedly reduced IgE/Ag-induced degranulation of LAD2 cells, without altering S1P- or ionomycin-induced degranulation (Figure 2B). Moreover, siSphK1 but not siControl significantly reduced secretion of TNF-α (Figure 2C) and IL-6 (Figure 2F), whereas the secretion of these cytokines in response to S1P or ionomycin, as expected, was unaffected.
Down-regulation of SphK1 decreases antigen-induced degranulation and cytokine and chemokine secretion from human LAD2 mast cells. LAD2 cells were transfected with control siRNA (□) or SphK1 siRNA (■) as described in “siRNA transfection.” (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK1 or anti-SphK2 antibodies. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and mRNA levels of SphK1 were determined by quantitative real-time PCR. Data are expressed relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-F) Duplicate cultures were treated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours (B-D,F) or 30 minutes (E). Degranulation (B) and secretion of TNF-α (C), CCL2 (D,E), and IL-6 (F) was determined by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
Down-regulation of SphK1 decreases antigen-induced degranulation and cytokine and chemokine secretion from human LAD2 mast cells. LAD2 cells were transfected with control siRNA (□) or SphK1 siRNA (■) as described in “siRNA transfection.” (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK1 or anti-SphK2 antibodies. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and mRNA levels of SphK1 were determined by quantitative real-time PCR. Data are expressed relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-F) Duplicate cultures were treated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours (B-D,F) or 30 minutes (E). Degranulation (B) and secretion of TNF-α (C), CCL2 (D,E), and IL-6 (F) was determined by ELISA. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
Similar to Ag, treatment of LAD2 cells with S1P also induced secretion of CCL2 (Figure 2D), previously known as monocyte chemoattractant protein (MCP-1), an important modulator of monocyte recruitment that plays a major role in a MC-dependent asthma model.21 siSphK1 also reduced the large increase of Ag-induced CCL2 secretion but not of that induced by S1P (Figure 2D). Even a stronger reduction of Ag-induced secretion of CCL2 was observed after a short period of stimulation (Figure 2E).
In contrast to recent studies with rodent MCs showing that ablating SphK2 expression abolishes Ag-induced degranulation,10 down-regulating SphK2 in LAD2 cells, which markedly and specifically reduced its mRNA and protein levels (Figure 3A), had no significant effect on degranulation induced by Ag, S1P, or ionomycin (Figure 3B). However, secretion of TNF-α and IL-6 in response to Ag was drastically reduced by 80% and 75%, respectively (Figure 3C,D). Of note, down-regulating SphK2 did not significantly affect secretion of these cytokines induced by S1P or ionomycin (Figure 3C,D). However, in contrast to down-regulation of SphK1, secretion of CCL2 in response to Ag was not significantly altered by down-regulation of SphK2 (Figure 3E).
Down-regulation of SphK2 does not alter antigen-induced degranulation, but impairs antigen-induced cytokine secretion from human LAD2 mast cells. LAD2 cells were transfected for 48 hours with control siRNA (□) or SphK2 siRNA ( ). (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK2. Lysate from cells overexpressing SphK2 was loaded as a positive control (left lane). Note that due to the high level of expression of SphK2, this lane from the same gel was developed for a much shorter time; a vertical line has been inserted to indicate this distinction. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and SphK2 mRNA levels were determined by quantitative real-time PCR. Data shown are relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-E) Duplicate cultures were stimulated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM) or ionomycin (1 μM). Degranulation (B) and secretion of TNF-α (C) and IL-6 (D) were determined after 2 hours, and secretion of CCL2 (E) was determined after 30 minutes. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
). (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK2. Lysate from cells overexpressing SphK2 was loaded as a positive control (left lane). Note that due to the high level of expression of SphK2, this lane from the same gel was developed for a much shorter time; a vertical line has been inserted to indicate this distinction. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and SphK2 mRNA levels were determined by quantitative real-time PCR. Data shown are relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-E) Duplicate cultures were stimulated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM) or ionomycin (1 μM). Degranulation (B) and secretion of TNF-α (C) and IL-6 (D) were determined after 2 hours, and secretion of CCL2 (E) was determined after 30 minutes. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
Down-regulation of SphK2 does not alter antigen-induced degranulation, but impairs antigen-induced cytokine secretion from human LAD2 mast cells. LAD2 cells were transfected for 48 hours with control siRNA (□) or SphK2 siRNA ( ). (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK2. Lysate from cells overexpressing SphK2 was loaded as a positive control (left lane). Note that due to the high level of expression of SphK2, this lane from the same gel was developed for a much shorter time; a vertical line has been inserted to indicate this distinction. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and SphK2 mRNA levels were determined by quantitative real-time PCR. Data shown are relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-E) Duplicate cultures were stimulated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM) or ionomycin (1 μM). Degranulation (B) and secretion of TNF-α (C) and IL-6 (D) were determined after 2 hours, and secretion of CCL2 (E) was determined after 30 minutes. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
). (A) Equal amounts of lysates (10 μg) were immunoblotted with anti-SphK2. Lysate from cells overexpressing SphK2 was loaded as a positive control (left lane). Note that due to the high level of expression of SphK2, this lane from the same gel was developed for a much shorter time; a vertical line has been inserted to indicate this distinction. Blots were stripped and reprobed with antitubulin to ensure equal loading and transfer. RNA was isolated and SphK2 mRNA levels were determined by quantitative real-time PCR. Data shown are relative to cells treated with control siRNA, calculated according to the 2−ΔΔCt method. (B-E) Duplicate cultures were stimulated with vehicle, IgE/Ag (30 ng/mL), S1P (100 nM) or ionomycin (1 μM). Degranulation (B) and secretion of TNF-α (C) and IL-6 (D) were determined after 2 hours, and secretion of CCL2 (E) was determined after 30 minutes. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01.
SphK1, but not SphK2, is required for migration of hMCs toward antigen
The expression of S1P1 in LAD2 cells is noteworthy since this receptor is critical for chemotaxis of rodent MCs toward S1P and Ag.4 Using transwell migration assays, we found that LAD2 cells migrate toward low concentrations of S1P (Figure 4A). Moreover, these cells also migrate toward increasing concentrations of Ag or S1P with typical bell-shaped responses (Figure 4A,B). Interestingly, sensitization with IgE also promoted their migration even in the absence of Ag (Figure 4B). It has been demonstrated that monomeric IgE can promote MC survival and activation,22-24 likely due to aggregation of FcϵRI in the absence of Ag. It is possible that monomeric IgE alone may increase migration of MCs by inducing secretion of soluble factors, such as S1P, that then act in an autocrine or paracrine manner.
Chemotaxis of mast cells toward antigen and S1P is pertussis toxin sensitive. Migration of (A) unsensitized LAD2 cells toward increasing concentrations of S1P (0.1, 1, 10, 20 μM) or FN (20 μg/mL) and (B) IgE-sensitized LAD2 cells toward increasing concentrations of Ag (10, 30, 100 ng/mL) was determined in transwell chambers. *P ≤ .01 compared with vehicle in unsensitized cells. **P ≤ .01 compared with vehicle in sensitized cells. (C) S1P (10 μM) or Ag (30 ng/mL) was added to either the bottom chamber ( ) or to both the top and bottom chambers (
) or to both the top and bottom chambers ( ), and migration of LAD2 was determined. (D) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,
), and migration of LAD2 was determined. (D) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,  ) were allowed to migrate toward vehicle, S1P (1 μM), IgE/Ag (30 ng/mL), or fibronectin (20 μg/mL). (E) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,
) were allowed to migrate toward vehicle, S1P (1 μM), IgE/Ag (30 ng/mL), or fibronectin (20 μg/mL). (E) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,  ) were treated with vehicle, S1P, anti-DNP IgE and DNP-HSA (IgE/Ag), or ionomycin (1 μM), and degranulation was determined by β-hexosaminidase release. (F,G) Chemotaxis of mast cells toward antigen is dependent on SphK1 but not on SphK2. (F) LAD2 cells were transfected with control siRNA (□) or siRNA targeted to SphK1 (■) or (G) siRNA targeted to SphK2 (
) were treated with vehicle, S1P, anti-DNP IgE and DNP-HSA (IgE/Ag), or ionomycin (1 μM), and degranulation was determined by β-hexosaminidase release. (F,G) Chemotaxis of mast cells toward antigen is dependent on SphK1 but not on SphK2. (F) LAD2 cells were transfected with control siRNA (□) or siRNA targeted to SphK1 (■) or (G) siRNA targeted to SphK2 ( ). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 5 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. Similar results were obtained in 3 independent experiments.
). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 5 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. Similar results were obtained in 3 independent experiments.
Chemotaxis of mast cells toward antigen and S1P is pertussis toxin sensitive. Migration of (A) unsensitized LAD2 cells toward increasing concentrations of S1P (0.1, 1, 10, 20 μM) or FN (20 μg/mL) and (B) IgE-sensitized LAD2 cells toward increasing concentrations of Ag (10, 30, 100 ng/mL) was determined in transwell chambers. *P ≤ .01 compared with vehicle in unsensitized cells. **P ≤ .01 compared with vehicle in sensitized cells. (C) S1P (10 μM) or Ag (30 ng/mL) was added to either the bottom chamber ( ) or to both the top and bottom chambers (
) or to both the top and bottom chambers ( ), and migration of LAD2 was determined. (D) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,
), and migration of LAD2 was determined. (D) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,  ) were allowed to migrate toward vehicle, S1P (1 μM), IgE/Ag (30 ng/mL), or fibronectin (20 μg/mL). (E) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,
) were allowed to migrate toward vehicle, S1P (1 μM), IgE/Ag (30 ng/mL), or fibronectin (20 μg/mL). (E) LAD2 cells pretreated for 1.5 hours without (□) or with pertussis toxin (PTX, 100 ng/mL,  ) were treated with vehicle, S1P, anti-DNP IgE and DNP-HSA (IgE/Ag), or ionomycin (1 μM), and degranulation was determined by β-hexosaminidase release. (F,G) Chemotaxis of mast cells toward antigen is dependent on SphK1 but not on SphK2. (F) LAD2 cells were transfected with control siRNA (□) or siRNA targeted to SphK1 (■) or (G) siRNA targeted to SphK2 (
) were treated with vehicle, S1P, anti-DNP IgE and DNP-HSA (IgE/Ag), or ionomycin (1 μM), and degranulation was determined by β-hexosaminidase release. (F,G) Chemotaxis of mast cells toward antigen is dependent on SphK1 but not on SphK2. (F) LAD2 cells were transfected with control siRNA (□) or siRNA targeted to SphK1 (■) or (G) siRNA targeted to SphK2 ( ). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 5 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. Similar results were obtained in 3 independent experiments.
). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 5 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. Similar results were obtained in 3 independent experiments.
We next determined whether the effects of Ag and S1P were mediated by enhanced directed migration in response to the gradient of chemoattractant (chemotaxis) or by increased random motility due to the presence of the chemoattractant itself (chemokinesis). The greatest numbers of cells were found to migrate along the chemotactic gradient, that is, toward increasing concentrations of Ag or S1P in the bottom chamber (Figure 4A-C). In sharp contrast, there was no increase in migratory cells when concentrations of Ag or S1P were the same in the top and bottom chambers (Figure 4C), indicating that Ag and S1P stimulate chemotactic rather than chemokinetic responses. Moreover, as with rodent MCs,4 pertussis toxin (PTX) ablated migration toward Ag and S1P, implicating a Gi-dependent pathway, without affecting migration toward fibronectin (Figure 4D). However, pertussis toxin did not inhibit degranulation in response to Ag or S1P (Figure 4E), in agreement with previous studies.4
Similar to previous results with rodent MCs, down-regulation of SphK1 expression markedly inhibited movement of LAD2 cells toward Ag (Figure 4F). As expected, down-regulation of SphK1 did not affect migration of these cells toward S1P, the product of SphK1. Down-regulating expression of SphK1 did not have a general inhibitory effect on motility of LAD2 cells because migration toward fibronectin was not affected (Figure 4F). Moreover, although siSphK2 markedly reduced SphK2 expression (Figure 3A), it had no significant effect on migration of these MCs toward Ag, S1P, or fibronectin (Figure 4G).
S1P is a strong inducer of hMC degranulation and secretion of CCL2
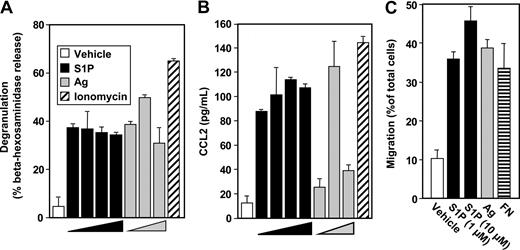
It was important to examine the effect of S1P and the roles of SphK1 and SphK2 in regulation of primary human MC functions. S1P potently induced degranulation of human cord blood-derived mast cells (hMCs; Figure 5A). Of note, S1P was nearly as effective as Ag (Figure 5A). As with LAD2 cells, degranulation was observed at a concentration of S1P as low as 1 nM, lower than the Kd for S1P receptors, suggesting that activation of only a limited number of S1P receptors is required. These results further substantiate that S1P is much more effective in degranulating human MCs than rodent MCs.
Effect of S1P on hMC functions. Purified cord blood–derived mast cells (hMCs) were treated with vehicle, increasing concentrations of S1P (1 nM, 10 nM, 100 nM, 1 μM), or ionomycin (1 μM) for 2 hours, or sensitized overnight with anti-DNP IgE (1 μg/mL) washed, and then stimulated with increasing concentrations of Ag (10, 30, 100 ng/mL) for 2 hours. Degranulation was assessed by β-hexosaminidase release (A), and secretion of CCL2 was measured by ELISA (B). Data are the means plus or minus SD of triplicate determinations from a single experiment. Similar results were obtained using cells from 2 different donors. (C) Migration of IgE-sensitized hMCs toward increasing concentrations of S1P (1, 10 μM), Ag (30 ng/mL), or FN (20 μg/mL) was determined in transwell chambers.
Effect of S1P on hMC functions. Purified cord blood–derived mast cells (hMCs) were treated with vehicle, increasing concentrations of S1P (1 nM, 10 nM, 100 nM, 1 μM), or ionomycin (1 μM) for 2 hours, or sensitized overnight with anti-DNP IgE (1 μg/mL) washed, and then stimulated with increasing concentrations of Ag (10, 30, 100 ng/mL) for 2 hours. Degranulation was assessed by β-hexosaminidase release (A), and secretion of CCL2 was measured by ELISA (B). Data are the means plus or minus SD of triplicate determinations from a single experiment. Similar results were obtained using cells from 2 different donors. (C) Migration of IgE-sensitized hMCs toward increasing concentrations of S1P (1, 10 μM), Ag (30 ng/mL), or FN (20 μg/mL) was determined in transwell chambers.
In agreement with previous results,25 hMCs generated and maintained in SCF secrete only minute amounts of TNF-α in response to FcϵRI cross-linking (data not shown). In contrast, passive sensitization and challenge of hMCs with Ag induced a significant amount of CCL2 secretion (Figure 5B). S1P also markedly enhanced release of CCL2 almost to the same extent as Ag (Figure 5B). Moreover, similar to LAD2 cells, hMCs also migrate toward Ag and S1P (Figure 5C).
SphK1 is important for Ag-induced degranulation and migration of hMCs, whereas both SphK1 and SphK2 are critical for TNF-α
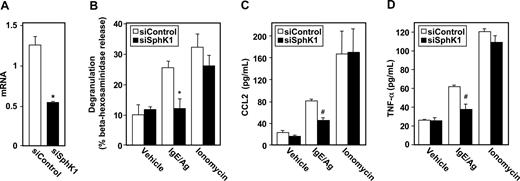
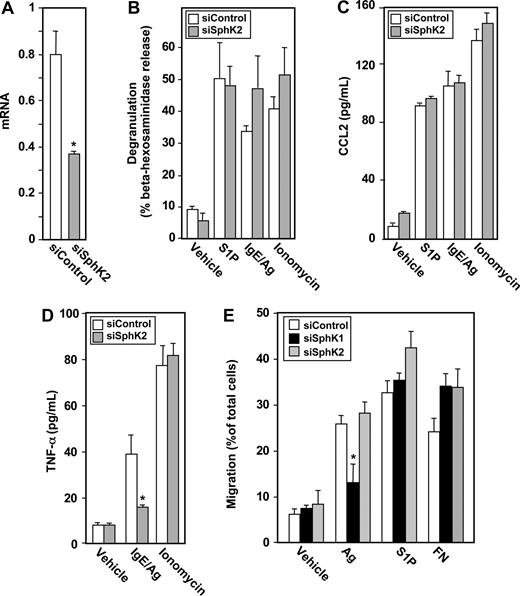
Because SphK1 and SphK2, the kinases responsible for production of S1P, are expressed at similar levels in hMCs, we next examined their involvement in Ag-induced degranulation. Down-regulation of SphK1 with isozyme-specific siRNA significantly and specifically reduced its mRNA (Figure 6A). Similar to the results obtained with LAD2 cells, siSphK1 nearly completely abrogated degranulation of hMCs induced by FcϵRI triggering, while scrambled siRNA did not exert any effect (Figure 6B). Ionomycin-induced degranulation was also unaffected by down-regulation of SphK1 (Figure 6B). Reducing expression of SphK1 in hMCs also significantly decreased secretion of CCL2 (Figure 6C) and TNF-α (Figure 6D) in response to Ag. As expected, secretion of CCL2 and TNF-α induced by ionomycin was unaltered (Figure 6C,D). In addition, similar to LAD2 cells, SphK1 was critical for migration of hMCs toward Ag (Figure 7E).
SphK1 is important for degranulation of hMCs and CCL2 and TNF-α secretion. Purified cord blood–derived mast cells (hMCs) were transfected with control siRNA (□) or SphK1 siRNA (■). (A) SphK1 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with 30 ng/mL Ag or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01, #P ≤ .05.
SphK1 is important for degranulation of hMCs and CCL2 and TNF-α secretion. Purified cord blood–derived mast cells (hMCs) were transfected with control siRNA (□) or SphK1 siRNA (■). (A) SphK1 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with 30 ng/mL Ag or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. *P ≤ .01, #P ≤ .05.
SphK2 is dispensable for degranulation and migration of hMCs but is required for secretion of TNF-α. Purified cord blood–derived human mast cells (hMCs) were transfected with control siRNA (□) or SphK2 siRNA ( ). (A) SphK2 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. (E) hMCs were transfected with control siRNA (□), siRNA targeted to SphK1 (■), or siRNA targeted to SphK2 (
). (A) SphK2 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. (E) hMCs were transfected with control siRNA (□), siRNA targeted to SphK1 (■), or siRNA targeted to SphK2 ( ). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 12 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. *P ≤ .01.
). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 12 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. *P ≤ .01.
SphK2 is dispensable for degranulation and migration of hMCs but is required for secretion of TNF-α. Purified cord blood–derived human mast cells (hMCs) were transfected with control siRNA (□) or SphK2 siRNA ( ). (A) SphK2 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. (E) hMCs were transfected with control siRNA (□), siRNA targeted to SphK1 (■), or siRNA targeted to SphK2 (
). (A) SphK2 mRNA levels normalized to GAPDH mRNA were measured by QPCR. (B-D) hMCs were sensitized overnight with IgE and then treated with Ag (30 ng/mL), S1P (100 nM), or ionomycin (1 μM) for 2 hours. Degranulation (B) and secretion of CCL2 (C) and TNF-α (D) were determined. Data are the means plus or minus SD of triplicate determinations. Similar results were obtained in 3 independent experiments. (E) hMCs were transfected with control siRNA (□), siRNA targeted to SphK1 (■), or siRNA targeted to SphK2 ( ). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 12 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. *P ≤ .01.
). Cells were then sensitized with 1 μg/mL anti-DNP IgE for 12 hours, and allowed to migrate toward vehicle, Ag (30 ng/mL), S1P (1 μM), or fibronectin (20 μg/mL) for 24 hours. Data are expressed as percentage migrating cells and are means plus or minus SD. *P ≤ .01.
Although siRNA targeted to specific sequences of SphK2 reduced its expression by more than 50% (Figure 7A), it had no significant effects on degranulation or secretion of CCL2 induced by Ag or ionomycin in hMCs (Figure 7B,C) or their migration toward Ag (Figure 7E). Nevertheless, Ag-induced, but not ionophore-induced secretion of TNF-α was decreased by approximately 50% by reducing SphK2 expression (Figure 7D).
Discussion
The observation that S1P levels are elevated in the BAL fluid of asthmatics when challenged with antigen1 suggested that it might play an important role in hMC responses. This finding led to studies mainly with murine mast cells that convincingly demonstrated that activated mast cells produce and secrete S1P upon antigen-induced activation of SphK1 and SphK2, the kinases that produce it. Moreover, secreted S1P regulates mast-cell degranulation and cytokine and chemokine production in an autocrine and/or paracrine manner through activation of their S1P receptors.4 In this study, we examined the roles of S1P formed by SphK1 and SphK2 in regulation of the functions of hMCs. We discovered that S1P is a much more potent regulator of human mast cells than of rodent mast cells, and is capable of inducing robust degranulation and cytokine and chemokine production at physiological concentrations.
MCs develop in peripheral tissues from bone marrow–derived, circulating committed progenitors, into 2 subpopulations that vary in the composition of their intragranular proteases: those expressing tryptase only are designated MCT, predominant in lung, whereas those that also contain chymase, designated MCTC, are predominant in skin. Importantly, S1P triggered degranulation of both LAD2 cells, which are mainly MCTCs, and SCF-dependent cord blood–derived MCs, which are MCTs. Degranulation of hMCs induced by S1P was accompanied by secretion of the proinflammatory cytokines IL-6 and TNF-α, which mediate acute inflammatory reactions, and the chemokine, CCL2/MCP-1, a major monocyte and macrophage chemoattractant that plays an important role at least in an MC-dependent rodent asthma model.21 Because CCL2 does not localize to secretory granules but rather in distinct intracytoplasmic vesicle-like clusters,26 this suggests that S1P has a broad effect on MCs affecting release of numerous mediators. Of note, a related lysophospholipid mediator, lysophosphatidic acid, also induces the generation of this proinflammatory chemokine by hMCs. However, in contrast to S1P, this required IL-4.27
Previous studies have shown that FcϵRI cross-linking stimulates SphK1 and SphK2.9 Down-regulation of SphK1, but not SphK2, with sequence-specific siRNA markedly reduced Ag-induced degranulation and CCL2 secretion in both LAD2 cells and primary human MCs. These results are consistent with previous findings with human BMMCs6 and murine BMMCs.4 In contrast, using fetal liver–derived mast cells from mice with a genetic deletion of SphK1 or SphK2, a recent study demonstrated a critical role for SphK2 and not SphK1 in degranulation.10 These important differences might result from known differences in responses of human mast cells compared with murine mast cells. Consistent with this, human MCs but not rodent MCs readily degranulate in response to exogenous S1P. Alternatively, SphK2 expression might be important for development of fully functional mast cells differentiated in vitro from fetal liver progenitors. This notion is supported by the inability of exogenous S1P to restore degranulation or calcium influx in SphK2−/− mast cells.10 It is also possible that SphK2 has an as-yet-unidentified role in mast cells that is independent of S1P and S1P receptors.
Similar to rodent mast cells,4 only SphK1 was critical for migration of human mast cells toward Ag. Although increased numbers of mast cells have been observed in pulmonary mucosa of asthmatic patients compared with healthy subjects,28 the underlying mechanism is not completely understood. Activation of SphK1 and secretion of S1P in response to FcϵRI triggering,4,5 as well as the increased level of S1P in the allergically stimulated lung of asthmatics,1 suggest that S1P may play an important role in the recruitment of mast cells to the lung. S1P may also mediate trafficking of mast cells that control food allergic responses.29
Although SphK2 was dispensable for degranulation and CCL2 secretion by activated hMCs as well as their migration toward Ag, its down-regulation significantly impaired secretion of TNF-α. This observation is reminiscent of the dependency on SphK2 expression for TNF-α secretion by fetal mouse liver-derived mast cells.10 These results indicate that SphK2 might play an important role in regulation of release of this cytokine that is important for exacerbation of allergic responses and is overproduced in asthmatic subjects.30
Altogether, our study reinforces the notion that SphK1 and S1P are critical for hMC activation and functions that have been implicated in allergic responses, such as asthma and anaphylaxis. Interestingly, passive systemic anaphylaxis was impaired in SphK1−/− mice, but not in SphK2−/− mice. Moreover, although circulating S1P levels were primarily determined by SphK1 expression outside of the mast-cell compartment, they correlated with histamine release and anaphylaxis.10 The exquisite sensitivity of human mast cells to extracellular S1P suggests that even small increases in circulating S1P could enhance and amplify their degranulation and as a consequence, allergic responses. Interestingly, S1P administration via the airways but not via the vasculature induces lung leakage and it has been suggested that spatially and mechanistically distinct S1P receptor subtypes have opposing effects on pulmonary epithelial and endothelial barrier functions.31,32 In contrast to activation of S1P1 receptor on endothelial cells leading to downstream assembly and stabilization of cell-cell junctions,33 increased S1P in the alveolar space causes activation of S1P3 in alveolar epithelium and results in increased permeability via tight junction opening likely through Rho.31 Therefore, regulation of lung endothelial and epithelial barriers by S1P constitutes another facet through which it might influence the vascular permeability changes seen in asthma and anaphylaxis. Because of the important role that SphK1 plays in regulating S1P levels within (this study) and outside10,11 of the mast-cell compartment, it is hoped that drugs that specifically target this isoenzyme would be useful to treat allergic diseases in humans, including asthma and anaphylaxis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Labor and Delivery team at the VCU Health System for kindly providing us with human umbilical cord blood samples; Drs D. D. Metcalfe and A. S. Kirshenbaum (NIH) for LAD2 cells; and Amgen for SCF.
This work was supported by NIH Grants K01AR053186 (C.A.O.) and RO1AI50094 (S.S.), and the Intramural Research Program of the National Institute of Mental Health (S.M.).
National Institutes of Health
Authorship
Contribution: C.A.O. made the initial discovery, performed research, analyzed data, and wrote the first draft; S.E.A., N.C.H., and M.M.P. performed research; S.M. analyzed data and wrote the paper; S.S. directed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah Spiegel, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, 1101 E Marshall St, Richmond, VA 23298-0614; e-mail: sspiegel@vcu.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal