Abstract
E3 ubiquitin ligases determine which intracellular proteins are targets of the ubiquitin conjugation pathway and thus play a key role in determining the half-life, subcellular localization and/or activation status of their target proteins. Itchy mice lack the E3 ligase, Itch, and show dysregulation of T lymphocytes and the induction of a lethal autoimmune inflammatory condition. Itch is widely expressed in hematopoietic and nonhematopoietic cells, and we demonstrate that disease is transferred exclusively by hematopoietic cells. Moreover, distinct manifestations of the autoimmune inflammatory phenotype are contributed by discrete populations of lymphocytes. The presence of Itch-deficient αβ T cells drives expansion of peritoneal B1b cells and elevated IgM levels, which correlate with itching and pathology. In contrast, Itch−/− interleukin-4–producing γδ T cells, even in the absence of αβ T cells, are associated with elevated levels of IgE and an inflammatory condition. These data indicate that disruption of an E3 ubiquitin ligase in αβ T cells can subvert a B-cell subpopulation, which normally functions to control particular microbial pathogens in a T-independent manner, to contribute to autoimmunity. In addition, disruption of Itch in innate γδ T cells can influence autoimmune pathology and might therefore require distinct therapeutic intervention.
Introduction
Engagement of receptors in cells of the immune system results in downstream signals whose final outcome is regulated by recruitment, interaction, and activation of proteins with both positive and negative activities.1 Among the negative regulators of signaling, E3 ubiquitin ligases catalyze the removal of transducer proteins from the signalosome through degradation either in the lysosomal2,3 or proteasomal4 compartments. In addition, ubiquitination modulates several regulatory pathways in a proteolysis-independent manner.5
The importance of E3 ligases and ubiquitination in cell regulation and effector function is stressed by several human pathological conditions and mouse models of autoimmunity caused by mutation of such proteins.6-10 In Itchy mice, deletion of the E3 ligase, Itch (also named AIP4), results in a severe immune dysregulation, characterized by itching of the skin, inflammatory disorder of stomach and lungs, hyperplasic lymph nodes, and splenomegaly.11 On a C57BL/6 background, Itch deficiency resulted in premature death at around 6 to 8 months of age, but symptoms and severity varied in other backgrounds.12
The molecular and cellular mechanisms underlying such immune disorders are currently not well defined. Itch has been implicated in the ubiquitin-dependent degradation of several proteins,13 including PKCθ and PLCγ,14 and Jun.15,16 Furthermore, Itch has been suggested to modulate NF-κB signaling through degradation of Bcl-10, a protein that is involved in TCR, BCR, and Notch signaling,17,18 and of c-FLIP downstream of TNF-αR.19 Itch is the murine orthologue of the Drosophila protein suppressor of Deltex (SuDx), which is a modifier of the Notch signaling pathway.20 Notch is also a primary target of Itch in cells of the mouse immune system,21 and Notch signaling has been implicated recently in both αβ and γδ T-cell differentiation,22,23 in polarization of Th1 or Th2 subsets,24 and in development of certain subsets of B cells, particularly B1 cells and marginal zone B (MZB) cells.25,26 In addition, Itch−/− T cells were demonstrated to show biased differentiation to the Th2 lineage upon activation, suggesting that a major influence in the immune disorder in Itchy mice was aberrant production of Th2 cells.15
Given the widespread expression and multifaceted roles of Itch in the immune system, we have investigated the nature of the autoimmune condition in Itchy mice, to try to clarify how lack of Itch contributes to pathology. Here we show that overt autoimmunity in Itchy mice depends on the absence of Itch in αβ T cells. Itch−/− αβ T cells induced an accumulation in the peritoneal cavity of B1 B cells, lacking the negative regulator CD5 (B1b cells), which were responsible for production of high levels of circulating IgM and the development of itching. Curiously, although Itch−/− mice displayed a marked increase in circulating IgE antibodies, their presence neither correlated with pathology nor depended on the presence of αβ T cells. Instead we found that γδ T cells from Itchy mice were competent to produce IL-4 ex vivo and were the most likely inducers of the hyper-IgE phenotype and contributed to the inflammatory condition. Therefore, even though a functional αβ T-cell compartment was necessary for initiation and maintenance of the autoimmune disorder, our data suggest that the propensity of Itch−/− αβ T cells to differentiate to a Th2 phenotype in vitro was not the only contributor to disease in vivo.
Methods
Mice
Itchy mice were obtained from MRC Harwell (Oxfordshire, United Kingdom) and maintained in our animal facility according to local and United Kingdom Home Office guidelines. The Itch mutation was backcrossed on the C57BL/10 (B10) background for 7 or more generations, on the Tcra−/− background, and on 4get mice.27
Immunization and enzyme-linked immunosorbent assay
Mice were immunized intraperitoneally with 25 μg TNP-Ficoll for T-independent responses; 100 μg keyhole lympet hemocyanin in MPL + TDM (Sigma-Aldrich, St Louis, MO) adjuvant for Th1 responses; or 200 μg endotoxin-free alum-precipitated ovalbumin (OVA; kind gift of Dr A. O'Garra) for Th2 responses. Ag-specific serum Igs were detected approximately 3 weeks after immunization by enzyme-linked immunosorbent assay (ELISA), using specific alkaline phosphatase–conjugated anti–mouse Igs (Southern Biotechnology, Birmingham, AL). Assay wells were coated with 50 μL Ag (50 μg/mL) in PBS, overnight at 4°C. For preimmune sera, plates were coated with 50 μL goat anti–mouse IgG + IgM (H + L; 10 μg/mL; Jackson ImmunoResearch, West Grove, PA) or of rat anti–mouse IgE (0.2 μg/mL; Southern Biotechnology). Isotype standards were purchased from Southern Biotechnology or Sigma. Serum antinuclear antibodies were detected by ELISA using Mouse ANA Elisa Kit (Alpha Diagnostic, San Antonio, TX).
Flow cytometry
The antibodies AlexaFluor-CD4, AlexaFluor-CD8, and allophycocyanin-B220 were from Invitrogen; PE anti-IgM, from Molecular Probes (Eugene, OR); and the remainder, from BD-Pharmingen (San Diego, CA) or eBioscience (San Diego, CA). Biotinylated Abs were revealed with peridinin-chlorophyll-protein–conjugated streptavidin (BD Pharmingen). Typically, 0.5 × 106 cells per well were incubated in a 96-well round-bottom plate (BD Biosciences, San Jose, CA) with pretitrated Abs. Samples were collected on FACSCalibur, LSR-II, or FACSCantoII flow cytometers (BD, Franklin Lakes, NJ) and analyzed using FlowJo software (TreeStar, Ashland, OR).
In vitro T-cell differentiation and activation
Naive CD4+ T cells were purified by sorting for CD62L+ CD44− CD4+ TCR-β+ using Dako Cytomation MoFlo (Beckman Coulter, Fullerton, CA). Cells were stimulated with plate-coated anti-CD3 plus soluble anti-CD28 (1 μg/mL) for 7 days, plus anti–IL-4 (10 μg) and anti–IL-12 (5 ng/mL) for Th1 conditions and anti–IFN-γ (10 μg/mL) plus IL-4 (5 ng/mL) for Th2 conditions, restimulated with anti-CD3/anti-CD28 for 6 hours, fixed, and permeabilized with 0.1% Tween20 for 3 minutes. Intracellular cytokines were detected by fluorescence-activated cell sorting (FACS).
Cytokine detection by cytometric bead analysis
CD4 T cells (105) were activated as described in the previous paragraph for 24, 48, and 72 hours with splenic mitomycin C–treated accessory cells (2 × 105 cells/well). Secretion of IFNγ, IL-2, IL-4, IL-5, IL-10, and IL-17 was analyzed by FlowCytomix Assay (Caltag Medsystems, Buckingham, United Kingdom).
Bone marrow chimeras and cell transfers
Bone marrow from adult mice was depleted for mature lymphocytes using anti-B220 and anti-Thy1.2 (BD Pharmingen). Cells were resuspended at 1.5 × 107 cells/mL and 0.3 × 107 cells per mouse were injected intravenously.
Single-cell suspensions of adult peritoneal exudates were incubated with a cocktail of biotinylated antibodies (anti-CD3, DX5, GL3, GR1, F4/80) and with low concentrations of anti-IgD. B1b cells were then negatively purified by using Streptavidin-magnetic beads (Invitrogen, Carlsbad, CA). Lymph node CD4+ T cells were positively selected using anti-CD4 microbeads (Miltenyi Biotech, Auburn, CA).
Histology and immunofluorescence
Kidneys were snap-frozen in OCT compound and stored at −70°C. Frozen sections (7 μm each) were stained with FITC-goat anti-mIgG (Jackson ImmunoResearch) or FITC-mouse anti-mIgMb plus AlexaFluor-488 amplification kit (Invitrogen). Sections in mounting medium (Dako Cytomation) were analyzed by Deltavision Systems (Applied Precision, Issaquah, WA). Alternatively, sections were stained with hematoxylin and eosin and observed with a Zeiss Axioplan and Lasersharp 2000 software (Carl Zeiss, Heidelberg, Germany).
Statistics
Data were analyzed by Student t test or Mann-Whitney U test.
Results
High concentration of IgM, IgE, and autoreactive Abs in Itchy mice
Itchy mice are characterized by an inflammatory autoimmune disorder with splenomegaly, lymphadenopathy, chronic inflammation in the skin, and lung infiltration. To investigate further the role of Itch in disease, we studied the immune response of Itch-deficient (Itchy) mice on a C57BL/10 background, backcrossed for 7 or more generations. Consistent with previous observations, infiltration of mononuclear cells was found in the lungs of older mice (data not shown). Increase in lymph node and spleen cell numbers (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) progressed with age from mild to severe, with an average 2-fold increase over wild-type (WT) controls. However the relative proportion of T (Figure S1B) and B (Figure S2A) cells in lymph nodes and spleen was not affected. We observed a slight decrease in CD4+ T-cell percentages, but not numbers (Figure S1C), and both CD4+ (Figure S1C-E) and CD8+ (Figure S1D) T cells showed an activated phenotype. In our specific pathogen-free (SPF) animal colony, up to 34% of the mice exhibited scratching from approximately 4 months of age. However, spontaneous lethality was low, in contrast to previous reports for the C57BL/6 background,11 most likely a result of either differences in background genes or the health status of the mice.
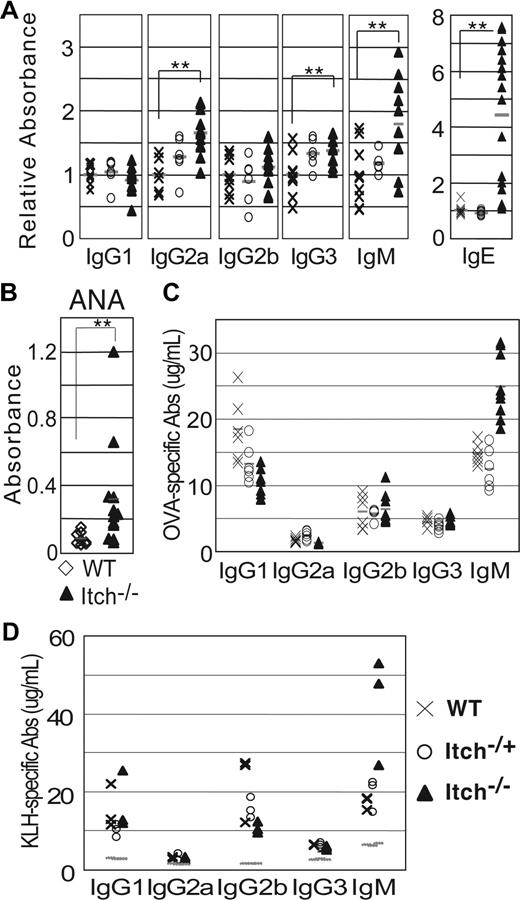
ELISA performed on unimmunized mice showed that basal serum levels of IgG1 and IgG2b were normal (Figure 1A), whereas there was a slight increase of IgG2a (P < .01) and IgG3 in Itch−/− compared with WT mice, with intermediate amounts of these Ab isotypes in Itch+/− mice. Most notably, Itch−/− mice showed a marked increase of IgM (P = .003) and, particularly, IgE (P < .001; Figure 1A). It has been reported previously that Itchy mice have bias toward production of Th2-derived cytokines, which are known to modulate a switch in Ig production by B cells to IgG1 and IgE.15 However our data only partially concur with these findings, as high production of IgM was not accompanied by increased concentration of IgG1 in our Itch−/− colony, but interestingly, it coexisted with augmented levels of IgE, as observed in some other models of autoimmunity.10,28,29
High concentration of IgM, IgE, and autoimmune antibodies in adult Itchy mice. (A) Serum Igs were measured in individual preimmune WT, Itch+/−, and Itch−/− mice by enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean fold increase of relative absorbance (OD405) over WT mice. **P < .01. (B) Antinuclear antibodies were detected by ELISA in the sera of WT and Itch−/− mice. (C,D) WT, Itch+/−, and Itch−/− mice were immunized with Th2 antigen OVA-alum (C) or KLH-MPL/TDM (D), and the concentration of Ag-specific Igs was measured 3 weeks later by ELISA. Preimmune background is shown as dotted lines for panel D. Symbols throughout are as follows: × indicates WT mice; ○, Itch+/− mice; and ▲, Itch−/− mice.
High concentration of IgM, IgE, and autoimmune antibodies in adult Itchy mice. (A) Serum Igs were measured in individual preimmune WT, Itch+/−, and Itch−/− mice by enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean fold increase of relative absorbance (OD405) over WT mice. **P < .01. (B) Antinuclear antibodies were detected by ELISA in the sera of WT and Itch−/− mice. (C,D) WT, Itch+/−, and Itch−/− mice were immunized with Th2 antigen OVA-alum (C) or KLH-MPL/TDM (D), and the concentration of Ag-specific Igs was measured 3 weeks later by ELISA. Preimmune background is shown as dotted lines for panel D. Symbols throughout are as follows: × indicates WT mice; ○, Itch+/− mice; and ▲, Itch−/− mice.
The symptoms characterizing Itchy mice were strongly suggestive of an inflammatory autoimmune disorder. Therefore we looked for the presence of autoreactive antibodies and the deposition of immune complexes in the glomeruli, a common feature of autoimmune pathologies such as systemic lupus erythematosus (SLE).30,31 Indeed, we detected a significant increase of serum antinuclear antibodies (P = .002, Figure 1B), which included antinuclear IgM (Figure S3A), and positive immunofluorescence stain for IgG and IgM deposits in kidney sections in Itch−/− mice but not in WT controls (Figure S3D,E). These data indicate that Itchy mice have a specific autoimmune condition in addition to more generalized inflammatory processes and expanded lymphocyte cellularity. Symptoms of autoimmunity in affected animals were unlikely to be due to defective T regulatory cell (Treg) functionality, as numbers of CD25+ CD4+ T cells, as well as their in vitro and in vivo suppressive potential, were not affected by Itch deficiency (A.-C.F., V.P., unpublished data, October 2007).
As we observed a noncanonical serum Th2 immunoglobulin pattern with no increase in IgG1 in Itch−/− mice, we investigated how the absence of Itch influenced development of a humoral response after immunization in vivo. Mice were immunized under condition that favor Th2-driven (OVA + alum; Figure 1C) or Th1-driven (KLH + MPL adjuvant; Figure 1D) responses, or T cell–independent antigens (TNP-Ficoll; Figure S3B and data not shown). IgG1 secretion by B cells is dependent on Th2 helper cells, and for both Itchy and control mice Ag-specific Th2 IgG isotypes were predominant in response to OVA + alum immunization, with amounts of specific IgG1 higher than IgG2a or IgG3, but with no significant differences between groups. Immunization under Th1 conditions also evoked comparable IgG profiles in both Itchy and WT mice. However, under all immunization conditions tested, production of Ag-specific IgM was elevated in Itch−/− mice compared with controls. These data show that the absence of Itch does not appear to impair or skew either T-dependent or T-independent response upon antigen stimulation in vivo.
Normal splenic B-cell populations but increased B1b cellularity in peritoneum of Itchy mice
High concentration of IgM and the presence of self-reactive antibodies in the serum of Itchy mice is a common trait of several autoimmune disorders.32,33 The potential for the production of self-reactive antibodies is intrinsic to 2 distinct B-cell populations, namely, marginal zone B (MZB) cells and B1 cells.
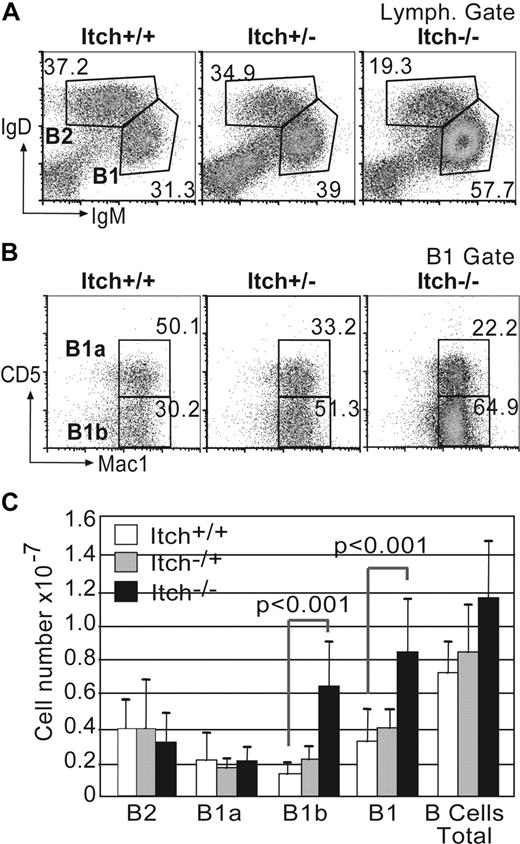
B1 B cells are most abundantly located in the peritoneal cavity, although a small population is found in the spleen, where initially they were identified as subset of CD5+ B cells that can produce natural low-affinity IgM Abs and respond to TI-antigen by producing high amounts of IgM. FACS analyses of spleens from Itchy mice older than 4 months showed that B-cell subsets were present in normal frequencies, including the proportion of splenic CD5+ B1b cells (data not shown), despite augmented cellularity consequent to splenomegaly (Figure S2A,B). In particular, the frequency of MZB cells in Itchy and WT mice was comparable (Figure S2D,E). Furthermore, Itchy splenic B cells showed no overt activation judged by their expression profiles of IgD, IgM, CD21, and CD23 (Figure S2C), indicating hyper-IgM production was unlikely to be caused by an activated splenic population. In contrast, peritoneal exudates from Itchy mice had increased overall cellularity (1.6 × 107 vs 1.1 × 107) and increased B-cell numbers (1.1 × 107 vs 0.7 × 107, P < .01; Figure 2C) compared with WT controls. Of particular significance was the detection of an increase in IgMhi IgDlo B1 cells (Figure 2A,C), most of which were Mac-1+ (Figure 2B), giving a skewed ratio of B1/B2 cells of 4.2:1 in Itch−/− mice compared with 0.9:1 in WT mice.
Increased cellularity and skewed B1/B2 and B1b/B1a ratios in peritoneal exudate from Itchy mice. (A) Peritoneal exudate cells from WT, Itch+/−, and Itch−/− mice were stained for B2 (IgDhiIgMlo) and B1 (IgMhiIgDlo) cells after gating on lymphocytes using side and forward scatter profiles. The percentage of each population is indicated. (B) B1 cells were further gated for B1a (Mac1+CD5+) and B1b (Mac1+CD5−). The percentages of each subpopulation are indicated next to each gate. Results of representative mice (n = 12) are shown. (C) Total numbers of individual peritoneal B-cell subpopulations (± SD) are shown for WT (□; n = 12), Itch+/− ( ; n = 12), and Itch−/− (■; n = 22) mice.
; n = 12), and Itch−/− (■; n = 22) mice.
Increased cellularity and skewed B1/B2 and B1b/B1a ratios in peritoneal exudate from Itchy mice. (A) Peritoneal exudate cells from WT, Itch+/−, and Itch−/− mice were stained for B2 (IgDhiIgMlo) and B1 (IgMhiIgDlo) cells after gating on lymphocytes using side and forward scatter profiles. The percentage of each population is indicated. (B) B1 cells were further gated for B1a (Mac1+CD5+) and B1b (Mac1+CD5−). The percentages of each subpopulation are indicated next to each gate. Results of representative mice (n = 12) are shown. (C) Total numbers of individual peritoneal B-cell subpopulations (± SD) are shown for WT (□; n = 12), Itch+/− ( ; n = 12), and Itch−/− (■; n = 22) mice.
; n = 12), and Itch−/− (■; n = 22) mice.
Peritoneal B1 B cells can be further subdivided into 2 subsets depending on their expression of the surface marker CD5 (ie, CD5+ B1a B cells and CD5− B1b B cells). Itch−/− mice had a significant increase in the percentage and numbers of the IgM+ peritoneal B1b B cells lacking the negative regulator CD5 (Figure 2B,C), suggesting that this population might be responsible for the elevated serum IgM in Itchy mice.
Autoimmunity in Itchy mice is intrinsic to the hematopoietic system
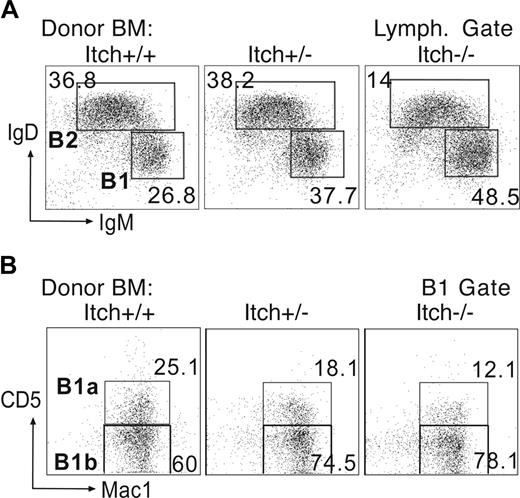
As Itch is expressed widely, we set out to investigate whether the increase of B1b B cells and the consequent autoimmune disorder in Itchy mice was intrinsic to the hematopoietic system or depending on a unique stromal and/or tissue milieu. We performed adoptive transfer of bone marrow (BM) cells from adult Itch−/−, Itch+/−, and WT control mice into sublethally irradiated Rag1−/− mice. Chimeras reconstituted with Itchy BM recapitulated the immune disorder of the donor mice characterized by hyperplasic lymph nodes, enlarged spleen, and severe itching, but with an earlier onset than in the donor strains (from 2-4 months after reconstitution). When Itch−/− BM was used for reconstitution, elevated serum IgM and autoimmune antibodies were detectable immediately preceding the onset of the disorder (Figure S3A), in parallel with augmented Mac1+ B1b cells and fewer IgDhi IgMlo B2 cells (Figure 3A). As expected, the B1a compartment was poorly reconstituted in all instances, due to the limited availability of precursors in adult BM.34,35 However, chimeras from Itch−/− BM donors had even greater skewing toward the B1b compartment than controls (Figure 3B), providing further correlation between the B1b, rather than the B1a subset, and development of autoimmunity.
Reconstitution of peritoneal B1b cells in bone marrow chimeras. Bone marrow from adult WT, Itch+/−, and Itch−/− mice was transferred into sublethally irradiated Rag1−/− recipients. (A) After 12 to 18 weeks, reconstitution of B2 (IgDhiIgMlo) and B1 (IgMhiIgDlo) cells was detected in the peritoneum. (B) The B1 population was comprised predominantly of B1b (Mac1+CD5−) cells with few B1a (Mac1+CD5+) cells. Data are representative of more than 5 experiments. The percentages of each population are indicated next to each gate.
Reconstitution of peritoneal B1b cells in bone marrow chimeras. Bone marrow from adult WT, Itch+/−, and Itch−/− mice was transferred into sublethally irradiated Rag1−/− recipients. (A) After 12 to 18 weeks, reconstitution of B2 (IgDhiIgMlo) and B1 (IgMhiIgDlo) cells was detected in the peritoneum. (B) The B1 population was comprised predominantly of B1b (Mac1+CD5−) cells with few B1a (Mac1+CD5+) cells. Data are representative of more than 5 experiments. The percentages of each population are indicated next to each gate.
Hyper-IgE production in Itchy mice is separate from autoimmunity and independent of αβ T-cell cognate help
The increase in B1 B cells in the peritoneal cavity of Itch−/− mice or chimeras might be dependent upon a developmental program intrinsic to either the B-cell compartment or to T cell–dependent mechanisms. To address this question, we crossed Itchy mutants with TCRa−/− mice. In these mice γδ T cells undergo normal development and maturation, while T cells of the αβ lineage fail to progress beyond the thymic CD4+ CD8+ double-positive stage of maturation due to the lack of functional TCR.
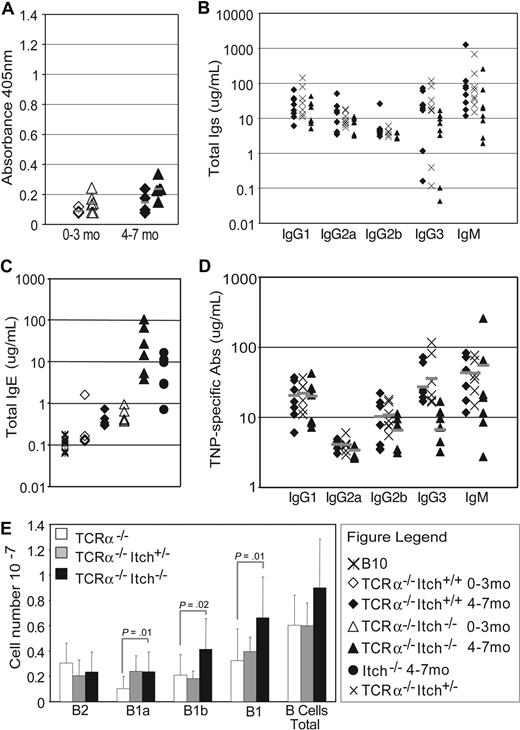
The absence of T cells ameliorated the autoimmune disorder and Itchy TCRa−/− mice did not develop the characteristic itching, despite a mild increase in cellularity in lymph nodes and spleen (data not shown). Unlike Itchy mice, Itchy TCRa−/− mice did not have a significant increase in serum concentrations of self-reactive Abs (Figure 4A) or of IgM or IgG isotypes, either before (Figure 4B) or after immunization with TNP-Ficoll (Figure 4D; compare with TNP-specific IgM in Itch−/−, Figure S3B), indicating no significant differences in the immune response of Itchy TCRa−/− and TCRa−/− control mice challenged with a TI-antigen. IgE serum concentration in TCRa−/− mice, initially equal to age-matched B10 control mice, increases significantly from 4 months of age (Figure 4C).36 Interestingly, serum IgE in young Itchy TCRa−/− mice was elevated comparably with older TCRa−/− WT controls and continued to increase with age, considerably beyond that found in T cell–competent Itchy mice or in TCRa−/− WT controls (Figure 4C). Thus in Itchy mice, hyper-IgM is dependent on a competent αβ T-cell compartment, whereas hyper-IgE is not.
Itch−/− αβ T cells are required for elevated IgM and autoimmune antibodies but dispensable for hyper-IgE. (A) No production of antinuclear antibodies was detected by ELISA in sera of TCRa−/− or TCRa−/−Itch−/− mice. Mice are divided in 2 age groups: 1 to 3 and 4 to 7 months of age. (B) Analysis of total Igs by ELISA from preimmune TCRa−/− WT, TCRa−/−Itch−/+, and TCRa−/−Itch−/− mice shows no significant difference among the groups for most of the isotypes. (C) However, IgE is substantially elevated in Itch−/− mice. (D) Ag-specific Igs in TCRa−/− WT, TCRa−/−Itch+/−, and TCRa−/−Itch−/− mice immunized with TNP-Ficoll were equivalent. (E) The average (± SD) numbers of total peritoneal B-cell subpopulations from TCRa−/− WT (□; n = 8), TCRa−/−Itch+/− ( ; n = 8), and TCRa−/−Itch−/− (■; n = 18) mice are shown. Subpopulations were identified by flow cytometry as follows: B2 = IgDhiIgMlo; B1 = IgMhiIgDlo; B1a = Mac1+CD5+; and B1b = Mac1+CD5−.
; n = 8), and TCRa−/−Itch−/− (■; n = 18) mice are shown. Subpopulations were identified by flow cytometry as follows: B2 = IgDhiIgMlo; B1 = IgMhiIgDlo; B1a = Mac1+CD5+; and B1b = Mac1+CD5−.
Itch−/− αβ T cells are required for elevated IgM and autoimmune antibodies but dispensable for hyper-IgE. (A) No production of antinuclear antibodies was detected by ELISA in sera of TCRa−/− or TCRa−/−Itch−/− mice. Mice are divided in 2 age groups: 1 to 3 and 4 to 7 months of age. (B) Analysis of total Igs by ELISA from preimmune TCRa−/− WT, TCRa−/−Itch−/+, and TCRa−/−Itch−/− mice shows no significant difference among the groups for most of the isotypes. (C) However, IgE is substantially elevated in Itch−/− mice. (D) Ag-specific Igs in TCRa−/− WT, TCRa−/−Itch+/−, and TCRa−/−Itch−/− mice immunized with TNP-Ficoll were equivalent. (E) The average (± SD) numbers of total peritoneal B-cell subpopulations from TCRa−/− WT (□; n = 8), TCRa−/−Itch+/− ( ; n = 8), and TCRa−/−Itch−/− (■; n = 18) mice are shown. Subpopulations were identified by flow cytometry as follows: B2 = IgDhiIgMlo; B1 = IgMhiIgDlo; B1a = Mac1+CD5+; and B1b = Mac1+CD5−.
; n = 8), and TCRa−/−Itch−/− (■; n = 18) mice are shown. Subpopulations were identified by flow cytometry as follows: B2 = IgDhiIgMlo; B1 = IgMhiIgDlo; B1a = Mac1+CD5+; and B1b = Mac1+CD5−.
FACS analysis of peritoneal exudate showed that Itchy TCRa−/− mice have an increased percentage of B1 cells compared with TCRa−/− mice (Figure S3C), reflecting a slight increase in numbers of both B1a and B1b subpopu-lations (Figure 4E). However, the rise in B1b cells (∼ 2-fold) is considerably less than that observed for T cell–competent Itchy mice (∼ 6-fold) and was comparable with the overall increase in lymphoid cellularity found in Itchy TCRa−/− mice.
αβ T cells initiate and maintain autoimmunity and instruct production of IgM, but not IgE, by B1 cells
To evaluate further the role of T cells in the autoimmune disorder and in B1 cell development, we generated mixed bone marrow (BM) chimeras (Table 1). By using combinations of BM from mice that could develop only B cells (TCRa−/− donors) or only T cells (μMT donors), we could restrict Itch deficiency to specific cell lineages within individual mice.
Peritoneal exudate cellularity in mixed bone marrow chimeras
| Group . | Donor mice . | Cell genotype . | Peritoneal subpopulation, cell no., ×107 . | ||||
|---|---|---|---|---|---|---|---|
| B cells . | T cells . | B1 . | B2 . | B1a . | B1b . | ||
| A | Itch−/− | −/− | −/− | 0.79* | 0.24* | 0.05* | 0.65* |
| B | B6 CD45.1 + B10 | WT | WT | 0.45 | 0.45 | 0.11 | 0.29 |
| C | μMT + Itch−/−TCRa−/− | −/− | WT | 0.27 | 0.33* | 0.05* | 0.20* |
| D | TCRa−/− CD45.1 + Itch−/− | WT & −/− | −/− | 0.62* | 0.24* | 0.05* | 0.51 |
| E | B6 CD45.1 + Itch−/−TCRa−/− | WT & −/− | WT | 0.32 | 0.62 | 0.05* | 0.23 |
| F | B6 CD45.1 + Itch−/− | WT & −/− | WT & −/− | 0.75* | 0.17* | 0.06* | 0.65* |
| Group . | Donor mice . | Cell genotype . | Peritoneal subpopulation, cell no., ×107 . | ||||
|---|---|---|---|---|---|---|---|
| B cells . | T cells . | B1 . | B2 . | B1a . | B1b . | ||
| A | Itch−/− | −/− | −/− | 0.79* | 0.24* | 0.05* | 0.65* |
| B | B6 CD45.1 + B10 | WT | WT | 0.45 | 0.45 | 0.11 | 0.29 |
| C | μMT + Itch−/−TCRa−/− | −/− | WT | 0.27 | 0.33* | 0.05* | 0.20* |
| D | TCRa−/− CD45.1 + Itch−/− | WT & −/− | −/− | 0.62* | 0.24* | 0.05* | 0.51 |
| E | B6 CD45.1 + Itch−/−TCRa−/− | WT & −/− | WT | 0.32 | 0.62 | 0.05* | 0.23 |
| F | B6 CD45.1 + Itch−/− | WT & −/− | WT & −/− | 0.75* | 0.17* | 0.06* | 0.65* |
Bone marrow cells from indicated donor mice were depleted from B220+ and Thy1.2+ cells and injected in a 1:1 ratio to a total of 6×106 cells per mouse (excluding group A where 3×106 cells per mouse were injected). Numbers of peritoneal exudate cells of mice are presented 8 to 10 weeks after adoptive transfer (n = 5).
P < .05 in t test with group B.
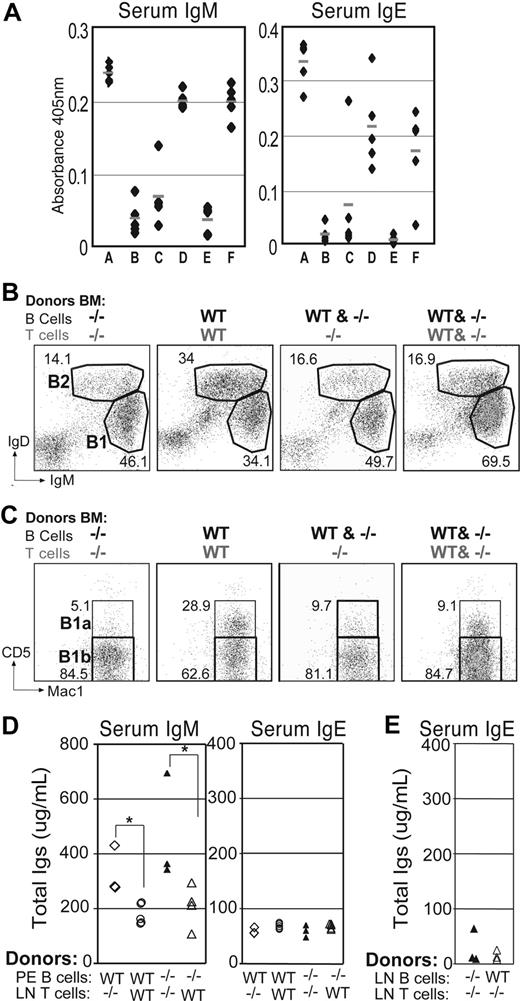
As expected, mice reconstituted with WT cells (group B) remained healthy for the duration of the experiment, while chimeras reconstituted exclusively with Itchy BM (group A) developed autoimmunity. Among the groups reconstituted with cells of mixed origin, only mice having T cells that were Itch deficient (groups A, D, and F) developed disease, regardless of the presence of WT T cells that also contain WT Tregs. The latter had elevated concentrations of serum IgM and IgE compared with the chimeras that did not develop disease (Figure 5A) and also showed a significant increase in numbers (Table 1) and percentages (Figure 5B) of B1 cells, and particularly B1b cells (Figure 5C; Table 1). As before (Figure 3B), given that the donor cells were from adult BM, there was limited reconstitution of peritoneal B1a cells. In some groups, by using the allotypic marker CD45.1, we were able to follow the development of cells with or without Itch within the same environmental milieu. Itch−/− B cells were less capable of entering the B1a compartment, in contrast to their WT counterparts in the same mouse (data not shown). Notably, mice reconstituted with Itch−/− B cells, but T cells from WT mice (group C), remained healthy and had serum IgM and IgE, and peritoneal B-cell subpopulations comparable with controls, indicating that the enhanced Igs and autoimmune phenotype in Itchy mice is not B-cell intrinsic.
Autoimmunity and expansion of B1b cells requires Itch−/− T cells.(A) Rag1−/− mice were reconstituted with combinations of bone marrow from WT or Itch−/− mouse strains (Table 1) and analyzed 14 to 18 weeks later. Anti-IgM and anti-IgE ELISA are shown for sera of mixed chimeras. (B) Representative FACS plots of peritoneal cells from mixed chimeric mice are shown for B cells after gating on lymphocytes using side and forward scatter profiles. (C) B1-gated peritoneal cells from the same mice were stained for B1a (Mac1+CD5+) and B1b (Mac1+CD5−) cells. (D) Rag1−/− mice were reconstituted with WT or Itch−/− purified peritoneal B1 cells together with purified Itch−/− or WT CD4+ T cells (LN), as indicated. Sera were analyzed 4 weeks later for total IgM (left) and IgE (right) by ELISA. (*P < .05). (E) Rag1−/− mice were reconstituted with WT or Itch−/− B cells (LN) together with purified Itch−/− or WT CD4+ T cells, as indicated. Sera were analyzed 4 weeks later for IgE by ELISA.
Autoimmunity and expansion of B1b cells requires Itch−/− T cells.(A) Rag1−/− mice were reconstituted with combinations of bone marrow from WT or Itch−/− mouse strains (Table 1) and analyzed 14 to 18 weeks later. Anti-IgM and anti-IgE ELISA are shown for sera of mixed chimeras. (B) Representative FACS plots of peritoneal cells from mixed chimeric mice are shown for B cells after gating on lymphocytes using side and forward scatter profiles. (C) B1-gated peritoneal cells from the same mice were stained for B1a (Mac1+CD5+) and B1b (Mac1+CD5−) cells. (D) Rag1−/− mice were reconstituted with WT or Itch−/− purified peritoneal B1 cells together with purified Itch−/− or WT CD4+ T cells (LN), as indicated. Sera were analyzed 4 weeks later for total IgM (left) and IgE (right) by ELISA. (*P < .05). (E) Rag1−/− mice were reconstituted with WT or Itch−/− B cells (LN) together with purified Itch−/− or WT CD4+ T cells, as indicated. Sera were analyzed 4 weeks later for IgE by ELISA.
To evaluate further the contribution of T cells and B1 cells to the onset of the hyper-IgM phenotype, we reconstituted Rag1−/− mice with purified lymph node (LN) CD4+ T cells together with peritoneal B1 cells from either WT or Itch−/− mice. Only mice reconstituted with Itch−/− CD4 T cells and peritoneal B cells (regardless of their genotype) displayed hyper-IgM and signs of autoimmunity by 4 weeks, whereas mice that received WT T cells did not (Figure 5D). In contrast to IgM, no overproduction of IgE was observed after transfer of Itch−/− αβ T cells together with WT or Itch−/− peritoneal or LN B cells (Figure 5D,E), indicating that different mechanisms and different B-cell subsets underlie the development of hyper-IgE and hyper-IgM symptoms in mice deficient for Itch. Unlike previous reports,37 we were unable to reconstitute peritoneal B-cell populations by transfer of LN B cells, although B cells were readily detectable in LN and spleen.
Dissociation between Th2-polarized CD4+ T cells and elevated serum IgE in Itch−/− mice: a role for γδ T cells
While overproduction of IgM in Itchy mice correlated with the presence of Itch−/− αβ T cells, the high levels of IgE found in the Itch−/−TCRa−/− mice together with the failure to see elevated IgE production in the presence of transferred Itch−/− CD4 T cells suggested that Itch−/− αβ T cells were not initiating hyper-IgE production in this model. This was surprising given that the propensity of Itch−/− CD4 T cells to develop along the Th2 lineage in vitro was suggested to contribute to autoimmunity.15 Moreover, we observed no particular overproduction of Th2-specific Ig isotypes after immunization of Itchy mice in vivo compared with wild-type controls, under conditions in which we might have expected exacerbation of the Th2 bias. Therefore, we asked whether Itch deficiency in the B10 background skewed T helper cell differentiation in vitro.
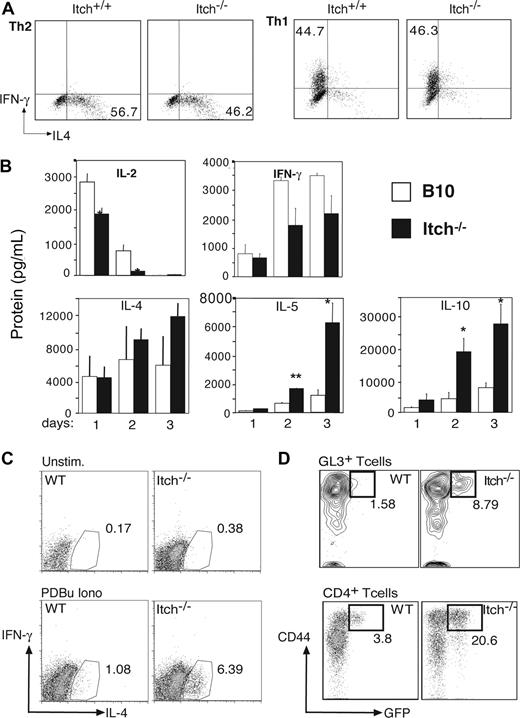
Naive CD4 T cells from B10 or Itchy mice were purified and cultured with anti-CD3, anti-CD28, and IL-2 under polarizing conditions for Th1 (IL-12 plus anti–IL-4 Abs) or Th2 (IL-4 plus anti-IFNγ Abs) responses. After 7 days, the differentiated cells were restimulated with plate-bound anti-CD3 and analyzed for intracellular cytokine production. The proportions of cells that produced either IFNγ or IL-2 under Th1 conditions, and those that produced IL-4 or IL-10 under Th2 conditions, were comparable with control cell cultures (Figure 6A and data not shown), whereas in nonpolarizing Th0 conditions the percentage of Itch−/− IL-4–producing cells is slightly higher (data not shown). More notably, when WT APCs, rather than polarizing cytokines and antibodies, were added to the stimulation of CD4+ T cells with anti-CD3 and anti-CD28, Itch−/− cells produced a Th2 cytokine profile with elevated IL-4 and particularly augmented IL-5 and IL-10 secretion in comparison with WT cells (Figure 6B). Therefore, TCR triggering in presence of APCs led to a more pronounced Th2-polarized response in Itch−/− CD4 T cells, suggesting that engagement of costimulatory molecules was required to direct the Th2 polarization in absence of Itch.
γδ T cells from Itch−/− mice produce IL-4 ex vivo. (A) Naive CD4+ T cells were stimulated under Th1- or Th2-polarizing conditions in vitro for 1 week before incubation for 4 hours with PDBu and ionomycin in presence of brefeldin A. IL-4 and IFN-γ production was evaluated by FACS after intracellular staining with specific fluorescent Abs. (B) WT (□) and Itch−/− (■) lymph node CD4+ T cells were stimulated for 1, 2, or 3 days with anti-CD3/anti-CD28 Abs in presence of CD11c+ spleen APCs. Cytokine levels were detected by a multiplex fluorescent bead immunoassay. Representative profiles of selected cytokines in 1 of 5 experiments are shown (*P < .05). (C) Splenic WT and Itch−/− γδ T cells were left unstimulated or activated with PDBu and ionomycin in presence of brefeldin A for 4 hours. IL-4 and IFN-γ production was evaluated by FACS after intracellular staining with fluorescent specific Abs. Representative profile in 1 of 3 experiments are shown. (D) IL-4 production in peritoneal γδ T cells and CD4+ αβ T cells was monitored directly ex vivo using 4get WT or 4get Itch−/− IL-4 reporter mice. Gated γδ T cells (GL3+ CD3+) or CD4+ αβ T cells (H57+) are shown, after gating on lymphocytes using side and forward scatter profile. Percentage of CD44+ GFP+ is indicated.
γδ T cells from Itch−/− mice produce IL-4 ex vivo. (A) Naive CD4+ T cells were stimulated under Th1- or Th2-polarizing conditions in vitro for 1 week before incubation for 4 hours with PDBu and ionomycin in presence of brefeldin A. IL-4 and IFN-γ production was evaluated by FACS after intracellular staining with specific fluorescent Abs. (B) WT (□) and Itch−/− (■) lymph node CD4+ T cells were stimulated for 1, 2, or 3 days with anti-CD3/anti-CD28 Abs in presence of CD11c+ spleen APCs. Cytokine levels were detected by a multiplex fluorescent bead immunoassay. Representative profiles of selected cytokines in 1 of 5 experiments are shown (*P < .05). (C) Splenic WT and Itch−/− γδ T cells were left unstimulated or activated with PDBu and ionomycin in presence of brefeldin A for 4 hours. IL-4 and IFN-γ production was evaluated by FACS after intracellular staining with fluorescent specific Abs. Representative profile in 1 of 3 experiments are shown. (D) IL-4 production in peritoneal γδ T cells and CD4+ αβ T cells was monitored directly ex vivo using 4get WT or 4get Itch−/− IL-4 reporter mice. Gated γδ T cells (GL3+ CD3+) or CD4+ αβ T cells (H57+) are shown, after gating on lymphocytes using side and forward scatter profile. Percentage of CD44+ GFP+ is indicated.
Although we observed a Th2 bias in Itch−/− CD4 T cells stimulated in vitro, it was uncertain whether such Th2-biased cognate help was relevant in vivo, particularly as there was discordance in the production of typical Th2 Ab isotypes. Moreover, given that spontaneous IgE levels were even higher in the complete absence of αβ T cells in TCRa−/−Itch−/− mice, we examined whether other cellular subsets could be contributing to this condition. To this end, we asked whether splenic γδ T cells from Itch−/− mice were able to produce IL-4 upon short restimulation with phorbol esters and ionomycin. Indeed, a substantial population of Itch-deficient γδ T cells could produce intracellular IL-4 ex vivo, in contrast to γδ T cells from WT mice (Figure 6C). Moreover, when Itch−/− mice were crossed with 4get reporter mice,27 we found that there were increased percentages of IL-4–expressing γδ and αβ T cells in the peritoneal lavage ex vivo (Figure 6D), although interestingly, the proportions of IL-4+ αβ and γδ T cells in the LN and spleens of these mice were similar (data not shown), at least at a young age. Activated T cells preferentially migrate to tissue sites such as the peritoneum, and exposure to gut flora and/or food antigens is thought to promote the development of CD44high memory/effector T cells in naive mice,38 therefore, it is not surprising that we could find IL-4–expressing cells in the peritoneum at this age. As the mice age, the numbers of CD44high cells in primary lymphoid sites accumulate, and in older mice we might expect to see more GFP-positive cells in lymph nodes and spleen. Although there are increased frequencies of both αβ and γδ T cells expressing IL-4 in Itch−/− mice, we propose that Itch−/− γδ T cells are required to initiate noncognate helper activity as transfer of Itch−/− αβ T cells alone was unable to drive IgE isotype switching, although IL-4–producing αβ T cells might further contribute to this phenotype once established.
Discussion
The autoimmune phenotype of Itchy mice is characterized by scratching, expansion of lymphoid tissue, increased production of particular Ig isotypes, and tissue inflammation. Itch is widely expressed by many different cell types, however, we show here that the primary cause of autoimmunity is the absence of Itch in T cells. Surprisingly, both Itch−/− αβ and γδ T cells contribute to disease, with the former driving expansion of B1b cells and hyper-IgM, while the latter was required to initiate hyper-IgE production. Moreover, pathology and scratching correlated with increased IgM rather than overproduction of IgE, which was associated with a milder lymphadenopathy.
T cells from Itchy mice were shown to have an expansion of CD4+ memory cells.15 In addition, the absence of Itch was shown to increase the stability of Jun family proteins in CD4 cells thus promoting Th2-biased T-cell differentiation.15,16 This, together with the reported elevation of IgE and IgG1 in Itchy mice, was suggested to be the primary contributor to disease. However, our data show dissociation between symptoms of disease and the production of Th2-type Ab isotypes, as hyper-IgE in our Itch−/− mice was not accompanied by increased expression of IgG1. This may be due to genetic background differences in the mice, but may also be a consequence of the help for IgE production being provided in a noncognate fashion.39 When Th2 immunization protocols that induce cognate T-cell help were used to immunize the mice, specific IgG1 Ab production was equivalent between Itchy and WT mice. However, in unimmunized animals we found that IL-4, the driving cytokine for IgE switching, was also produced by γδ T cells that might induce IgE production outside of germinal centers (GCs). Erazo et al40 described recently that IgE-producing cells could develop very quickly into plasma cells outside GCs, whereas IgG1 was produced exclusively within GCs. Other examples of γδ T cell–driven hypergammaglobulinemia, such as mice with particular mutations in the LAT adapter molecule,41 show increased IgE together with IgG1, however such mice also show chronic γδ T-cell activation, which was not apparent in Itchy mice. Interestingly, increased IgE without increased IgG1 was found in Ndfip1−/− mice.42 Ndfip1 was shown to interact with and regulate the activity of Itch, and the absence of Ndfip1 led to increased production of Th2 cytokines also, although whether these were produced by γδ T cells was not described.
We could readily demonstrate a Th2 bias in Itchy αβ T cells after stimulation in vitro, as others have described. However, the presence of dendritic cells (DCs) together with stimulating anti-CD3/CD28 Abs was necessary to see elevated IL-4 production starting from highly purified naive Itch−/− T cells. Discrepancies with previous observations may reflect the purity of the starting T-cell populations, for example, from using FACS sorting versus magnetic bead selected cells. The latter may contain memory phenotype cells (expanded in Itchy mice) and APCs. Indeed, highly purified naive Itch−/− T cells activated in absence of accessory DCs showed no more cytokine bias than WT cells, in our hands, suggesting that costimulatory factors provided by DCs, rather than direct TCR activation, might account for the Th2-skewed response in Itch−/− T cells.
Although dispensable for the production of elevated IgE in Itchy mice, Itch−/− αβ T cells were essential for the development of autoimmunity. Autoantibody production and itching, particularly in adult mice, occurred only in presence of Itch−/− αβ T cells and were inevitably associated with augmented B1b cellularity and hyper-IgM. Itch has been identified as a negative modulator of Notch,21,43 whose signaling is fundamental for the development of both MZB44 and B1 cells, and both these subsets have the potential to produce self-reactive Abs, particularly IgM.45 It was shown that conditional knockout mice for the Notch signaling modulator, RBP-J, had a severe reduction of MZB cells, but normal serum levels of IgM and IgG3, indicating that these Abs are produced by other cell subsets,46 such as peritoneal B1 cells that were not affected by this mutation. B1 cells have been implicated in several animal models of autoimmunity, and mice carrying mutations in negative regulators of BCR signaling have expansion of B1 cells, with the potential of producing autoreactive IgM.33 Itch−/− mice showed no perturbation in the MZB subset, and we found that hyper-IgM production was transferred in recipients of Itchy PE B1 cells, strongly implicating this population as the major cause of disease in Itchy mice.
Opinions on the genesis of peritoneal B1b cell subpopulation are divided as to whether these cells originate from B1a B cells or from the B2 population.47 Recent data have shown that CD45Rlo-neg CD19+ CD138int perinatal bone marrow precursor cells are the primary source of B1a cells, whereas adult bone marrow cells with the same markers give rise preferentially to B1b cells.48,49 Adult bone marrow was shown to contain low numbers of B1a precursors compared with fetal tissue,50 and, unsurprisingly, we observed that recipients of adult bone marrow had poor reconstitution of the CD5+ B1a compartment. In contrast, in the presence of Itch−/− αβ T cells, a large expansion of B1b cells occurred, regardless of the B-cell genotype in the mixed bone marrow chimeras and concomitant with normal B2 cell reconstitution. It is noteworthy that mice reconstituted with Itch−/− B cells in the presence of WT T cells showed neither expanded B1b cells nor evidence of disease, suggesting that the propensity to produce IgM and IgE is not intrinsic to Itch−/− B cells. B1b cell expansion was shown to occur in mice transgenic for the cytokine IL-9,51 and it is possible that production of this or similar cytokines by Itch−/− T cells drives expansion of the B1b population.
In chimeric mice with mixed WT and Itch−/− B cells, we observed that Itch−/− B cells were less capable of entering the B1a compartment than their WT counterparts, suggesting Itch might influence some aspects of B-cell differentiation. There are a number of mechanisms by which lack of Itch could influence B-cell development. For example, Itch has been identified as an E3 ubiquitin ligase for Bcl10,18 a modulator of NF-κB downstream of the BCR. Mice deficient for Bcl10 or for other modulators of NF-κB have severely reduced or absent B1 cells, reduced serum IgM, and fewer MZ B cells,17 highlighting a role for NF-κB in B-cell development and function. Given that the strength of signaling through the BCR significantly influences B-cell development,52 it is conceivable that the absence of a negative regulator, such as Itch, could cause an increase in BCR signaling output resulting in differentiation to B1b rather than to B1a or MZB cells.
In conclusion, our studies show that there can be multiple components to the immune dysregulation resulting from loss of a widely expressed intracellular signaling molecule. Our data point to a role for the innate immune system, particularly γδ T cells, in some aspects of disease, notably expansion of the lymphoid compartment, even though lethality seems to correlate primarily with dysregulation of αβ T cells. Production of Th2-type cytokines by αβ T cells may well contribute to disease, as IL-5 production has been linked to recruitment of B1 IgM-producing cells to local tissue, which together with eosinophil recruitment can result in contact sensitivity.53 It is likely that local dysregulation of cytokine production is important for disease induction, and resident innate cells may well be important contributors to this process.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Meenaxi Chavda for her technical contribution and Dr Brigitta Stockinger and Dr Alexandre Potocnik for insightful discussions. This work was supported by the Medical Research Council, United Kingdom.
Authorship
Contribution: V.P., A.-C. F., P.D.T., and M.A.B. performed experiments; V.P. and R.Z. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rose Zamoyska, Institute of Immunology and Infection Research, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK; e-mail: rose.zamoyska@ed.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal