Abstract
Human Dectin-1 (hDectin-1) is a member of the C-type lectin–like receptor family that was shown to be the major receptor for fungal beta-glucans and to play an important role in the cellular responses mediated by these carbohydrates. In this study, we demonstrate that hDectin-1 is involved in the uptake and cross-presentation of cellular antigens. Furthermore, activation of monocyte-derived dendritic cells (MDCs) with toll-like receptor 3 (TLR3) ligand but not with TLR2 ligand or TLR7 ligand resulted in down-regulation of hDectin-1 expression and reduced phagocytosis of apoptotic tumor cells as well as presentation of pp65-derived T-cell epitopes upon engulfment of cytomegalovirus (CMV)–infected human foreskin fibroblasts.
Introduction
Apoptosis, in contrast to necrosis, is a well-regulated process of controlled cell death and plays a pivotal role throughout the lifespan of each organism starting from morphologic changes in embryogenesis to the maintenance of adult tissue homeostasis.1 Under physiological conditions, these apoptotic cells or cell fragments have to be cleared rapidly by phagocytes or neighboring cells to avoid loss of membrane integrity and the release of intracellular contents. This process, called secondary (postapo-ptotic) necrosis, can result in harmful inflammation and induce clinically relevant autoimmune reactions and diseases.2
Dendritic cells (DCs) are professional antigen-presenting cells that were shown to be involved in the uptake and clearance of such cell corpses.3 DCs can engulf both apoptotic and necrotic cells and the kind of incorporated remnant directly influences their immunologic behavior. Antigens delivered to DCs via apoptotic cells normally induce tolerance, but under certain circumstances, may also induce DC maturation and immunity like necrotic cell material.4
The removal of these dying cells is initiated through the recognition of special molecules, so-called eat-me signals, on the cell's surface.5 The best characterized and probably most important one is phosphatidylserine (PS) that is translocated from the inner to the outer leaflet of the plasma membrane.6 It is recognized by the recently cloned PS receptor whose blockade abolishes the engulfment of apoptotic cells by macrophages.7 In addition, there are other molecules described to function as eat-me signals, including oxidized low-density lipoprotein-like sites,8 thrombospondin-1–binding sites,9 and sites for binding the complement proteins C1q or C3b.10,11 Furthermore, other C-type lectins were shown to be involved in this process and several binding sites for different collectins such as the mannose-binding lectin (MBL) or the lung surfactant proteins A and D (SP-A, SP-D) were described.11
Human Dectin-1 (hDectin-1; GenBank accession number AY009090), a recently identified member of the C-type lectin receptor family, has been the focus of intensive research for the last several years.12-15 It is a small type II transmembrane protein that consists of the characteristic single extracellular carbohydrate recognition domain, a stalk and a transmembrane region, and an intracellular cytoplasmic tail that harbors an immunoreceptor tyrosine-activation motif (ITAM)–like motif. It is alternatively spliced in several isoforms of which only the hDectin-1a and -1b are functional in binding to zymosan, a β-glucan–rich particle derived from the cell wall of Saccharomyces cerevisiae.14 These 2 splice variants differ in the presence or absence of the stalk region, a membrane proximal part of the extracellular domain (ECD) between the carbohydrate recognition domain and the transmembrane domain.
hDectin-1 is expressed mainly on human DCs and macrophages and to a lesser extent in some other hematopoietic cell types.12,15,16 It was demonstrated that hDectin-1 triggers nonopsonic phagocytosis of yeast and synergizes with TLR2 to increase proinflammatory cytokine responses to the pathogens.17-19 Furthermore, using a whole-cell binding assay, it could be shown that hDectin-1 binds to T lymphocytes in a carbohydrate-independent manner, indicating that additional receptors or binding sites may exist on mammalian cells.14 We and others12,20 found that it exhibits stimulatory properties, as hDectin-1b–transfected HeLa cells are able to induce proliferation of cocultured T lymphocytes.
In this study, we analyzed the possible involvement of hDectin-1 in recognition and clearing of human cells.
Methods
Cells
All cells were cultured in RP10 medium (RPMI 1640 with glutamax I, supplemented with 10% inactivated FCS and antibiotics [Invitrogen, Karlsruhe, Germany]). The cell lines used in the experiments were HEK-293 (embryonal kidney), K562 (chronic myeloid leukemia [CML] in blast crisis), CCRF-CEM (T-cell acute lymphoblastic leukemia [T-ALL]), and HeLa (cervix carcinoma), all from the DSMZ (Braunschweig, Germany).
Generation of MDCs
Monocyte-derived dendritic cells (MDCs) were generated from peripheral blood monocytes by magnetic cell sorting (MACS) as described previously.21 In brief, peripheral blood mononuclear cells were isolated by Ficoll/Paque (Biochrom, Berlin, Germany) density gradient centrifugation of blood obtained from buffy coats of healthy volunteers from the blood bank of the University of Tübingen. For MACS, CD14 magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used and purities of more than 95% were achieved. Monocytes were plated (106 cells/3 mL per well) into 6-well plates (BD-Falcon, Heidelberg, Germany) in RP10 medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF, 100 ng/mL, sargramostim; Berlex, Richmond, VA) and IL-4 (20.0 ng/mL; R&D Systems, Wiesbaden, Germany). Cytokines were added to differentiating MDCs every 2 to 3 days. In some experiments, MDC maturation was induced on day 6 by adding one of the following toll-like receptor (TLR) ligands: pam3cys (TLR2L, 5.0 μg/mL; EMC Microcollection, Tübingen, Germany), poly I:C (TLR3L, 50.0 μg/mL; Sigma-Aldrich, Deisenhofen, Germany), and R848 (TLR7/8L, 2.0 μg/mL; InvivoGen, San Diego, CA), respectively. Phenotypic analyses were done by flow cytometry (fluorescence-activated cell sorting [FACS]).
PAGE and Western blotting
For the preparation of whole-cell lysates, cells were lysed in a buffer containing 1.0% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 2.0 mM EDTA, 1.0 mM phenylmethylsulfonyl fluoride, and 2.0 μg/mL aprotinin. Protein concentrations were determined using a BCA assay (Pierce, Perbio Science, Bonn, Germany). Protein extracts (20.0 μg) were separated on a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The blot was probed with antibodies recognizing hDectin-1 (rabbit polyclonal, generated against a synthetic peptide representing the amino terminus of hDectin-1)12 or GAPDH (6C5, mouse monoclonal; HyTest, Turku, Finland), which was followed by incubation with an appropriate horseradish peroxidase–conjugated secondary antibody. Bands were visualized using the enhanced chemiluminescence (ECL) staining system (Amersham Biosciences, Freiburg, Germany).
Quantitative reverse transcriptase–polymerase chain reaction
Quantification of hDectin-1 transcripts was done on a Taqman device (Applied Biosystems, Darmstadt, Germany). RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and subjected to cDNA synthesis with the Superscript II cDNA synthesis system (Invitrogen). For amplification of hDectin-1a-d, the following primers were used in different combinations: FP hDectin Ex3 int, 5′-tcctaccaaagctgtcaaaacca-3′; FP hDectin Ex3 spl, 5′-ctgggtaccatgggggttct-3′; RP hDectin Ex5 int, 5′-aaaatgaattatcaggttgggaagac-3′; RP hDectin Ex5 spl, 5′-gtagctgtggttctgatctgaaatca-3′. In all hDectin-1 polymerase chain reactions (PCRs), the probe hDectin Ex4 5′-6-FAM-caatgctggcaactgggctctaatctcc-TAMRA-3′ was used. As an endogenous control, amplification with primers specific for GAPDH was done: FP GAPDH, 5′-ccacatcgctcagacaccat-3′; RP GAPDH, 5′-ggcaacaatatccactttaccagagt-3′; probe GAPDH, 5′-6-FAM-accaaatccgttgactccgaccttca-TAMRA-3′. Data analysis was done using the ΔCT method for relative quantification.
Recombinant protein expression
The coding region of the hDectin-1b ECD (nt 275–677) was amplified by reverse transcriptase (RT)-PCR using MDC cDNA and inserted in the pCR2.1 TOPO vector (Invitrogen). For subcloning, the insert was excised and ligated into the BamHI and SalI sites of the expression vector pQE-30 (Qiagen) that codes for an additional N-terminal 6xHis tag. The protein was expressed in Escherichia coli and after cell lysis inclusion bodies were solubilized in 8.0 M urea/PBS and purified using Ni-NTA-Agarose (Qiagen). After several rounds of dialysis against 50 mM Tris (pH 8.0), 19.0 mM NaCl, 0.8 mM KCl, 2.0 mM GSH, 0.2 mM GSSG with decreasing concentrations of urea, refolding was performed and purity was examined by SDS-PAGE and following silver staining. Dihydrofolate reductase (DHFR) was expressed in an analogous manner from the plasmid pQE-40 (Qiagen).
ECD staining and induction of apoptosis
Cell staining with the hDectin-1b ECD was performed by incubating the cells using approximately 10.0 μg/100 μL ECD, followed by incubation with 2.0 μg/100 μL anti-His antibody (His-1, mouse monoclonal; Sigma) and an appropriate FITC-conjugated polyclonal antimouse antibody (BD-Biosciences, Heidelberg, Germany). All incubation steps were done for 1 hour at 4°C in PBS (Invitrogen) containing 0.5% BSA (Roche Diagnostics, Mannheim, Germany). For precipitation experiments, hDectin-1 ECD was mixed with zymosan particles and incubated for 5 minutes at 4°C. Afterward the suspension was cleared and the supernatant was used for cell staining. For characterization studies of the ligand of hDectin-1, K562 cells were treated with N-glycosidase F (10.0 U/mL; Roche Diagnostics), pronase (5.0 mg/mL; Calbiochem, Merck Biosciences, Schwalbach, Germany), or proteinase K (5.0 mg/mL, Calbiochem, Merck Biosciences), respectively, for 10 minutes at 37°C in PBS. Apoptosis was induced by resuspending HEK-293 cells in PBS followed by UV irradiation in Petri dishes with 100.0 mJ on a Stratalinker 2400 (Stratagene, Amsterdam, The Netherlands). Cells were immediately transferred in RP10 medium for overnight cultivation and were harvested the next day for staining and phagocytosis experiments. For annexin V–FITC and propidium iodide (PI) staining, the Annexin-V-Fluos Staining Kit (Roche Diagnostics) was used according to the manufacturer's instructions. The mean of annexin V–positive apoptotic cells was 59.3% (± SD = 2.4) throughout all experiments.
Phagocytosis assay
For FACS analysis in phagocytosis experiments, MDCs and HEK-293 cells or human foreskin fibroblasts (HFFs) were labeled with PKH67 (green) or PKH26 (red) membrane dyes, respectively, according to the manufacturer's instructions (Sigma). In the case of HEK-293 cells, staining was done after UV irradiation. For phagocytosis, 105 MDCs and 105 HEK-293 cells or HFF were cocultivated in 24-well plates in 1 mL RP10 medium. In some experiments, 10.0 μg/mL hDectin-1b-ECD, 10.0 μg/mL recombinant DHFR, 106 zymosan particles, or 100.0 μg/mL curdlan was added. After incubation for 24 hours at 37°C, cells were harvested and either analyzed by FACS or used as stimulators in IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays.
CMV infection of human foreskin fibroblasts (HFFs)
HLA-A2− HFFs were cultured in MEM medium (Invitrogen) containing 2.4 mM glutamine, 100.0 μg/mL gentamycin, and 5% FCS. HFFs were used for experiments between passages 10 and 25. For preparation of virus stocks, HFFs were infected at a multiplicity of infection (moi) of 0.1 with human cytomegalovirus (CMV) strain AD169. Supernatants of infected cultures were harvested 6 days after infection, cells and cell debris were removed by centrifugation, and cell-free virus preparations were then used for infection of HFF cultures. The medium was removed and replaced by virus preparations for 120 minutes at 37°C. Subsequently, virus preparations were removed, and cells were washed with fresh medium and used for immunologic assays.
IFN-γ ELIspot assay
A CMV-specific HLA-A2+ cytotoxic T lymphocyte (CTL) line was generated in vitro using autologous MDCs loaded with the HLA-A2–specific pp65-peptide NLVPMVATV (amino acids 495-503; A*0201), as described previously.22 After one restimulation, cells were collected and incubated at a concentration of 105 or 5 × 104 cells/well in an antihuman IFN-γ-antibody (antihuman IFN-γ, 10 μg/mL; Hölzel Diagnostika, Köln, Germany)–coated 96-well plate with 104 autologous MDCs. As positive or negative controls, MDCs were pulsed with the specific pp65-CMV-peptide or an irrelevant HLA-A2-binding peptide derived from human immunodeficiency virus (HIV; ILKEPVHGV; amino acids: 476-484; pol HIV-1 reverse transcriptase), respectively. Furthermore, MDCs that were cocultivated with irradiated (96 Gy, Gammacell 1000; Atomic Energy of Canada, Mississauga, ON) CMV-uninfected or -infected HFFs in the presence or absence of zymosan, curdlan, or the hDectin-1b ECD were used as stimulators, respectively. After 48 hours of incubation, visualization of IFN-γ release was done using a biotin-labeled antihuman IFN-γ antibody (antihuman IFN-γ biotin, 2.0 μg/mL; Hölzel Diagnostika). Spots were counted using an automated ELIspot reader (Immunospot Analyzer; CTL Analyzers, Cleveland, OH).
Standard 51Cr-release assays (CTL assays)
A CMV-specific HLA-A2+ CTL line was generated in vitro as described in the preceding section. CTL assays were performed as described previously.21 In brief, target cells were generated by loading autologous MDCs with the specific pp65-CMV-peptide, an irrelevant HLA-A2–binding HIV peptide or by coculture of MDCs with UV-irradiated CMV-uninfected or -infected HFFs. In addition, MDCs were incubated with HFFs electroporated with in vitro transcript (IVT) coding for pp65 or EGFP protein and used as targets in the assay.22,23 Target cells were labeled with 51Cr sodium chromate in X-VIVO 20 (Lonza Verviers, Verviers, Belgium) medium for 1 hour at 37°C. Target cells (104) were transferred to a well of a round-bottomed 96-well plate. Various numbers of CTLs were added to a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, supernatants (50 μL/well) were harvested and counted in a beta-plate counter. The percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximal release − spontaneous release). Spontaneous and maximal releases were determined in the presence of either X-VIVO 20 (BioWhittaker) medium or 2% Triton X-100, respectively. Inhibition of HLA class I molecules was achieved by incubating target cells for 1 hour prior to the assay with the monoclonal antibody W6/32 (20 μg/mL) directed against HLA class I molecules (kindly provided by S. Stevanovic, University of Tübingen). To analyze the pathway of presentation, target cells were incubated with brefeldin A (5 μg/mL), chloroquine (50 μM), or lactacystin (50 μM) (all from Sigma-Aldrich, Taufkirchen, Germany) as described previously.24,25
Statistical analysis
Paired t test was performed to compare means of measured data (GraphPad Prism V 4.0; GraphPad Prism Software, San Diego, CA). Results were considered statistically significant with P values of less than .05.
Results
Quantitative analysis of hDectin-1 mRNA expression in different DC populations
CD14+ peripheral blood monocytes from healthy donors were cultured with GM-CSF and IL-4 to generate MDCs. On day 7, the cells had the typical DC morphology with its dendrite shape. These cells were analyzed by FACS and showed the characteristic phenotype with CD1a and HLA-DR expression and the absence of CD14 (data not shown). Stimulation of these cells on day 6 with TLR ligands led to an up-regulation of CD54, CD80, CD83, CD86, CD40, and HLA-A, HLA-B, HLA-C, and CCR7 (Figure 1 and data not shown).
Phenotypic analysis of in vitro–generated MDCs. MDCs were generated from CD14+ monocytes with GM-CSF and IL-4. Maturation was induced on day 6 by adding pam3cys (TLR2L), poly I:C (TLR3L), or R848 (TLR7/8L). DMSO was used as the solvent control. Overlay diagrams show expression of indicated surface molecules. Solid histograms: labeling with isotype-matched irrelevant MoAb. Numbers represent mean fluorescence intensity.
Phenotypic analysis of in vitro–generated MDCs. MDCs were generated from CD14+ monocytes with GM-CSF and IL-4. Maturation was induced on day 6 by adding pam3cys (TLR2L), poly I:C (TLR3L), or R848 (TLR7/8L). DMSO was used as the solvent control. Overlay diagrams show expression of indicated surface molecules. Solid histograms: labeling with isotype-matched irrelevant MoAb. Numbers represent mean fluorescence intensity.
By analyzing expression kinetics (Figure 2A) of the hDectin-1 isoforms a to d in monocytes differentiating into immature MDCs (without TLR agonists) by quantitative RT-PCR, it became clear that hDectin-1 transcripts were already detectable after the first hour of differentiation and reached expression levels of immature MDCs after 18 hours. Furthermore, it revealed that hDectin-1b is the major isoform expressed in these in vitro–cultured cells and that hDectin-1a and -1d were found to be expressed at a much lower extent, while hDectin-1c was hardly detectable.
Quantitative analysis of hDectin-1 mRNA expression in different cell populations. The expression of hDectin-1 was analyzed in different in vitro–generated cell populations. Quantitative real-time RT-PCR was performed using primers discriminating the 4 isoforms hDectin-1a (■), hDectin-1b ( ), hDectin-1c (
), hDectin-1c ( ), and hDectin-1d (□). Expression is shown in peripheral blood monocytes differentiating into immature MDCs over a 7-day period without TLR agonist (A) and in MDCs that were matured with different TLR ligands (B). Shown are the relative expression levels in comparison with the endogenous control GAPDH. 7d or GM/IL-4 indicate immature MDCs on day 7. The data shown are representative of 3 independent experiments.
), and hDectin-1d (□). Expression is shown in peripheral blood monocytes differentiating into immature MDCs over a 7-day period without TLR agonist (A) and in MDCs that were matured with different TLR ligands (B). Shown are the relative expression levels in comparison with the endogenous control GAPDH. 7d or GM/IL-4 indicate immature MDCs on day 7. The data shown are representative of 3 independent experiments.
Quantitative analysis of hDectin-1 mRNA expression in different cell populations. The expression of hDectin-1 was analyzed in different in vitro–generated cell populations. Quantitative real-time RT-PCR was performed using primers discriminating the 4 isoforms hDectin-1a (■), hDectin-1b ( ), hDectin-1c (
), hDectin-1c ( ), and hDectin-1d (□). Expression is shown in peripheral blood monocytes differentiating into immature MDCs over a 7-day period without TLR agonist (A) and in MDCs that were matured with different TLR ligands (B). Shown are the relative expression levels in comparison with the endogenous control GAPDH. 7d or GM/IL-4 indicate immature MDCs on day 7. The data shown are representative of 3 independent experiments.
), and hDectin-1d (□). Expression is shown in peripheral blood monocytes differentiating into immature MDCs over a 7-day period without TLR agonist (A) and in MDCs that were matured with different TLR ligands (B). Shown are the relative expression levels in comparison with the endogenous control GAPDH. 7d or GM/IL-4 indicate immature MDCs on day 7. The data shown are representative of 3 independent experiments.
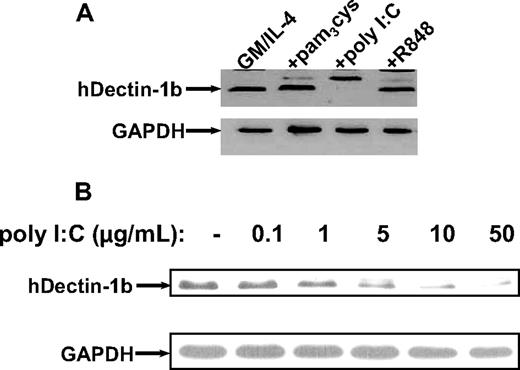
To analyze hDectin-1 expression in matured MDCs, immature MDCs were additionally activated with different compounds for another 24 hours (Figure 2B). Surprisingly, stimulation with the TLR ligand poly I:C (TLR3L) led to a dramatic down-regulation of hDectin-1 mRNA expression. In contrast, maturation with the other TLR ligands pam3cys (TLR2L) or R848 (TLR7/8L) did not substantially affect hDectin-1 transcription, thus indicating that TLR ligand–mediated stimuli may evoke distinct activation signals that result in different functional properties of DCs.
To further extend and confirm the expression of hDectin-1 in different DC subpopulations, we performed Western blot analysis using a polyclonal antibody generated against a synthetic peptide derived from the N-terminus of the protein.12 As shown in Figure 3 in line with the results from quantitative RT-PCR, stimulation of immature MDCs with the TLR ligand poly I:C led to a concentration-dependent complete down-regulation of hDectinb-1b, while the level of protein expression was not affected by other stimuli.
Analysis of hDectin-1 protein levels in MDCs exposed to TLR agonists. Western blotting was performed to examine the hDectin-1b protein expression using a polyclonal rabbit anti–hDectin-1 antibody. (A) Lysates from immature and matured (with different compounds: pam3cys, 5.0 μg/mL; poly I:C, 50.0 μg/mL; and R848, 2.0 μg/mL) MDCs are shown. (B) Titration with increasing amounts of poly I:C. The blots were reprobed with a GAPDH-specific antibody to confirm equal loading. The data shown are representative of 3 independent experiments.
Analysis of hDectin-1 protein levels in MDCs exposed to TLR agonists. Western blotting was performed to examine the hDectin-1b protein expression using a polyclonal rabbit anti–hDectin-1 antibody. (A) Lysates from immature and matured (with different compounds: pam3cys, 5.0 μg/mL; poly I:C, 50.0 μg/mL; and R848, 2.0 μg/mL) MDCs are shown. (B) Titration with increasing amounts of poly I:C. The blots were reprobed with a GAPDH-specific antibody to confirm equal loading. The data shown are representative of 3 independent experiments.
Recombinant expression of the ECD of hDectin-1b
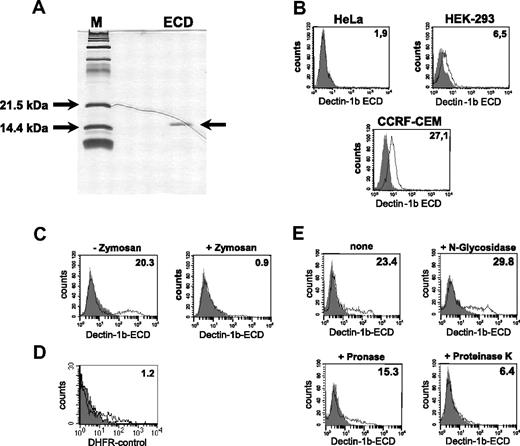
Some of the known C-type lectins such as DC-SIGN were shown to be involved in the uptake of cells or cellular material. To analyze the possible involvement of hDectin-1 in this process, we recombinantly expressed the ECD of hDectin-1b fused to a 6xHis-tag in E coli and used it to stain tumor cells. The ECD was purified through immobilized metal chelate affinity chromatography, renatured, and analyzed for purity in SDS-PAGE followed by silver staining (Figure 4A). As demonstrated in Figure 4B, the recombinant ECD of hDectin-1b bound not or only marginally to different cell lines such as HeLa or HEK-293, respectively. Further experiments resulted in the identification of 2 cell lines with good binding efficiencies: CCRF-CEM (T-ALL; Figure 4B) and K562, derived from a CML in blast crisis (Figure 4C). Due to the better binding ability of the ECD, the K562 line was used in further experiments. To confirm the specificity of the binding and the correct structure of the ECD, inhibition experiments were performed. In these assays, we included zymosan, a crude cell wall extract from S cerevisiae, that binds to hDectin-1.14 Recognition of this particle through the intact recombinant ECD led to its precipitation and therefore to a reduced extent of cell labeling (Figure 4C). As a control to exclude unspecific binding, we used DHFR, an irrelevant protein produced in the same way as the ECD (Figure 4D). To further characterize the ligand of hDectin-1, K562 cells were treated with digestive enzymes affecting the glycan or protein molecules on the cell surface. As demonstrated in Figure 4E, treatment of cells with proteinase K and to a lesser extent with pronase reduced the binding of the hDectin-1b ECD while N-glucosidase had no effect.
Recombinant expression and functional analysis of the hDectin-1b ECD. The cloned ECD of hDectin-1b was recombinantly expressed in E coli, purified, and subjected to a renaturation procedure. (A) Purity of the protein was analyzed in SDS-PAGE following silver staining. (B-D) Analysis of the binding behavior of the ECD. Shown are FACS stainings with (open histogram) or without (solid histogram) the ECD of hDectin-1. Detection was done using an antibody that recognizes the 6xHis tag of the ECD followed by incubation with an appropriate FITC-conjugated secondary antibody. (B) Analysis of the binding of the ECD to different tumor cell lines. (C) Specific binding of the hDectin-1b ECD was verified by precipitating the recombinant protein with macromolecular zymosan particles prior to the incubation of the staining solution with K562 cells. (D) Control staining with DHFR, an irrelevant protein produced in an analogous manner as the ECD. (E) To characterize the ligand for hDectin-1 on K562, cells were treated for 10 minutes with N-glycosidase (10.0 U/mL), pronase (5.0 mg/mL), or proteinase K (5.0 mg/mL) or were left untreated. The percentage of positive cells (B,D,E) or the mean fluorescence intensity (E) is shown in the upper right corner of the histograms. The data shown are representative of at least 3 independent experiments.
Recombinant expression and functional analysis of the hDectin-1b ECD. The cloned ECD of hDectin-1b was recombinantly expressed in E coli, purified, and subjected to a renaturation procedure. (A) Purity of the protein was analyzed in SDS-PAGE following silver staining. (B-D) Analysis of the binding behavior of the ECD. Shown are FACS stainings with (open histogram) or without (solid histogram) the ECD of hDectin-1. Detection was done using an antibody that recognizes the 6xHis tag of the ECD followed by incubation with an appropriate FITC-conjugated secondary antibody. (B) Analysis of the binding of the ECD to different tumor cell lines. (C) Specific binding of the hDectin-1b ECD was verified by precipitating the recombinant protein with macromolecular zymosan particles prior to the incubation of the staining solution with K562 cells. (D) Control staining with DHFR, an irrelevant protein produced in an analogous manner as the ECD. (E) To characterize the ligand for hDectin-1 on K562, cells were treated for 10 minutes with N-glycosidase (10.0 U/mL), pronase (5.0 mg/mL), or proteinase K (5.0 mg/mL) or were left untreated. The percentage of positive cells (B,D,E) or the mean fluorescence intensity (E) is shown in the upper right corner of the histograms. The data shown are representative of at least 3 independent experiments.
Recognition of apoptotic cells by hDectin-1
In our experiments analyzing the binding of the hDectin-1b ECD to tumor cell lines, we observed that the staining of some lines depended on the cell culture conditions. This suggested that the cell viability might affect the binding properties of the ECD. We therefore examined the ability of hDectin-1 to bind to apoptotic cells. To accomplish this, we used HEK-293 cells that were demonstrated to have a very low binding capacity of hDectin-1 (Figure 4B) and induced apoptotic death in these cells by UV irradiation in a reproducible manner. As shown in Figure 5, binding of the ECD to HEK-293 cells is increased upon induction of apoptosis. To confirm recognition of the apoptotic cell population, staining with annexin V and PI was performed to exclude secondary necrosis as described recently (data not shown).26
Binding of the hDectin-1b ECD to apoptotic HEK-293 cells. HEK-293 cells were UV irradiated (100 mJ) to induce apoptosis (+UV) or were left untreated (−UV). After overnight incubation, cells were stained with (open histogram) or without (solid histogram) the hDectin-1b ECD followed by detection with an antibody that recognizes the 6xHis tag of the ECD and an appropriate FITC-conjugated secondary antibody. The percentages of positive cells are shown in the upper right corner. The data shown are representative of 3 independent experiments.
Binding of the hDectin-1b ECD to apoptotic HEK-293 cells. HEK-293 cells were UV irradiated (100 mJ) to induce apoptosis (+UV) or were left untreated (−UV). After overnight incubation, cells were stained with (open histogram) or without (solid histogram) the hDectin-1b ECD followed by detection with an antibody that recognizes the 6xHis tag of the ECD and an appropriate FITC-conjugated secondary antibody. The percentages of positive cells are shown in the upper right corner. The data shown are representative of 3 independent experiments.
Phagocytosis of apoptotic cells
Based on the results derived from the expression data of hDectin-1 and the “staining” of apoptotic cells with the ECD, we sought to determine a possible involvement of hDectin-1 in the phagocytic activity of immature and mature MDCs and its regulation by different TLR ligands.
Initially, we analyzed the phenotype of MDCs incubated with HEK-293 cells. Addition of untreated or UV-irradiated apoptotic HEK-293 cells to immature MDCs had no effect on their phenotype (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
In the next series of experiments, we labeled MDCs and the apoptotic cells with different membrane dyes for FACS analyses and coincubated them for 24 hours in the presence or absence of zymosan or the hDectin-1b ECD, respectively (Figure 6). Immature MDCs showed a strong phagocytic activity and uptake of large amounts of apoptotic cells could be observed (Figure 6 dot blots; shift to red, upper right) as expected. Surprisingly, pam3cys-activated MDCs displayed a phagocytic rate similar to immature MDCs. In contrast, the poly I:C–treated MDCs exhibited a diminished uptake of apoptotic cells, which correlated with the reduced hDectin-1 expression (Figures 2,3). Furthermore, the addition of the ECD of hDectin-1b, and to a stronger extent zymosan (Figure 6A,B) or the more hDectin-1–specific linear (1→3)-β-D-glucan polymer curdlan27 (Figure 6C,D), to the cocultures led to a significant decrease of the uptake of apoptotic cells. As controls, phagocytosis was performed in the presence of DHFR as an irrelevant protein produced in an analogous manner as the ECD (Figure 6C,D). In addition, labeled cells were coincubated at 4°C or were mixed shortly before FACS analysis to exclude unspecific diffusion of membrane dyes (data not shown).
Phagocytosis of apoptotic cells. (A) Immature or matured (with different TLR ligands) MDCs (green) and apoptotic HEK-293 cells (red) were labeled with membrane dyes and were cocultivated for 24 hours. In some experiments, 106 zymosan particles or 10.0 μg/mL of the ECD of hDectin-1b was added to the cultures to inhibit hDectin-1–mediated recognition. Uptake of apoptotic HEK-293 cells by MDCs is represented as the double-positive cell fraction. Representative 2-color dot plots are shown. Percentage of double-positive cells is shown in the upper right quadrant. (B) The diagram represents mean (± SD) of 3 independent experiments using different donors. *P < .05 (paired t test). (C) Inhibition of the uptake of apoptotic tumor cells. Addition of the hDectin-1–specific (1→3)-β-D-glucan curdlan led to a reduction of the phagocytosis of apoptotic tumor cells (red) through immature MDCs (green). Addition of an irrelevant protein (DHFR; 10.0 μg/mL) or the buffer control (NaOH/HCl solvent of curdlan) did not affect the uptake of cellular material. Representative 2-color dot plots are shown. Percentage of double-positive cells is shown in the upper right quadrant. (D) The diagram represents mean (± SD) of 3 independent experiments. *P < .01 (paired t test).
Phagocytosis of apoptotic cells. (A) Immature or matured (with different TLR ligands) MDCs (green) and apoptotic HEK-293 cells (red) were labeled with membrane dyes and were cocultivated for 24 hours. In some experiments, 106 zymosan particles or 10.0 μg/mL of the ECD of hDectin-1b was added to the cultures to inhibit hDectin-1–mediated recognition. Uptake of apoptotic HEK-293 cells by MDCs is represented as the double-positive cell fraction. Representative 2-color dot plots are shown. Percentage of double-positive cells is shown in the upper right quadrant. (B) The diagram represents mean (± SD) of 3 independent experiments using different donors. *P < .05 (paired t test). (C) Inhibition of the uptake of apoptotic tumor cells. Addition of the hDectin-1–specific (1→3)-β-D-glucan curdlan led to a reduction of the phagocytosis of apoptotic tumor cells (red) through immature MDCs (green). Addition of an irrelevant protein (DHFR; 10.0 μg/mL) or the buffer control (NaOH/HCl solvent of curdlan) did not affect the uptake of cellular material. Representative 2-color dot plots are shown. Percentage of double-positive cells is shown in the upper right quadrant. (D) The diagram represents mean (± SD) of 3 independent experiments. *P < .01 (paired t test).
hDectin-1 mediates cross-presentation of antigens derived from engulfed infected cells
Uptake of cellular material was shown to result in cross-presentation of epitopes derived from engulfed proteins and stimulation of MHC class I–restricted CTLs.24,28-31 We therefore analyzed the involvement of hDectin-1–mediated uptake of cells in the presentation of peptide antigens to human CTLs. To test this, we used a CTL line generated from an HLA-A2– and CMV-positive donor by performing serial restimulations with an HLA-A2–binding peptide derived from the pp65 CMV antigen. As shown in Figure 7A, this in vitro–generated CTL line specifically recognized the cognate pp65-peptide. Stimulation of T cells with autologous MDCs pulsed with an irrelevant HIV peptide resulted only in background IFN-γ production as analyzed in an ELIspot assay. In the next set of experiments, autologous MDCs were incubated with HLA-A2–negative HFFs either infected with CMV or left untreated and were used as stimulators in ELIspot assays. As shown in Figure 7B, immature MDCs efficiently stimulated IFN-γ secretion by CMV-specific autologous CTLs when they were cocultured overnight with CMV-infected HFFs. In contrast, addition of untreated HFFs to the MDC cultures had no effect as was the case if phagocytosis was performed at 4°C (data not shown). Likewise, the uptake of apoptotic cells and in succession stimulation of the CTLs was reduced significantly upon addition of zymosan (Figure 7B), curdlan (Figure 7D), or the ECD of hDectin-1b (Figure 7B,E). Furthermore, in line with the results from previous experiments, activation of MDCs with poly I:C (TLR3L) led to a reduced activation of the pp65-specific HLA-A2–restricted CTLs (Figure 7C). Addition of the irrelevant DHFR protein control had no effect on the IFN-γ production by the CTLs (Figure 7D).
Uptake of CMV-infected human foreskin fibroblasts (HFF) and cross-presentation of CMV-specific peptides. Phagocytosis of CMV strain AD169 infected (CMV+) or uninfected (CMV−) HFFs (HLA-A2 negative) and subsequent cross-presentation of CMV-derived peptides on HLA-A2 molecules was analyzed in IFNγ-ELIspot assays. (A) The generated CTL line raised against a HLA-A2–binding CMV pp65-derived peptide specifically recognized autologous MDCs loaded with the corresponding peptide but not an irrelevant HIV-derived peptide. (B) Infected or uninfected HFFs were cocultured with immature MDCs in the presence or absence of zymosan or the hDectin-1b ECD, respectively (representative experiment). (C) Uptake of infected HFFs by MDCs that were matured using different TLR ligands or were left in an immature state (GM/IL-4) (representative experiment). (D) Addition of the hDectin-1–specific (1→3)-β-D-glucan curdlan to the coculture of MDCs and infected HFFs led to a reduction of IFNγ production by the CMV-specific CTL line, while addition of an irrelevant protein (DHFR; 10.0 μg/mL) or the buffer control did not (representative experiment). (E) The diagram represents mean (± SD) of 3 independent experiments using different donors. *P < .05 (paired t test).
Uptake of CMV-infected human foreskin fibroblasts (HFF) and cross-presentation of CMV-specific peptides. Phagocytosis of CMV strain AD169 infected (CMV+) or uninfected (CMV−) HFFs (HLA-A2 negative) and subsequent cross-presentation of CMV-derived peptides on HLA-A2 molecules was analyzed in IFNγ-ELIspot assays. (A) The generated CTL line raised against a HLA-A2–binding CMV pp65-derived peptide specifically recognized autologous MDCs loaded with the corresponding peptide but not an irrelevant HIV-derived peptide. (B) Infected or uninfected HFFs were cocultured with immature MDCs in the presence or absence of zymosan or the hDectin-1b ECD, respectively (representative experiment). (C) Uptake of infected HFFs by MDCs that were matured using different TLR ligands or were left in an immature state (GM/IL-4) (representative experiment). (D) Addition of the hDectin-1–specific (1→3)-β-D-glucan curdlan to the coculture of MDCs and infected HFFs led to a reduction of IFNγ production by the CMV-specific CTL line, while addition of an irrelevant protein (DHFR; 10.0 μg/mL) or the buffer control did not (representative experiment). (E) The diagram represents mean (± SD) of 3 independent experiments using different donors. *P < .05 (paired t test).
To further confirm the cross-presentation of CMV-derived antigens, we performed 51Cr-release assays using the pp65-peptide–specific CTL line generated from an HLA-A2– and CMV-positive donor as above (Figure 7). As target cells, we used autologous MDCs pulsed with the pp65-peptide or incubated with HLA-A2–negative HFFs that were either infected with CMV (CMV+) or left untreated (CMV−). As shown in Figure 8A, the CTL line recognized and efficiently lysed autologous MDCs pulsed with the cognate pp65-peptide as well as MDCs cocultured with CMV+ HFFs with increasing E/T ratios. There was no recognition of MDCs incubated with CMV− HFFs, an irrelevant HIV peptide, or supernatant (SN) obtained from the CMV+ HFF cell cultures. The specific lysis could be blocked using a monoclonal antibody directed against HLA class I molecules indicating that the elicited T-cell responses were HLA class I restricted. Consequently, the antibody directed against HLA class II molecules did not inhibit the lysis of the MDCs presenting CMV-pp65 epitopes. In addition, we included MDCs that were incubated with HFFs transfected with pp65-IVT in these assays. These targets were recognized by the pp65-specific CTLs, whereas MDCs that were cocultured with HFFs transfected with an irrelevant EGFP-IVT were spared (Figure 8B).
51Cr-release assays using a CTL line generated from an HLA-A2– and CMV-positive donor. (A) 51Cr-release assays using a pp65-peptide–specific CTL line generated from an HLA-A2– and CMV-positive donor. Autologous MDCs pulsed with the pp65-peptide or incubated with HLA-A2–negative HFFs that were either infected with CMV (CMV+) or left untreated (CMV−) served as target cells. MDCs incubated with an irrelevant HIV peptide or supernatant (SN) from infected HFFs were applied as controls. Inhibition of HLA class I or class II was performed by incubating MDCs prior to the assay with anti (α)–HLA class I or II antibodies. MDCs that were cocultured with HFFs transfected with pp65-IVT as well as MDCs that were cocultured with HFFs transfected with an irrelevant EGFP-IVT as a control were included. (B) Cross-presentation of pp65-derived HLA-A*02–binding T-cell epitopes is sensitive to proteasome inhibitor lactacystin or the blocker of the MHC class I processing pathway brefeldin A but not to the lysosomotropic agent chloroquine. Data represent means (± SD) of quadruplicates. One representative experiment is shown.
51Cr-release assays using a CTL line generated from an HLA-A2– and CMV-positive donor. (A) 51Cr-release assays using a pp65-peptide–specific CTL line generated from an HLA-A2– and CMV-positive donor. Autologous MDCs pulsed with the pp65-peptide or incubated with HLA-A2–negative HFFs that were either infected with CMV (CMV+) or left untreated (CMV−) served as target cells. MDCs incubated with an irrelevant HIV peptide or supernatant (SN) from infected HFFs were applied as controls. Inhibition of HLA class I or class II was performed by incubating MDCs prior to the assay with anti (α)–HLA class I or II antibodies. MDCs that were cocultured with HFFs transfected with pp65-IVT as well as MDCs that were cocultured with HFFs transfected with an irrelevant EGFP-IVT as a control were included. (B) Cross-presentation of pp65-derived HLA-A*02–binding T-cell epitopes is sensitive to proteasome inhibitor lactacystin or the blocker of the MHC class I processing pathway brefeldin A but not to the lysosomotropic agent chloroquine. Data represent means (± SD) of quadruplicates. One representative experiment is shown.
In our next experiments, we sought to analyze if cross-presentation of pp65-derived HLA-A*02–binding epitopes requires proteasome degradation. Cytosolic protein degradation is performed by the proteasome, a large multicatalytic protease complex. Lactacystin specifically inhibits the 20S and 26S proteasome activity by targeting the catalytic subunit.32-35 MDCs were preincubated with lactacystin, cocultured together with HLA-A2–negative CMV-infected HFFs, and used as targets in standard 51Cr-release assays. As demonstrated in Figure 8B, addition of lactacystin completely inhibited the presentation of pp65-derived peptides indicating the involvement of the proteasome in these processes.
The fungal product brefeldin A blocks the MHC class I processing pathway by specifically inhibiting the vesicular egress from the ER and the Golgi complex.36,37 In line with previous findings, incubation with brefeldin A almost completely abolished the lysis of MDCs incubated with CMV+ HFFs.
To further analyze whether the cross-presentation of CMV-derived peptides on HLA class I molecules was dependent on lysosomal proteases, MDCs that were coincubated with HFFs as above were treated with the lysosomotropic agent chloroquine that prevents acidification of the lysosomal compartment involved in the exogenous pathway of antigen presentation. As shown in Figure 8B, the addition of chloroquine had no effect on the cross-presentation of CMV-derived epitopes on HLA class I molecules. However, in line with previous findings, the addition of curdlan or poly I:C but not of R848 significantly reduced the specific lysis of target cells.
Discussion
Apoptotic cell death and the removal of cells dying by apoptosis is an essential process in maintaining normal tissue homeostasis. Several receptors and ligands have been reported to be important in the uptake of apoptotic cells. The present study demonstrates that hDectin-1, a member of the C-type lectin–like receptor family, binds to apoptotic cells, facilitates their uptake by DCs, and mediates cross-presentation of cell-derived antigens.
Pattern recognition receptors (PRRs) are part of the innate immune system that is important in specifically recognizing pathogens and mediates their uptake by phagocytic cells. The most prominent member representing these receptors is the TLR family that acts mainly to sense the environment and submit danger signals to the cell.38 Further on, there are several other molecules involved in recognition and uptake of antigen, such as C-type lectins, including the soluble collectins lung surfactant protein A (SP-A) and SP-D or the mannose-binding lectin (MBL) that opsonizes pathogens and mediates uptake through its specific receptors.39 Several membrane-bound C-type lectins such as MMR, Dec205, or DC-SIGN are reported to directly bind and internalize their ligands, which leads to efficient antigen presentation in the case of DCs. In recent years, it became clear that these receptors function not only in pathogen recognition but are also involved in the binding or uptake of endogenous ligands.40 For example, SP-A, SP-D, and MBL are not only a component of the innate immune system but also facilitate the binding of phagocytes to apoptotic cells.
hDectin-1 has been shown to be the major β-glucan receptor that is expressed mainly on human DCs and macrophages. Until now, it has been known that hDectin-1 mediates the nonopsonic phagocytosis of yeast and that it synergizes with the TLR2 in increasing proinflammatory cytokine production and their release in response to these pathogens. Furthermore, binding of this receptor to T lymphocytes in a carbohydrate-independent manner and induction of proliferation in these cells using the recombinant protein or transfected cells suggests that there is/are further ligand(s) for hDectin-1 endogenously expressed on mammalian cells. These data and the observed binding behavior of the hDectin-1 ECD to cell lines cultured under different conditions prompted us to investigate if there is a selective increase in the expression of this potential ligand on these cells. In our experiments, we could show that the binding of the ECD of hDectin-1b increases upon induction of apoptosis in HEK-293 cells. Furthermore, we could observe in phagocytosis experiments that uptake of apoptotic cells by immature MDCs is dramatically reduced upon cocultivation with the linear (1→3)-β-D-glucan polymer curdlan or zymosan particles. This particle not only consists of β-glucans that are recognized by hDectin-1 but is instead a crude mixture of different cell wall components for which binding to other receptors of mammalian cells such as DC-SIGN,41 which binds to mannan contents, is described. Therefore, the ECD of hDectin-1b was used as a competitive inhibitor to directly measure the participation of this molecule in apoptotic cell clearing. Although it was revealed as not as efficient as zymosan or curdlan in inhibiting apoptotic cell uptake, a significant reduction of phagocytosis could be observed. The remaining phagocytic effect is probably due to other molecules involved in phagocytosis.5 Interestingly, in the case of MDCs that were matured with poly I:C but not with pam3cys or R848, a reduced phagocytic activity could be observed that was accompanied with a decrease in hDectin-1 expression as demonstrated in quantitative real-time PCR and Western blot experiments. This might be due to the different signaling pathways engaged upon activation of the specific TLRs.38 TLR2 and TLR7 signaling is MyD88 dependent and leads to the activation and nuclear localization of NFκB. This is also true for TLR4-mediated signal transduction, but ligand binding by this receptor, likewise stimulation of TLR3, could also induce activation of a MyD88-independent pathway that leads to activation of the transcription factor IRF-3. This molecule is known to be involved in IFN-stimulated gene transcription that functions as a direct response to viral infection and is potently involved in inhibiting viral replication.42 However, the impact of the function of IRF-3 in the light of hDectin-1 regulation remains to be clarified.
hDectin-1 also seems to be involved in the uptake of CMV-infected HFFs leading to cross-presentation of CMV-derived peptides on MHC class I molecules and activation of CMV pp65-specific CD8+ T lymphocytes. This is mediated at least in part in an hDectin-1–dependent manner as uptake and presentation of CMV antigens could be inhibited through addition of zymosan, curdlan, or hDectin-1 ECD to the cocultures of MDCs and HFFs. In line with the phagocytosis experiments using apoptotic HEK-293 cells, stimulation of MDCs with the TLR3 ligand led to a reduced activation of pp65-specific HLA-A2–restricted CTLs. Presentation of CMV-specific peptides is likely to be mediated by cross-presentation and not due to secondary infection of MDCs in the coculture because the laboratory strand of CMV (AD169) used shows a HFF-specific cell tropism and does not infect DCs.43,44 Furthermore, we used HLA-A2–negative HFFs in our experiments. Incubation of MDCs with viral particle containing supernatant obtained from infected HFFs had no effect on stimulation of CMV-specific CTLs (data not shown). These results were further confirmed using HFFs electroporated with the pp65-IVT in the CTL assays.
The observed down-regulation of hDectin-1 upon activation of MDCs with poly I:C (Figures 2 and 3) may reveal as a novel escape mechanism from immune recognition of host invading microorganism. These pathogens could activate TLR3 and hence would not be recognized by the heterodimeric receptor complex that is formed by TLR2 and hDectin-1.19 Furthermore, our data demonstrate that the production and secretion of β-glucan by pathogens may interfere with the uptake of cellular antigens derived from infected cells and thus support their spread and inhibit their recognition and elimination by the immune system. This assumption is confirmed by recent studies in which it was shown that several pathogenic microorganisms45,46 and even tumor cells47 take advantage of TLRs to evade immune recognition and destruction.
In summary, the data reported here show that hDectin-1 not only functions as a pattern recognition receptor in innate immunity but is also involved in the clearing of apoptotic cells and cross-presentation of cellular antigens on MHC class I molecules to specific CTLs. Furthermore, we demonstrate that TLR ligands differentially affect this process.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Laib-Sampaio (Institute of Medical Virology, University of Tübingen, Tübingen, Germany) for their help with the CMV experiments as well as Bruni Drotlef, Sylvia Stephan, and Solveig Daecke for excellent technical assistance.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 685, Project A5).
Authorship
Contribution: M.M.W. designed research, performed research, and wrote the paper; S.A., D.W., and A.B. performed research; C.S. contributed vital new reagents; F.G. and P.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, University of Bonn, Department of Hematology and Oncology, Wilhelm str. 35-37, 5311 Bonn, Germany; e-mail: peter.brossart@ukb.uni-bonn.de.
References
Author notes
*P.B. and F.G. contributed equally to this work.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal