Abstract
To determine the effect of posttransplantation immunotherapy with IL-2 on the progression-free survival (PFS) and overall survival (OS) of patients with non-Hodgkin lymphoma (NHL) after autologous stem-cell transplantation (PBSCT), patients with previously treated NHL were treated with cyclophosphamide, etoposide, total body irradiation (TBI), and PBSCT. Twenty-eight to 80 days after PBSCT, patients were randomized to IL-2 versus observation. Three hundred seventy-six eligible patients were registered (with 4-year PFS of 34% and 4-year OS of 52%), and 194 eligible patients were randomized to continuous infusion intravenous IL-2 (9 million units/m2/day for 4 days followed 5 days later by 1.6 million units/m2/day for 10 days) versus observation. In randomized patients, there was no significant difference in PFS (hazard ratio of IL-2 to observation = 0.90; P =.56) or in OS (hazard ratio of IL-2 to observation = 0.88; P =.55). There were no deaths related to IL-2 treatment. Grade 4 IL-2–related toxicities (n = 14) were reversible. These results confirm earlier SWOG findings that cyclophosphamide, etoposide, TBI, and PBSCT can be administered to patients with relapsed/refractory NHL with encouraging PFS and OS. Posttransplantation IL-2 given at this dose and schedule of administration had no significant effect on PFS or OS. This study is registered at www.clinicaltrials.gov as NCT00002649.
Introduction
Patients who relapse after achieving complete remission (CR) or never achieve CR of non-Hodgkin lymphoma (NHL) can rarely be cured with further chemotherapy. However, high-dose chemoradiotherapy with autologous bone marrow or peripheral blood stem-cell rescue is curative in some patients and is superior to conventional chemotherapy.1 Chemosensitivity of the lymphoma is one of the most important predictors of outcome with autologous transplantation.2 Intensification of conditioning regimens has not resulted in an increase in disease-free survival but is associated with an increase in transplantation-related deaths. The success of high-dose chemoradiotherapy and stem-cell transplantation (PBSCT) for patients with advanced hematologic malignancies is limited primarily by a high incidence of relapse.3
The cytoreductive regimen used in this protocol consisted of high-dose cyclophosphamide, etoposide, and total body irradiation (TBI). Petersen et al performed a phase 1 study determining that high-dose combinations of etoposide, cyclophosphamide, and TBI were tolerable in autologous transplantation patients,4 and these results were confirmed and extended in other single-institution trials.5-8 SWOG conducted a phase 2 multi-institutional trial of etoposide, cyclophosphamide, and TBI followed by autologous bone marrow transplantation for relapsed/refractory NHL and found that patients with resistant disease appeared to have an improved outcome compared with historical controls treated with less intensive preparative regimens.9
IL-2 stimulates the proliferation of antigen-specific T cells, induces the secretion of other cytokines such as interferon-γ and tumor necrosis factor, and augments the cytolytic function of cytotoxic T lymphocytes and natural killer cells.10,11 Treatment with IL-2 has induced responses, complete and partial, in some patients with advanced lymphoma.12 Two phase 1b trials of continuous intravenous infusion of IL-2 after autologous marrow or PBSCT defined a regimen that was tolerable and induced significant immunomodulation.10,13,14 This study was designed to test the hypothesis that posttransplantation immunotherapy, administered early after transplantation, might be effective in eliminating tumor cells that survived the preparative regimen or that were infused with the stem cells, and thereby reduce relapses.
Methods
Patient eligibility included previously treated, biopsy proven, low-, intermediate-, or high-grade NHL except small lymphocytic or lymphoblastic lymphoma (Working Formulation Groups A and I). Patients with low-grade lymphoma must have (1) received debulking chemotherapy to determine whether the relapsed disease was sensitive to chemotherapy, (2) received at least 2 prior chemotherapy regimens, and (3) had a remission duration of less than a year with their most recent complete response. Patients with intermediate- or high-grade lymphoma must have either never responded to prior therapy or relapsed after achieving a CR or partial response (PR) to prior therapy. All patients, except those with intermediate- or high-grade lymphoma whose disease did not respond to, or progressed during initial chemotherapy, were required to have debulking (salvage) chemotherapy to determine chemosensitivity. Debulking chemotherapy was administered prior to the initial patient registration. The debulking regimen could be determined by each center; however, the DHAP regimen (dexamethasone, high-dose cytarabine, and cisplatin) was recommended.
Patients with a response to induction therapy followed by relapse and then response to debulking therapy were coded as having sensitive disease. Patients with a response to induction therapy followed by relapse and then no response to debulking therapy were coded as having resistant disease. In addition, patients with no response to induction therapy were coded as having resistant disease.
All patients must have reached their 16th but not their 61st birthday and must have had a SWOG performance status of 0 or 1 at the time of initial registration. Patients must have had serum creatinine level of 1.5× or less the institutional upper limit of normal (IULN); bilirubin level of 1.5× or less the IULN; serum glutamic oxoloacetic transaminase/serum glutamic pyruvic transaminase of 2× or less the IULN; normal cardiac history and physical examination or normal ejection fraction determined by echocardiogram or multiple-gated acquisition (MUGA) scan; no electrocardiogram (ECG) evidence of active cardiac disease; no medications to control congestive heart failure (CHF) or arrhythmias; no chest x-ray findings compatible with CHF, diffusing capacity of the lung for carbon monoxide (DLCO), or forced expiratory volume in one second (FEV1) of 65% or more predicted; a negative HIV test; and an adequate collection of peripheral blood stem cells. All participants signed informed consent in accordance with the Declaration of Helsinki based on each institution's institutional review board guidelines.
A complete list of investigators and institutions of the SWOG can be found in Document 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Eligibility for randomization after the transplantation included the following: 28 to 80 days after PBSCT; absence of known recurrent or progressive disease; absolute neutrophil count higher than 0.5 × 109/L (500/μL) and platelet count more than 20 × 109/L (20 000/μL) with one or no transfusions per day; adequate organ function; grade 1 or lower cardiac, pulmonary, CNS, renal, hepatic, or gastrointestinal toxicity, and lower than grade 3 mucosal toxicity; performance status 0 to 2; and no active cardiopulmonary disease.
Treatment regimen
The transplant regimen is listed as follows: Patients were to receive prophylactic trimethoprim-sulfamethoxazole on days −8 through −2. Patients in relapse with a high tumor burden prior to PBSCT received allopurinol on days −10 through −1. Hyperfractionated TBI was administered at 150 cGy/dose (dose rate of 5-20 cGy/min) twice daily on days −8 through −5 with doses separated by 5 or more hours. The preferred treatment position was anterior-posterior/posterior-anterior with standard lung blocks used for the final 450 cGy. The dose was calculated at midplane, central axis. Thermoluminescent dosimeters were used at the first dose with deviations adjusted to be less than 10% of the planned dose. Corrections for lung inhomogeneity were not performed.
Patients were subsequently treated with high-dose etoposide and cyclophosphamide.15 Etoposide was administered at 60 mg/kg (based on actual body weight) as a single 4-hour intravenous infusion on day −4. Cyclophosphamide was administered at 100 mg/kg (based on ideal body weight unless the patient's body weight was less than 95% of the ideal body weight) on day −2. PBSCT were infused intravenously on day 0. Appropriate intravenous hydration, antiemetics, and sedatives were given. To prevent hemorrhagic cystitis, continuous bladder irrigation for 24 hours was recommended.
IL-2 was mixed in 500 mL 5% dextrose in water (D5W)/day containing 0.1% human serum albumin (HSA). Patients received prophylactic vancomycin 1 g intravenously every 12 hours for the first 4 days of IL-2, and ciprofloxacin 500 mg by mouth for 12 hours beginning one day before, and continuing throughout the IL-2 therapy. Patients were not allowed to receive corticosteroids, pentoxifylline, methotrexate, or other cytokines during IL-2 therapy. Maintenance IL-2 therapy was mixed in D5W containing 0.1% HSA and infused using an ambulatory pump. Treatment with IL-2 was continued until grade 3 or 4 toxicity (or grade 2 cardiac or neurologic toxicity; grade 4 neutrophils or bilirubin; and/or platelets < 10 × 109/L (10 000/μL) with transfusions) occurred, at which time IL-2 treatment was discontinued until a return to baseline or grade 1 toxicity. IL-2 was then resumed at 50% of the initial dose. If toxicity recurred after resumption, IL-2 was discontinued and no further IL-2 was administered. Any missed doses of IL-2 were not given, and total duration of IL-2 therapy was not extended beyond 19 days.
Statistical considerations
All eligible patients were included in the analysis of progression-free survival (PFS) and OS. For the randomized comparison, PFS was measured from the date of randomization until progression, relapse, or death from any cause. OS was measured from the date of randomization until death from any cause. PFS and OS were estimated by the Kaplan-Meier method.16 NCI Common Toxicity Criteria (version 2.0; National Cancer Institute, Bethesda, MD) was used to define toxicities.
Patients were described at the time of initial registration by (1) histology, (2) response to initial chemotherapy, and (3) response to the most recent chemotherapy. Patients were stratified at the time of randomization for posttransplantation therapy (protocol step 2) by the same factors plus the patient's current performance status (0-1 vs 2). The accrual goal was 206 randomized patients for the comparison of IL-2 versus observation as posttransplantation therapy. Study results were monitored by the SWOG data and safety monitoring committee, and interim analyses were planned and conducted when approximately 50%, 80%, and 100% of posttransplantation patients were randomized. The statistical design specified early stopping guidelines for both efficacy and futility. Each of the interim analyses tested the null and alternative hypothesis (hazard ratio = 1.5) for PFS at the one-sided alpha .005 level. Due to a decreasing accrual rate, the data monitoring committee recommended closure of the study to new patient registrations in July 2004, after reaching 95% of planned accrual.
All statistical comparisons of PFS and OS in this report are 2-sided P values and were adjusted for the design-specified randomization stratification factors (1) histology (low vs intermediate or high), (2) response to initial chemotherapy (CR or PR vs < PR), (3) response to the most recent chemotherapy (CR or PR vs < PR), and (4) current performance status (0-1 vs 2) using Cox proportional hazards model.17
Results
Patient characteristics
Three hundred ninety-four patients were registered between January 1995 and July 2004. Eighteen patients were ineligible. Reasons for ineligibility included the following: no relapse after response to prior treatment (5), ongoing concurrent treatment or prior therapy not meeting protocol specifications (3), laboratory value outside protocol-specified limits (2), inadequate collection of stem cells (2), age older than 61 years at registration (2), active cardiac problems (1), treatment started more than 1 day after registration (1), low-grade NHL with most recent CR duration longer than 1 year (1), and concurrent untreated basal cell cancer (1). Patient characteristics of the 376 eligible patients at baseline are listed in Table 1. Most patients, 84%, were of intermediate/high-grade histology (consisting of working formulation [WF] D, 9%; WF E, 7%; WF F, 11%, WF G, 45%; WF H, 10%; and WF J, 2%), compared with 16% low-grade histology (consisting of working formation (WF) B, 9%; and WF C, 7%) based on institutional pathology review. The disposition of patients is summarized in Figure 1. The characteristics of the 194 eligible patients who were randomized are shown in Table 2. The patients were well balanced by histology, response to salvage chemotherapy, and performance status.
Patient characteristics at baseline
| . | TBI + Chemo + PBSCT, no. (%), n = 376 . |
|---|---|
| Age, y | |
| Median | 48.3 |
| Minimum | 16.2 |
| Maximum | 60.5 |
| Sex | |
| Males | 243 (65) |
| Females | 133 (35) |
| Hispanic | |
| Yes | 25 (7) |
| No | 351 (93) |
| Race | |
| White | 346 (92) |
| Black | 17 (5) |
| Other | 10 (3) |
| Unknown | 3 (1) |
| Histology | |
| Low | 61 (16) |
| Intermediate/high | 315 (84) |
| Induction and salvage therapy response | |
| Induction therapy CR or PR followed by relapse, then CR or PR to salvage therapy (sensitive) | 277 (74) |
| Induction therapy CR or PR followed by relapse, but no response to salvage therapy (resistant) | 52 (14) |
| No response to induction therapy (resistant) | 47 (12) |
| . | TBI + Chemo + PBSCT, no. (%), n = 376 . |
|---|---|
| Age, y | |
| Median | 48.3 |
| Minimum | 16.2 |
| Maximum | 60.5 |
| Sex | |
| Males | 243 (65) |
| Females | 133 (35) |
| Hispanic | |
| Yes | 25 (7) |
| No | 351 (93) |
| Race | |
| White | 346 (92) |
| Black | 17 (5) |
| Other | 10 (3) |
| Unknown | 3 (1) |
| Histology | |
| Low | 61 (16) |
| Intermediate/high | 315 (84) |
| Induction and salvage therapy response | |
| Induction therapy CR or PR followed by relapse, then CR or PR to salvage therapy (sensitive) | 277 (74) |
| Induction therapy CR or PR followed by relapse, but no response to salvage therapy (resistant) | 52 (14) |
| No response to induction therapy (resistant) | 47 (12) |
Disease characteristics at randomization
| . | IL-2, no. (%), n = 99 . | Observation, no. (%), n = 95 . |
|---|---|---|
| Histology | ||
| Low | 16 (16) | 13 (14) |
| Intermediate/high | 83 (84) | 82 (86) |
| Induction and salvage therapy response | ||
| Induction therapy CR or PR followed by relapse, then CR or PR to salvage therapy (sensitive) | 77 (78) | 76 (80) |
| Induction therapy CR or PR followed by relapse, but no response to salvage therapy (resistant) | 12 (12) | 9 (9) |
| No response to induction therapy (resistant) | 10 (10) | 10 (11) |
| Performance status | ||
| 0 or 1 | 95 (96) | 87 (92) |
| 2 | 4 (4) | 8 (8) |
| . | IL-2, no. (%), n = 99 . | Observation, no. (%), n = 95 . |
|---|---|---|
| Histology | ||
| Low | 16 (16) | 13 (14) |
| Intermediate/high | 83 (84) | 82 (86) |
| Induction and salvage therapy response | ||
| Induction therapy CR or PR followed by relapse, then CR or PR to salvage therapy (sensitive) | 77 (78) | 76 (80) |
| Induction therapy CR or PR followed by relapse, but no response to salvage therapy (resistant) | 12 (12) | 9 (9) |
| No response to induction therapy (resistant) | 10 (10) | 10 (11) |
| Performance status | ||
| 0 or 1 | 95 (96) | 87 (92) |
| 2 | 4 (4) | 8 (8) |
Toxicity
Toxicity of the transplantation.
Two patients were removed from treatment immediately after initial registration, and no toxicity documentation was reported for 3 other patients. These patients are coded as not evaluable for toxicity. Therefore, 371 eligible patients were evaluated for toxicity from the transplantation procedure as shown in Table 3. Thirty-one patients (8%) had treatment-related deaths. Grade 5 toxicities include infection (17); lung (10), cardiovascular (5), hemorrhage (3), or neurologic (2) syndromes; and one case each of clotting, hematologic toxicity, renal/bladder, and miscellaneous toxicity (acute myeloid leukemia). One ineligible patient died of grade 5 lung toxicity. Grade 4 hematologic toxicity was reported for most patients.
Adverse experiences of patients undergoing TBI plus chemo plus PBSCT, n = 371
| Adverse event . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| Unk . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Cardiovascular | 0 | 152 | 75 | 65 | 53 | 21 | 5 |
| Clotting | 0 | 365 | 3 | 0 | 2 | 0 | 1 |
| Dermatologic | 0 | 85 | 93 | 152 | 35 | 6 | 0 |
| Ear | 0 | 362 | 4 | 5 | 0 | 0 | 0 |
| Endocrine | 1 | 359 | 6 | 4 | 1 | 0 | 0 |
| Eye | 1 | 351 | 7 | 9 | 3 | 0 | 0 |
| Flulike symptoms | 1 | 53 | 91 | 173 | 47 | 6 | 0 |
| Gastrointestinal | 0 | 2 | 11 | 54 | 178 | 126 | 0 |
| Hematologic | 0 | 2 | 0 | 0 | 5 | 363 | 1 |
| Hemorrhage | 3 | 250 | 69 | 16 | 28 | 2 | 3 |
| Immunologic | 0 | 348 | 18 | 4 | 1 | 0 | 0 |
| Infection | 2 | 127 | 1 | 4 | 212 | 8 | 17 |
| Liver | 0 | 140 | 109 | 72 | 41 | 9 | 0 |
| Lung | 1 | 214 | 62 | 43 | 24 | 17 | 10 |
| Metabolic | 0 | 230 | 25 | 61 | 46 | 9 | 0 |
| Miscellaneous | 0 | 357 | 9 | 3 | 1 | 0 | 1 |
| Musculoskeletal | 0 | 363 | 4 | 2 | 1 | 1 | 0 |
| Neurologic | 1 | 163 | 86 | 74 | 34 | 11 | 2 |
| Pain | 1 | 171 | 114 | 52 | 28 | 5 | 0 |
| Renal/bladder | 1 | 241 | 87 | 28 | 10 | 3 | 1 |
| Secondary malignancy | 0 | 370 | 0 | 0 | 0 | 1 | 0 |
| Sexual/reproductive function | 0 | 366 | 3 | 2 | 0 | 0 | 0 |
| Syndromes | 0 | 357 | 2 | 0 | 8 | 2 | 2 |
| Maximum grade any adverse event, no. | 0 | 2 | 0 | 0 | 3 | 335 | 31 |
| Adverse event . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| Unk . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Cardiovascular | 0 | 152 | 75 | 65 | 53 | 21 | 5 |
| Clotting | 0 | 365 | 3 | 0 | 2 | 0 | 1 |
| Dermatologic | 0 | 85 | 93 | 152 | 35 | 6 | 0 |
| Ear | 0 | 362 | 4 | 5 | 0 | 0 | 0 |
| Endocrine | 1 | 359 | 6 | 4 | 1 | 0 | 0 |
| Eye | 1 | 351 | 7 | 9 | 3 | 0 | 0 |
| Flulike symptoms | 1 | 53 | 91 | 173 | 47 | 6 | 0 |
| Gastrointestinal | 0 | 2 | 11 | 54 | 178 | 126 | 0 |
| Hematologic | 0 | 2 | 0 | 0 | 5 | 363 | 1 |
| Hemorrhage | 3 | 250 | 69 | 16 | 28 | 2 | 3 |
| Immunologic | 0 | 348 | 18 | 4 | 1 | 0 | 0 |
| Infection | 2 | 127 | 1 | 4 | 212 | 8 | 17 |
| Liver | 0 | 140 | 109 | 72 | 41 | 9 | 0 |
| Lung | 1 | 214 | 62 | 43 | 24 | 17 | 10 |
| Metabolic | 0 | 230 | 25 | 61 | 46 | 9 | 0 |
| Miscellaneous | 0 | 357 | 9 | 3 | 1 | 0 | 1 |
| Musculoskeletal | 0 | 363 | 4 | 2 | 1 | 1 | 0 |
| Neurologic | 1 | 163 | 86 | 74 | 34 | 11 | 2 |
| Pain | 1 | 171 | 114 | 52 | 28 | 5 | 0 |
| Renal/bladder | 1 | 241 | 87 | 28 | 10 | 3 | 1 |
| Secondary malignancy | 0 | 370 | 0 | 0 | 0 | 1 | 0 |
| Sexual/reproductive function | 0 | 366 | 3 | 2 | 0 | 0 | 0 |
| Syndromes | 0 | 357 | 2 | 0 | 8 | 2 | 2 |
| Maximum grade any adverse event, no. | 0 | 2 | 0 | 0 | 3 | 335 | 31 |
Unk indicates unknown.
Twenty-one eligible patients (6%) had at least one second primary, as shown in Table 4.
Second malignancies
| Second malignancy . | No. of patients . | Time from randomization, y . |
|---|---|---|
| SCCA skin | 3 | 3.9, 4.7, 5.7 |
| Prostate ca | 3 | 0.5, 4.3, 7.6 |
| ALL | 2 | 0.4, 1.0 |
| AML | 2 | 0.2, 4.2 |
| AML/MDS | 2 | 2.4, 3.6 |
| Melanoma | 2 | 1.1, 4.6 |
| Parotid ca | 1 | 7.8 |
| Nonmelanoma skin ca | 1 | 5.1 |
| Lymphoma, follicular | 1 | 5.1 |
| SCCA lung & skin | 1 | 0.1, 3.9 |
| Colon ca | 1 | 4.2 |
| Adenoca duodenum | 1 | 3.4 |
| Sarcoma | 1 | 2.4 |
| Second malignancy . | No. of patients . | Time from randomization, y . |
|---|---|---|
| SCCA skin | 3 | 3.9, 4.7, 5.7 |
| Prostate ca | 3 | 0.5, 4.3, 7.6 |
| ALL | 2 | 0.4, 1.0 |
| AML | 2 | 0.2, 4.2 |
| AML/MDS | 2 | 2.4, 3.6 |
| Melanoma | 2 | 1.1, 4.6 |
| Parotid ca | 1 | 7.8 |
| Nonmelanoma skin ca | 1 | 5.1 |
| Lymphoma, follicular | 1 | 5.1 |
| SCCA lung & skin | 1 | 0.1, 3.9 |
| Colon ca | 1 | 4.2 |
| Adenoca duodenum | 1 | 3.4 |
| Sarcoma | 1 | 2.4 |
SCCA indicates squamous cell carcinoma; ca, carcinoma; ALL, acute lymphocytic lymphoma; AML, acute myelogenous leukemia; and MDS, myelodysplastic syndrome.
Toxicity of IL-2.
Ninety-nine patients were randomized to posttransplantation IL-2 therapy. Five patients received no treatment and are not evaluable for toxicity. No toxicity documentation was submitted for one other patient who is also coded as not evaluable for toxicity. Therefore, 93 eligible randomized patients were evaluated for IL-2 toxicity as shown in Table 5. There were no deaths related to IL-2 treatment. Fourteen patients had at worst grade 4 toxicity. Grade 4 toxicities included hematologic (10), cardiovascular (4), renal/bladder (2), flulike symptoms (1), gastrointestinal (1), liver (1), lung (1), metabolic (1), and neurologic (1).
Adverse experiences in patients randomized to IL-2, n=93
| Adverse event . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| Unk . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Cardiovascular | 0 | 33 | 13 | 28 | 15 | 4 | 0 |
| Dermatologic | 1 | 38 | 30 | 20 | 4 | 0 | 0 |
| Ear | 0 | 90 | 2 | 1 | 0 | 0 | 0 |
| Endocrine | 0 | 90 | 0 | 3 | 0 | 0 | 0 |
| Eye | 0 | 89 | 2 | 2 | 0 | 0 | 0 |
| Flulike symptoms | 0 | 12 | 28 | 43 | 9 | 1 | 0 |
| Gastrointestinal | 1 | 20 | 40 | 21 | 10 | 1 | 0 |
| Hematologic | 0 | 8 | 7 | 20 | 48 | 10 | 0 |
| Hemorrhage | 0 | 86 | 4 | 2 | 1 | 0 | 0 |
| Immunologic | 0 | 85 | 7 | 1 | 0 | 0 | 0 |
| Infection | 0 | 79 | 2 | 4 | 8 | 0 | 0 |
| Liver | 0 | 36 | 28 | 23 | 5 | 1 | 0 |
| Lung | 0 | 70 | 11 | 5 | 6 | 1 | 0 |
| Metabolic | 0 | 69 | 7 | 12 | 4 | 1 | 0 |
| Miscellaneous | 0 | 87 | 6 | 0 | 0 | 0 | 0 |
| Neurologic | 0 | 59 | 16 | 16 | 1 | 1 | 0 |
| Pain | 0 | 54 | 24 | 12 | 3 | 0 | 0 |
| Renal/bladder | 0 | 59 | 19 | 11 | 2 | 2 | 0 |
| Sexual/reproductive function | 0 | 90 | 1 | 0 | 2 | 0 | 0 |
| Maximum grade any adverse event, no. | 0 | 2 | 3 | 16 | 58 | 14 | 0 |
| Adverse event . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| Unk . | 0 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Cardiovascular | 0 | 33 | 13 | 28 | 15 | 4 | 0 |
| Dermatologic | 1 | 38 | 30 | 20 | 4 | 0 | 0 |
| Ear | 0 | 90 | 2 | 1 | 0 | 0 | 0 |
| Endocrine | 0 | 90 | 0 | 3 | 0 | 0 | 0 |
| Eye | 0 | 89 | 2 | 2 | 0 | 0 | 0 |
| Flulike symptoms | 0 | 12 | 28 | 43 | 9 | 1 | 0 |
| Gastrointestinal | 1 | 20 | 40 | 21 | 10 | 1 | 0 |
| Hematologic | 0 | 8 | 7 | 20 | 48 | 10 | 0 |
| Hemorrhage | 0 | 86 | 4 | 2 | 1 | 0 | 0 |
| Immunologic | 0 | 85 | 7 | 1 | 0 | 0 | 0 |
| Infection | 0 | 79 | 2 | 4 | 8 | 0 | 0 |
| Liver | 0 | 36 | 28 | 23 | 5 | 1 | 0 |
| Lung | 0 | 70 | 11 | 5 | 6 | 1 | 0 |
| Metabolic | 0 | 69 | 7 | 12 | 4 | 1 | 0 |
| Miscellaneous | 0 | 87 | 6 | 0 | 0 | 0 | 0 |
| Neurologic | 0 | 59 | 16 | 16 | 1 | 1 | 0 |
| Pain | 0 | 54 | 24 | 12 | 3 | 0 | 0 |
| Renal/bladder | 0 | 59 | 19 | 11 | 2 | 2 | 0 |
| Sexual/reproductive function | 0 | 90 | 1 | 0 | 2 | 0 | 0 |
| Maximum grade any adverse event, no. | 0 | 2 | 3 | 16 | 58 | 14 | 0 |
Unk indicates unknown.
Treatment outcome
Overall transplantation outcome.
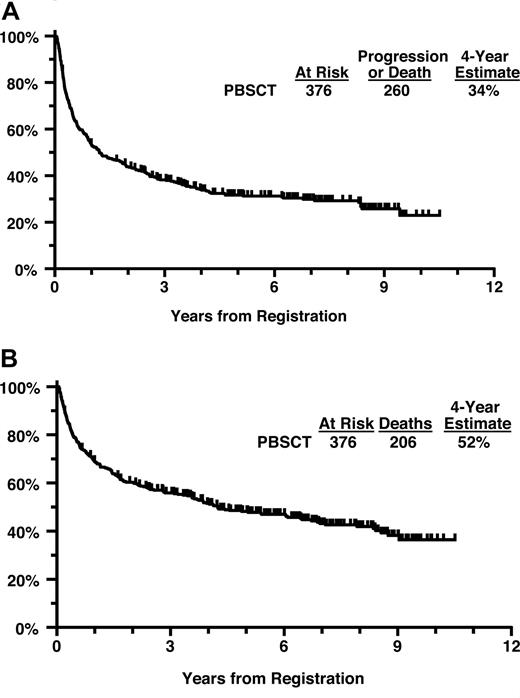
The 2 patients removed from treatment immediately after registration and 1 patient who received a dose of cyclophosphamide 50% too high were coded as having had major protocol deviations. Of the 376 eligible patients, the 4-year PFS estimate is 34% and the 4-year OS estimate is 52% (Figure 2A,B). Four-year PFS for patients with intermediate/high-grade histology versus low-grade histology was 35% versus 28%. Four-year OS for patients with intermediate/high-grade histology versus low-grade histology was 50% versus 61%.
(A) Progression-free survival from initial registration (all patients). (B) Overall survival from initial registration (all patients).
(A) Progression-free survival from initial registration (all patients). (B) Overall survival from initial registration (all patients).
One hundred eighty-two eligible patients were not randomized to posttransplantation therapy. The primary reasons for not being randomized include refusal (46), grade 5 toxicity (31), progressive disease documented within 80 days after transplantation (28), grade 4 or lower toxicity (31), disease symptoms (10), money or insurance reasons (8), family considerations (3), and other selected reasons (physician decision [3], other medical conditions [3], death prior to randomization [2], other treatment received [2], institutional error [2], administrative reasons [1], logistics [1], no protocol treatment received [1], and second primary [3]). For 9 patients, no reason was reported for not advancing to randomization.
PFS and OS by chemosensitivity.
The majority of patients had chemosensitive disease (n = 277, 74%). Four-year PFS for sensitive patients was 37% versus 27% for resistant patients (Figure 3A). Four-year OS for sensitive patients was 57% versus 36% for patients with resistant disease (Figure 3B).
(A) Progression-free survival from initial registration by chemosensitivity status. (B) Overall survival from initial registration by chemosensitivity status.
(A) Progression-free survival from initial registration by chemosensitivity status. (B) Overall survival from initial registration by chemosensitivity status.
In the subgroup of patients with resistant disease (n = 99), 52 had a response to initial induction therapy and 47 did not. Four-year PFS for patients with a response to prior induction therapy was 30% versus 23% for patients with no response. Four-year OS for patients with a response to induction therapy was 42% versus 30% for patients with no response.
Randomized comparison: IL-2 versus observation
For patients randomized to receive IL-2, the median time on treatment was 17 days (range, 2-21 days). Seventy-five percent of patients were determined to have received the full course of induction plus maintenance IL-2. On the IL-2 arm, 59 of 99 patients have progressed or died, with a 4-year PFS estimate of 43% (Figure 4A). On the observation arm, 57 of 95 randomized patients have progressed or died, with a 4-year PFS estimate of 46%. After adjusting for the protocol-specified stratification factors, there were no differences in PFS by treatment arm (HR of IL-2 to observation = 0.90; 95% CI, 0.62-1.30; P = .56).
(A) Progression-free survival from randomization by treatment arm. (B) Overall survival from randomization by treatment arm.
(A) Progression-free survival from randomization by treatment arm. (B) Overall survival from randomization by treatment arm.
On the IL-2 arm, 41 of 99 patients have died, with a 4-year OS estimate of 66% (Figure 4B). On the observation arm, 42 of 95 randomized patients have died, with a 4-year OS estimate of 60%. There were no differences in OS by treatment arm adjusting for the protocol-specified stratification factors (HR of IL-2 to observation = 0.88; 95% CI, 0.57-1.35; P = .55).
As an exploratory analysis, we tested whether disease type (sensitive vs resistant) interacted with treatment with respect to survival. However, no evidence of interaction was found between disease type and treatment for either progression-free survival (P =.21) or overall survival (P =.32). In addition, no evidence of interaction was found between histology (Working Formulation intermediate/high vs low) and either progression-free survival (P = .16) or overall survival (P = .17).
Discussion
The primary cause of failure of high-dose chemotherapy or chemoradiotherapy and autologous stem-cell transplantation in relapsed/refractory NHL is relapse. A variety of strategies have been explored to address the problem of relapse, including the following: intensification of the preparative regimen; involved field radiation therapy; and immunotherapy with cytokines, monoclonal antibodies, or allogeneic stem-cell transplantation. The primary purpose of this trial was to test the role of posttransplantation consolidative immunotherapy with a regimen of IL-2 derived from phase 1/2 trials.
Theoretical reasons for giving IL-2 after stem-cell transplantation are that (a) lymphocytes are induced to exert cytotoxic effects against lymphoma cells by treatment with IL-2 after transplantation; (b) the mechanism of action of IL-2 therapy is thought to be non–cross-resistant with chemoradiotherapy; and (c) immunotherapy with IL-2 can be administered at a time of minimal residual disease after transplantation when the effectiveness of immunotherapy would likely be greatest. Any or all of these reasons may contribute to an antilymphoma effect. Based on data from phase 1/2 trials using a regimen of posttransplantation IL-2 developed in Seattle, it was projected that patients would have a 2-year relapse-free survival of 54%, substantially higher than the 40% projected without IL-2.
Despite encouraging preliminary clinical data, the results of immunotherapy with IL-2 after transplantation in this randomized phase 3 trial revealed no significant benefit. Three-fourths of patients received the full planned dose of IL-2; hence, the negative results are likely not a result of inadequate delivery of the planned doses of IL-2. The lack of efficacy of IL-2 also did not result from a regimen of IL-2 using doses that were too low. The incidence of grade III/IV toxicities and the clinical impression of the transplantation teams confirm that higher doses of IL-2 would not have been feasible, particularly given early after transplantation in this heavily pretreated patient population. Only approximately 50% of the registrants underwent randomization, illustrating the difficulty of delivering even a moderately toxic regimen of immunotherapy in this setting. Alternative approaches that delay treatment with intensive IL-2 therapy might permit additional recovery from the toxicities of the preparative regimen and thereby improve the tolerability of posttransplantation IL-2. Slavin and colleagues used a regimen of high-dose IL-2 in combination with interferon, given 2 to 10 months after transplantation for relapsed lymphoma, with a survival benefit in comparison with historical controls (Nagler et al18 ). However, since many patients relapse very early after autologous transplantation, their results may reflect the treatment of a favorable-risk subset of patients, and have not been confirmed in randomized trials. It is possible that the relatively brief, intensive course of IL-2 therapy in this study may not have provided sufficient duration of immunostimulation. Other groups have investigated the use of prolonged, lower-dose IL-2 immunotherapy with or without IL-2–activated lymphocytes beginning immediately after transplantation,19 or 1 to 2 months after transplantation.20 Although immunostimulatory changes of potential antitumor significance were reported, these researchers did not observe beneficial effects on relapse or survival in comparison with historical controls.
Effective posttransplantation immunotherapy will require new approaches, such as the use of monoclonal antibodies, alone or in combination with lymphokines.21,22 IL-2 might increase the effectiveness of rituximab by enhancing antibody-dependent cellular cytotoxicity or by increasing delivery of the antibody to tumor cells via changes in vascular permeability.23,24 The results of this study demonstrate again the critical need for phase 3 trials to objectively test results from phase 2 protocols. The fact that 10 years were required to complete this trial, despite outstanding collaboration from multiple excellent transplantation centers, illustrates the difficulty of asking experimental questions in the complicated context of stem-cell transplantation.
Several important findings emerge from this trial, which is the largest prospective randomized trial of autologous stem-cell transplantation for NHL. The results confirm the observation by others that patients with chemosensitive disease have better PFS and OS compared with those with resistant disease, particularly in the early years after transplantation.2,9 However, with follow-up beyond 6 years in this study, both the PFS and OS curves converge. This suggests that chemosensitivity may have less impact on long-term, durable responses to autologous PBSCT. Among patients who never responded to initial induction chemotherapy, a subset appears to be salvaged by high-dose chemoradiotherapy and autologous PBSCT. The 4-year PFS and OS rates of 23% and 30%, respectively, observed in this trial are comparable with results from the Autologous Bone Marrow Transplantation (ABMT) registry.25 The incidence of secondary hematologic malignancies as well as solid tumors is consistent with reports from other groups, and continued surveillance is warranted to assess the risk of second malignancy at 10 or more years after transplantation.26 The treatment-related mortality (TRM) of 8% observed in this trial is unacceptably high, in comparison with other studies of autologous PBSCT conducted within a similar time frame that reported TRM rates from 2.6% to 6.5%.25,27 The TRM observed in the current trial may be due to enrollment of many heavily pretreated patients; by contrast, the same preparative regimen used in different patient populations who were less heavily pretreated was associated with TRM of only 1% to 4%.28,29 Non–TBI-containing preparative regimens should be recommended in heavily pretreated patients with relapsed/refractory NHL. The use of radioimmunotherapy is one approach under development to avoid TBI, and this technique for delivery of radiotherapy as part of the preparative regimen may be as effective if not more effective than TBI.30,31
In conclusion, the current study confirms and extends prior findings from SWOG that a regimen of total body irradiation, cyclophosphamide, and etoposide, followed by autologous PBSCT can be administered in a multicenter setting with encouraging PFS and OS for patients with relapsed or refractory NHL. Although posttransplantation high-dose interleukin-2 was administered successfully in this setting, IL-2 given at this dose and schedule of administration had no significant effect on posttransplantation relapse, progression-free survival, or overall survival. New strategies are needed to reduce the rate of relapse after autologous PBSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the following Public Health Service (PHS) Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA46368, CA46282, CA20319, CA58348, CA35431, CA45377, CA35192, CA46441, CA63845, CA35090, CA14028, CA04920, CA13612, CA11083, CA58415, CA35281, and CA58686, and supported in part by Novartis.
National Institutes of Health
Authorship
Contribution: J.A.T. was the SWOG Study Coordinator and wrote the paper; R.I.F. was the SWOG Lymphoma Committee chair; M.L. and J.M.U. were SWOG Lymphoma Committee statisticians; S.J.F. was the SWOG Stem Cell Transplantation Committee chair; O.W.P., A.P.N., P.J.S., and S.H.P. were SWOG Lymphoma Committee members and major accruers to the clinical trial; A.F. designed the clinical trial.
A complete list of investigators and institutions of the SWOG can be found on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John A. Thompson, Seattle Cancer Care Alliance, 825 Eastlake Ave East, Mailstop G4-830, Seattle, WA 98109-1023; e-mail: jat@u.washington.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal