Abstract
In principle, bone marrow transplantation should offer effective treatment for disorders originating from defects in mesenchymal stem cells. Results with the bone disease osteogenesis imperfecta support this hypothesis, although the rate of clinical improvement seen early after transplantation does not persist long term, raising questions as to the regenerative capacity of the donor-derived mesenchymal progenitors. We therefore studied the kinetics and histologic/anatomic pattern of osteopoietic engraftment after transplantation of GFP-expressing nonadherent marrow cells in mice. Serial tracking of donor-derived GFP+ cells over 52 weeks showed abundant clusters of donor-derived osteoblasts/osteocytes in the epiphysis and metaphysis but not the diaphysis, a distribution that paralleled the sites of initial hematopoietic engraftment. Osteopoietic chimerism decreased from approximately 30% to 10% by 24 weeks after transplantation, declining to negligible levels thereafter. Secondary transplantation studies provided evidence for a self-renewing osteopoietic stem cell in the marrow graft. We conclude that a transplantable, primitive, self-renewing osteopoietic cell within the nonadherent marrow cell population engrafts in an endosteal niche, like hematopoietic stem cells, and regenerates a significant fraction of all bone cells. The lack of durable donor-derived osteopoiesis may reflect an intrinsic genetic program or exogenous environmental signaling that suppresses the differentiation capacity of the donor stem cells.
Introduction
There are no obvious reasons why bone marrow transplantation (BMT) could not be used to correct a far greater range of stem-cell disorders than is commonly reported. This potential resides in the heterogeneity of the bone marrow cell population, which comprises precursors of several different tissues other than blood, including bone, cartilage, and muscle. Indeed, in animal models and clinical studies, transplanted marrow-derived mesenchymal cells migrate to and become incorporated into the recipient's bone and muscle,1-7 suggesting a therapeutic capacity for these cells
Prompted by encouraging preclinical studies,8,9 we undertook a series of clinical trials of allogeneic bone marrow cell therapy as treatment for children with severe osteogenesis imperfecta (OI),2,3,10 a genetic disorder in which generalized osteopenia leads to bony deformities, excessive bone fragility with fracturing, and short stature. The underlying defect is a mutation in one of the 2 genes encoding type I collagen, the primary structural protein of bone. After transplantation of unmanipulated whole bone marrow, we demonstrated marked improvement in total body mineral content and the microscopic structure of abnormal bone that were associated with accelerated linear growth and decreased fracture rates.2,10 Similarly, after transplantation of isolated donor-derived marrow mesenchymal stromal cells (MSCs) in the same patients, we observed a second phase of striking accelerated linear growth.3 In all instances, however, the observed acceleration of growth was not sustained with time after transplantation.
Thus, the inability of BMT to produce a sustained rate of improvement in the abnormalities of OI in children could be due either to engraftment of an osteopoietic progenitor with limited self-renewal and differentiation capacity or to engraftment of an osteopoietic stem cell with robust self-renewal properties that are subject to intrinsic or environmental regulatory mechanisms which prevent sustained therapeutic levels of donor-derived osteopoiesis. To address these possibilities, we studied the kinetics, histologic pattern, and anatomic distribution of osteopoietic donor cells after transplantation of nonadherent marrow cells in mice. Our selection of the nonadherent cell population was based on its more robust transplantable osteoprogenitor activity, compared with that of MSCs. Our findings, that the primary source of donor cell–derived osteopoiesis in this model is indeed a primitive cell with self-renewing capacity, but that the extent of osteoblast repopulating activity is limited by mechanisms that regulate the differentiation of these stem cells.
Methods
Transduction and transplantation of murine marrow cells
Donor and recipient (male and female, respectively; 6 to 8 weeks old) FVB/N mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in the animal facility at St Jude Children's Research Hospital under standard conditions. Donor marrow cells were harvested, transduced with a green fluorescent protein (GFP)–expressing retroviral vector, and transplanted into lethally irradiated (1125 cGy) recipient mice as previously described.5 Secondary transplantation studies used GFP-transgenic mice11 as primary donors. All animal protocols were approved by the Institutional Animal Use and Care Committee.
Immunohistochemical staining
Formalin-fixed, decalcified, and paraffin-embedded sections were stained with standard hematoxylin and eosin. (H&E; Sigma-Aldrich, St Louis, MO). Single and double immunohistochemical analyses were performed with rabbit anti-GFP antibody (1:300; Invitrogen, Carlsbad, CA), rabbit anti-collagen I (Col I) antibody (1:100; Chemicon International, Temecula, CA), and rabbit anti-osteocalcin (1:100; Cosmo Bio, Tokyo, Japan), with a goat anti-rabbit biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA) as previously described.5 Horseradish peroxidase was visualized with both NOVARed (red) and diaminobenzidine salts (Nickel-DAB, black; Vector Laboratories).
All slides were counterstained with Harris hematoxylin (Surgipath Medical Industries, Richmond, IL). Negative control specimens were sections taken from animals given transplants of mock-transduced marrow cells and immunostained with the anti-GFP antibody. As an additional negative control, bone sections from the experimental mice were immunostained with an isotypic primary antibody (Vector Laboratories). Background staining was not apparent in any of the cases.
Microscopy
Stained slides were examined on a Nikon E800 (Nikon, Melville, NY) with either a 10×/0.3 NA or a 40×/0.95 NA dry objective. Photomicrographs were acquired using the attached Nikon DXM1200 color camera and Nikon ACT-1 Version 2.11 software (Nikon, Melville, NY). Images were cropped and labeled using Photoshop 7.0 and Illustrator 10.0 (Adobe Systems, San Jose, CA).
Flow cytometry
Peripheral blood and bone marrow were analyzed for GFP expression by flow cytometry as previously described.12
Statistics
Analyses were performed with the Excel 2003 program (Microsoft, Redmond, Washington) or Prism, version 4 (GraphPad, San Diego, CA). A 2-tailed P value of .05 or less from the Student t test was considered statistically significant.
Results
Kinetics and histologic pattern of osteopoietic engraftment
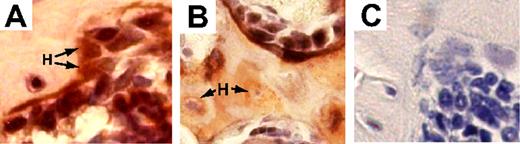
To more accurately define the kinetics of osteopoietic engraftment after BMT, we used our murine transplantation model, in which we previously demonstrated the capacity of nonadherent bone marrow cells to engraft in bone.5 After transplanting lethally irradiated FVB/N recipient mice with 2 × 106 nonadherent bone marrow cells (FVB/N donors), transduced with a GFP-expressing retroviral vector, we used immunohistochemical staining to identify donor cells. Relatively large, cuboidal cells, often showing abundant cytoplasm and eccentrically placed nuclei, distributed along the endosteal surface were considered to be osteoblasts, while solitary, stellate-shaped cells within the lacunae of bone were regarded as osteocytes. Double staining of representative sections of bone at 4 weeks after transplantation demonstrated that these bone cells coexpressed GFP and osteocalcin (Figure 1A), or GFP and collagen I (Figure 1B), confirming their identity as osteoblasts and osteocytes.
Nonadherent donor bone marrow cells engraft as osteoblasts and osteocytes after transplantation. (A) Representative photomicrograph of a bone/bone marrow section taken from a mouse after nonadherent marrow cell transplantation double stained with anti-GFP (black) and antiosteocalcin (red) antibodies. Several double-positive donor osteoblasts are distributed along the surface of endosteal bone, with a donor (GFP+) osteocyte embedded in bone. Osteocalcin-expressing host cells (H; red stain without GFP) are indicated by arrows. (B) Bone/bone marrow section double stained with anti-GFP (black) and anti-collagen l (red) antibodies. Donor osteoblasts and osteocytes were detected by red/black colocalization. Collagen-expressing host osteocytes (H) are indicated by arrows. (C) Control bone section from a mouse transplanted with nontransduced nonadherent bone marrow cells and stained with anti-GFP and isotype control antibodies. Original magnification for all panels, 1600× (400× optical, 4× digital).
Nonadherent donor bone marrow cells engraft as osteoblasts and osteocytes after transplantation. (A) Representative photomicrograph of a bone/bone marrow section taken from a mouse after nonadherent marrow cell transplantation double stained with anti-GFP (black) and antiosteocalcin (red) antibodies. Several double-positive donor osteoblasts are distributed along the surface of endosteal bone, with a donor (GFP+) osteocyte embedded in bone. Osteocalcin-expressing host cells (H; red stain without GFP) are indicated by arrows. (B) Bone/bone marrow section double stained with anti-GFP (black) and anti-collagen l (red) antibodies. Donor osteoblasts and osteocytes were detected by red/black colocalization. Collagen-expressing host osteocytes (H) are indicated by arrows. (C) Control bone section from a mouse transplanted with nontransduced nonadherent bone marrow cells and stained with anti-GFP and isotype control antibodies. Original magnification for all panels, 1600× (400× optical, 4× digital).
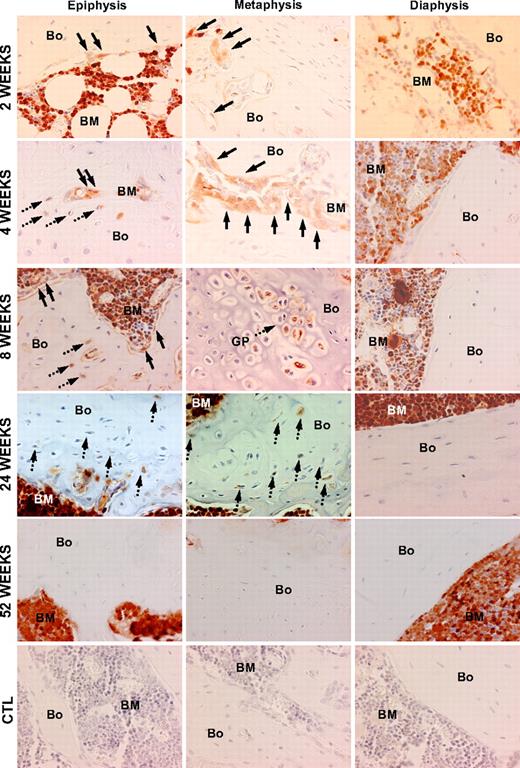
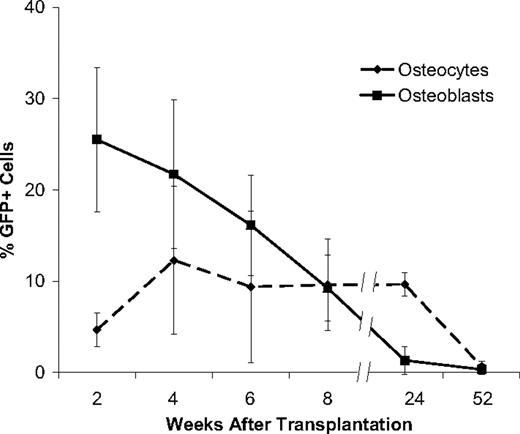
We then traced the fate of transplanted, GFP-transduced nonadherent marrow cells in the osteoblast and osteocyte niches of bone (Figures 2,3). At 2 weeks, 25.5% plus or minus 7.8% (mean ± SD) of the osteoblasts in the metaphysis and epiphysis were of donor origin, while only rare donor-derived osteoblasts (< 1%) were found in the diaphysis (Figure 2). The donor cells appeared as several small clusters of GFP+ cells along the endosteal surface, invariably adjacent to GFP+ hematopoietic cells within the marrow space. In contrast to the substantial donor contribution to the osteoblast population, only 4.6% plus or minus 1.7% of osteocytes in the metaphysis and epiphysis, and none in the diaphysis, were donor derived at 2 weeks after transplantation. As with the donor-derived osteoblasts, these osteocytes were arranged as clusters within the trabecular bone in close proximity to the endosteal surface.
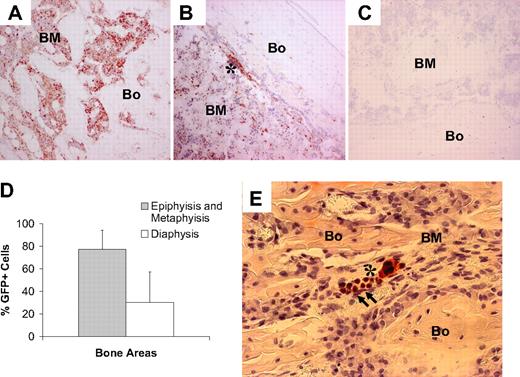
Patterns of bone engraftment with increasing time after transplantation of nonadherent bone marrow cells. Sections taken at 2 to 52 weeks after transplantation from different regions of bone (Bo) and bone marrow (BM) were stained with anti-GFP antibody (red). At 2 weeks, GFP+ osteoblasts (arrows) engrafted as clusters next to host (GFP−) osteoblasts in the epiphysis and in the metaphysis, while in the diaphysis the osteoblasts remained almost exclusively host derived. Beginning at 4 weeks, GFP+ osteocytes (dashed arrows) were consistently detected in both the metaphysis and epiphysis. At 8 weeks, in the metaphysis, in particular, Bo-embedded GFP+ cells could be identified under the growth plate (GP) as cell clusters. Again, the donor contribution was virtually undetectable in the diaphysis. At 24 weeks, a similar pattern of donor-derived osteopoiesis was evident. By 52 weeks, neither donor Bo nor BM contained GFP+ osteoblasts or osteocytes. The bottom row consists of photomicrographs of control (CTL) Bo/BM sections from a mouse that received a transplant of untransduced cells that were stained with anti-GFP antibody. Original magnification of all panels, 400×.
Patterns of bone engraftment with increasing time after transplantation of nonadherent bone marrow cells. Sections taken at 2 to 52 weeks after transplantation from different regions of bone (Bo) and bone marrow (BM) were stained with anti-GFP antibody (red). At 2 weeks, GFP+ osteoblasts (arrows) engrafted as clusters next to host (GFP−) osteoblasts in the epiphysis and in the metaphysis, while in the diaphysis the osteoblasts remained almost exclusively host derived. Beginning at 4 weeks, GFP+ osteocytes (dashed arrows) were consistently detected in both the metaphysis and epiphysis. At 8 weeks, in the metaphysis, in particular, Bo-embedded GFP+ cells could be identified under the growth plate (GP) as cell clusters. Again, the donor contribution was virtually undetectable in the diaphysis. At 24 weeks, a similar pattern of donor-derived osteopoiesis was evident. By 52 weeks, neither donor Bo nor BM contained GFP+ osteoblasts or osteocytes. The bottom row consists of photomicrographs of control (CTL) Bo/BM sections from a mouse that received a transplant of untransduced cells that were stained with anti-GFP antibody. Original magnification of all panels, 400×.
Kinetics of bone engraftment after transplantation. Robust GFP+ osteoblast engraftment was detected at 2 and 4 weeks after transplantation with significant declines thereafter: 6 weeks (P = .02), 8 weeks (P = .04), 24 weeks (P = .01). GFP+ osteocyte engraftment showed a different kinetic profile characterized by a significant increase at 4 weeks (P = .04), a plateau phase and a significant decrease to a negligible level at 52 weeks (P = .004). To quantify the engraftment of donor GFP + cells in bone, we scored 20 random 400× fields in both the epiphysis and the metaphysis of 10 bone sections taken from each mouse at different times after transplantation (n ≥ 4 mice per group). Experiments were performed in triplicate. The reported values are mean (+SD) percentages of GFP+ cells per 400× microscopic field.
Kinetics of bone engraftment after transplantation. Robust GFP+ osteoblast engraftment was detected at 2 and 4 weeks after transplantation with significant declines thereafter: 6 weeks (P = .02), 8 weeks (P = .04), 24 weeks (P = .01). GFP+ osteocyte engraftment showed a different kinetic profile characterized by a significant increase at 4 weeks (P = .04), a plateau phase and a significant decrease to a negligible level at 52 weeks (P = .004). To quantify the engraftment of donor GFP + cells in bone, we scored 20 random 400× fields in both the epiphysis and the metaphysis of 10 bone sections taken from each mouse at different times after transplantation (n ≥ 4 mice per group). Experiments were performed in triplicate. The reported values are mean (+SD) percentages of GFP+ cells per 400× microscopic field.
At 4 weeks after transplantation, the proportion of donor-derived osteoblasts had decreased to 21.7% plus or minus 8.1% (Figure 3). The cells were arranged as clusters without GFP− host cells, although numerous GFP− cells were seen between the clustered (GFP+) donor osteoblasts (Figure 2). There was an increased proportion of donor derived osteocytes in clusters of 10 to 15 GFP+ cells each, accounting for 12.2% plus or minus 7.5% of all osteocytes in the metaphysis and epiphysis. The donor cells were most often found toward the middle of the trabeculae in the histologic sections. GFP+ cells were not detectable in the diaphysis.
The contribution of donor-derived cells to the osteoblast compartment of the metaphysis and epiphysis steadily declined from the peak at 2 weeks to 16.1% plus or minus 8.1% at 6 weeks and 1.5% plus or minus 1.3% at 24 weeks after transplantation. By 1 year, donor cells were minimally detectable in bone (0.3% ± 0.3%). By contrast, the donor fraction of osteocytes rose from 2 to 4 weeks, remaining statistically stable from 6 weeks (9.2% ± 3.5%) to 24 weeks (9.6% ± 1.0%). Donor osteocytes were rarely seen (0.6% ± 0.4%) at 1 year after transplantation (Figure 3).
Kinetics and histologic pattern of hematopoietic engraftment
If, as we propose,5 the transplantable osteopoietic cells are derived from a common nonadherent hematopoietic-osteopoietic progenitor, what might account for the lack of durable osteopoietic engraftment in our murine model? One explanation might be that the donor cells were defective in their long-term regenerative capacity overall.
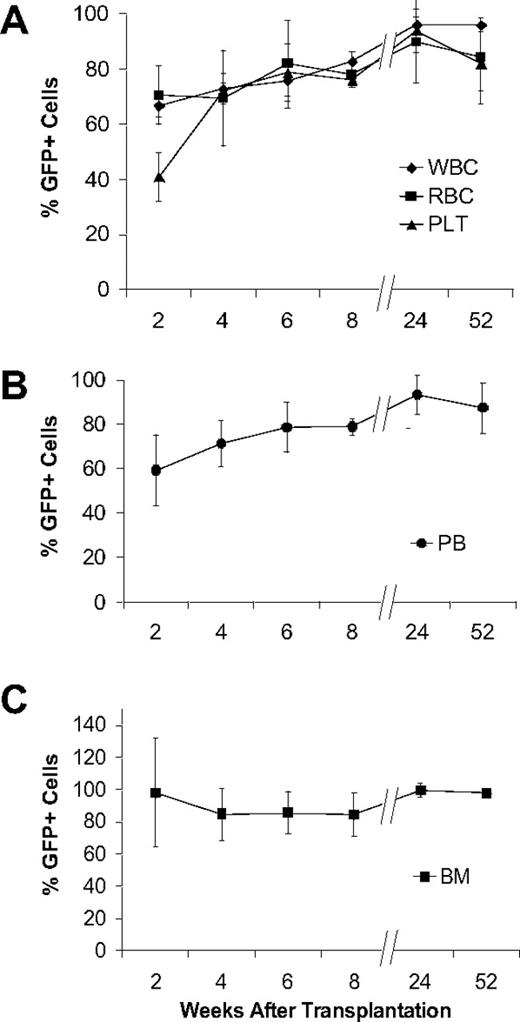
To test this prediction, we first analyzed the contribution of GFP+ cells to subsets of hematopoietic cells in the peripheral blood using flow cytometry. From 2 to 8 weeks after transplantation, the proportion of leukocytes, erythrocytes, and platelets increased substantially to 82.7% plus or minus 3.4%, 78.0% plus or minus 1.2%, and 76.1% plus or minus 2.9%, respectively (Figure 4A). Long-term analysis showed that the overall contribution of donor cells to blood increased from 8 weeks to 24 weeks (79.0% ± 3.7% to 93.3% ± 8.1%) remaining stable at 52 weeks (Figure 4B). The total marrow cellularity, determined by histologic examination of sequential bone marrow sections, increased from 49.6% plus or minus 12.7% at 2 weeks to 90% plus or minus 4.0% at 8 weeks, remaining stable at 52 weeks (93.3% ± 2.5%). The fraction of GFP+ bone marrow cells was about 98% at 2 weeks, 82% to 84% at 4 to 8 weeks, and more than 95% at 24 weeks, a level that was maintained through 52 weeks (Figure 4C).
Nonadherent bone marrow cells can produce stable hematopoietic engraftment. (A) GFP expression by white blood cells (WBC), red blood cells (RBC), and platelets (PLT) assessed by flow cytometry with the whole peripheral blood analyses. The findings are reported as mean (+SD) percentages of GFP+ cells representing each of the 3 hematopoietic lineages. Values for all lineages were significantly increased at 24 weeks (WBC, P < .001; RBC, P = .03; PLT, P = .008) compared with 2 weeks, becoming stable thereafter. (B) Flow cytometric analysis of GFP expression in whole peripheral blood (PB) collected from the mice that underwent transplantation (n ≥ 4 per group) at increasing times after transplantation. The findings are reported as mean (+SD) percentages of GFP+ cells. (C) GFP expression in bone marrow assessed at different times after transplantation. We scored 20 randomly selected 400× fields in representative sections from each mouse (n ≥ 4 mice per group). The values were derived from immunohistochemical analyses of bone/bone marrow sections stained with anti-GFP antibody and are expressed as mean (+SD) percentages of GFP+ cells in each microscopic field. All experiments were performed in triplicate.
Nonadherent bone marrow cells can produce stable hematopoietic engraftment. (A) GFP expression by white blood cells (WBC), red blood cells (RBC), and platelets (PLT) assessed by flow cytometry with the whole peripheral blood analyses. The findings are reported as mean (+SD) percentages of GFP+ cells representing each of the 3 hematopoietic lineages. Values for all lineages were significantly increased at 24 weeks (WBC, P < .001; RBC, P = .03; PLT, P = .008) compared with 2 weeks, becoming stable thereafter. (B) Flow cytometric analysis of GFP expression in whole peripheral blood (PB) collected from the mice that underwent transplantation (n ≥ 4 per group) at increasing times after transplantation. The findings are reported as mean (+SD) percentages of GFP+ cells. (C) GFP expression in bone marrow assessed at different times after transplantation. We scored 20 randomly selected 400× fields in representative sections from each mouse (n ≥ 4 mice per group). The values were derived from immunohistochemical analyses of bone/bone marrow sections stained with anti-GFP antibody and are expressed as mean (+SD) percentages of GFP+ cells in each microscopic field. All experiments were performed in triplicate.
At 2 weeks after transplantation, the total marrow and GFP+ marrow cells preferentially localized to the trabecular bone of the metaphysis and epiphysis (cellularity, 77.4% ± 16.8%) as compared with the diaphysis (cellularity, 30.1% plus or minus 27.1%; P < .001; Figure 5A-D). The marrow cells appeared in clusters that gave a patchy appearance to engraftment. Although several groups of donor (GFP+) marrow cells were found along a bone surface lacking donor (GFP+) osteopoietic cells, all areas of donor osteopoietic engraftment bordered donor marrow cell clusters (Figure 5E). From 4 to 52 weeks after transplantation, there was no evidence of anatomic variation of the total and GFP+ marrow cellularity (P > .05). Thus, these mice exhibit an expected pattern of hematopoietic reconstitution after transplantation of GFP-marked nonadherent cells. Together, these data suggest that hematopoietic stem cell niches capable of engrafting transplanted, or circulating, hematopoietic stem cells reside in the metaphysis and epiphysis, coincident with the anatomic distribution of donor-derived osteopoietic cells.
Nonadherent bone marrow cells engraft preferentially in metaphysis and epiphysis in the early regenerative phases after BMT. (A) Photomicrograph of a bone (Bo)/bone marrow (BM) section taken from the epiphysis of a mouse that underwent transplantation killed at 2 weeks after transplantation and stained with anti-GFP antibody (red). Original magnification, 100×. (B) Low level of GFP+ cells engrafted as clusters in the diaphysis, mainly in close proximity to the endosteal bone surface (asterisk). (C) Section of bone from a mouse that received a transplant of untransduced nonadherent marrow cells and stained with anti-GFP antibody (negative control). (D) Comparison of GFP+ engraftment in the diaphysis versus the metaphysis/epiphysis at 2 weeks after transplantation. A total of 20 random 400× fields of bone sections from each of 4 mice were studied. The reported values are mean (+SD) percentages of GFP+ cells per field. The difference in engraftment is highly significant (P < .001). (E) Photomicrograph of a bone (Bo)/bone marrow (BM) section taken from the metaphysis of a transplanted mouse killed at 2 days after transplantation and stained with anti-GFP antibody (red). The cluster of early osteopoietic engraftment (arrows) is adjacent to donor (GFP+) hematopoietic cells (asterisk). Original magnification, 400×.
Nonadherent bone marrow cells engraft preferentially in metaphysis and epiphysis in the early regenerative phases after BMT. (A) Photomicrograph of a bone (Bo)/bone marrow (BM) section taken from the epiphysis of a mouse that underwent transplantation killed at 2 weeks after transplantation and stained with anti-GFP antibody (red). Original magnification, 100×. (B) Low level of GFP+ cells engrafted as clusters in the diaphysis, mainly in close proximity to the endosteal bone surface (asterisk). (C) Section of bone from a mouse that received a transplant of untransduced nonadherent marrow cells and stained with anti-GFP antibody (negative control). (D) Comparison of GFP+ engraftment in the diaphysis versus the metaphysis/epiphysis at 2 weeks after transplantation. A total of 20 random 400× fields of bone sections from each of 4 mice were studied. The reported values are mean (+SD) percentages of GFP+ cells per field. The difference in engraftment is highly significant (P < .001). (E) Photomicrograph of a bone (Bo)/bone marrow (BM) section taken from the metaphysis of a transplanted mouse killed at 2 days after transplantation and stained with anti-GFP antibody (red). The cluster of early osteopoietic engraftment (arrows) is adjacent to donor (GFP+) hematopoietic cells (asterisk). Original magnification, 400×.
Secondary bone marrow transplantation
The above results led us to consider that osteopoietic engraftment might be a time-limited process in our model due to reconstitution from a short-term osteopoietic stem cell distinct from the long-term hematopoietic stem cell that gave rise to normal hematopoietic reconstitution of bone marrow. If so, the putative osteopoietic cell would be expected to have limited self-renewal capacity, rendering it incapable of maintaining a stem cell pool, in contrast to stem cells with of long-term repopulating activity. This possibility was assessed by studying osteopoietic engraftment animals that underwent serial transplantation.
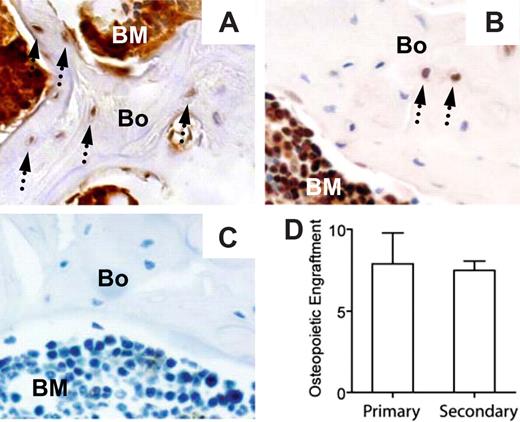
Lethally irradiated normal FVB/N mice (n = 3) received transplants with 2 × 106 nonadherent bone marrow cells harvested from a GFP-transgenic mouse11 (FVB/N genetic background). At 8 weeks after transplantation, the bone marrow was more than 98% donor derived (GFP+ cells) by flow cytometric analysis, and the bone cells (osteoblasts and osteocytes) were 7.9% plus or minus 4.2% donor-derived (GFP+) by immunohistochemical staining and microscopic examination (Figure 6). Bone marrow cells (98% GFP+), harvested from the primary recipients at 8 weeks after transplantation, were transplanted into lethally irradiated secondary recipients (5 × 106 cells/mouse; n = 10 mice). At 3 weeks later, the peripheral blood (80.1% ± 10.1%) and bone marrow (84.5% ± 8.2%) were predominately from donor cells. Most importantly, donor cell osteopoiesis accounted for 7.5% plus or minus 1.3% of the bone cells in the epiphysis and metaphysis. These data suggest that the primitive transplantable marrow cells responsible for the restoration of osteopoiesis also undergo self-renewal, a primary characteristic of stem cells with multilineage potential.
The osteopoietic capacity of nonadherent bone marrow cells is preserved in secondary recipients. Immunohistochemical identification of donor-derived bone cells (arrows) in primary (A) and secondary (B) recipients. (C) Section of bone from a mouse that received a transplant of untransduced nonadherent marrow cells and stained with anti-GFP antibody (negative control). (D) Comparison of the percentages of donor-derived osteopoiesis in primary (n = 3 mice) versus secondary (n = 10 mice) recipients. The reported values are mean (± SD) percentages of GFP+ cells. Bone engraftment was quantified as described in Figure 3; P > .05.
The osteopoietic capacity of nonadherent bone marrow cells is preserved in secondary recipients. Immunohistochemical identification of donor-derived bone cells (arrows) in primary (A) and secondary (B) recipients. (C) Section of bone from a mouse that received a transplant of untransduced nonadherent marrow cells and stained with anti-GFP antibody (negative control). (D) Comparison of the percentages of donor-derived osteopoiesis in primary (n = 3 mice) versus secondary (n = 10 mice) recipients. The reported values are mean (± SD) percentages of GFP+ cells. Bone engraftment was quantified as described in Figure 3; P > .05.
Discussion
The identification of the hematopoietic stem-cell niche13,14 has directed much effort toward understanding the mechanism(s) by which stem cells engraft and differentiate in the marrow microenvironment after transplantation. This question is especially intriguing in the case of bone disorders, as little is known about the nature of the transplantable marrow osteoprogenitor cells and curiously, all of the available data indicate early engraftment of osteopoietic cells but a lack of long-term repopulating capacity.1,5,15 In this report, we provide key elements needed to understand these critical issues and to begin to devise alternative means to ensure durable engraftment of osteopoietic cells.
In our murine model, clusters of GFP+ osteoblasts and osteocytes were found in the epiphysis and metaphysis of the long bones with negligible concentrations of donor cells in the diaphysis. This pattern of osteopoietic engraftment paralleled hematopoietic seeding early after BMT, consistent with previous reports16 suggesting that the endosteal stem-cell niche17-19 resides in the metaphysis and epiphysis as opposed to the diaphysis. Moreover, bone growth in the diaphysis of mice is predominantly due to periosteal matrix appostion, in contrast to endosteal bone formation of the metaphysis.20 Thus, endosteum of the metaphysis and epiphysis appears to harbor the niche for both hematopoietic stem cells and endogenous primitive osteoprogenitors, and perhaps a bipotential hematopoietic-osteopoietic stem cell.5 This constellation of engraftment and the anatomic distribution of endogenous progenitors support a close functional and developmental relationship between blood and bone. Interestingly, Onyia et al compared the transplantation efficiency of adherent MSCs directly injected into the metaphysis and the diaphysis, and found a complete lack of engraftment in the diaphysis, in contrast to the high level seen in the metaphysis,21 suggesting that ex vivo expanded MSCs may also engraft in this osteopoietic stem-cell niche. The low level of MSC engraftment in the marrow seen by these authors and others8,22,23 after systemic infusion may be due to the lack of appropriate marrow homing signals. In our studies, the transplantable osteopoietic cells do seem to home to the marrow space and engraft in specific sites. Moreover, we have previously shown that engraftment of the transplantable marrow osteoprogenitor is saturable24 ; thus, direct intrafemoral injection of our nonadherent marrow cells would likely yield results similar to what we have observed after intravenous infusion.
The robust level of donor-derived osteopoiesis observed early after transplantation in our murine model, followed by decreasing donor osteopoietic chimerism over time, corresponds closely to the abbreviated course and temporary measurable osteopoietic engraftment in children with severe OI. This relationship supports our hypothesis that the clinical improvement secured early in patients with OI arose from the engraftment and differentiation of donor-derived marrow cells and validates further use of the mouse to test clinically relevant predictions regarding the fate of transplantable nonadherent cells in the bone and marrow microenvironments. Moreover, early robust osteopoietic engraftment with early functional improvement of bone followed by decreasing donor osteopoietic chimerism and waning of bone function seems to be a general characteristic of osteopoietic engraftment by transplanted marrow cells, and not limited to specific diseases. This profile was found in normal mice previously,8 in normal mice in this report, in children with severe OI, and in a child who underwent bone marrow transplantation as therapy for infantile hypophosphatasia.25 Finally, transplantation of bone marrow cells in a murine model of a severe genetically linked osteoporosis was followed by rapid amelioration of the pathologic phenotype with a slow reversion to the pretreatment status over time.26,27
How, then, does one explain high levels of osteopoietic engraftment early after BMT with diminishing osteopoietic chimerism over time? Bone is a dynamic tissue undergoing continuous remodeling with high cell and matrix turnover. The life span of a murine osteoblast is only 10 to 20 days,28,29 and both osteoblasts and osteocytes show high rates of apoptosis,19,30 which are linked to repopulation by primitive osteoprogenitors and preosteoblasts during physiologic bone turnover.31 Thus, the progressive decrease of donor-derived osteopoiesis likely reflects the normal physiologic turnover of osteoblasts and osteocytes, but this mechanism does not explain the lack of newly differentiated osteoblasts from the engrafted donor primitive cells.
One possibility is that the donor-derived osteoblasts do not differentiate according to known physiologic pathways. Labeling transplantable marrow cells with a GFP-encoding retroviral vector afforded us a unique opportunity to track the fate of undifferentiated cells in the marrow microenvironment along the osteopoietic differentiation pathway after transplantation, because GFP expression in our system is not affected by cell differentiation.12 Evidence that donor-derived osteopoietic cells were developing in the correct pathways was provided by the 1:5 ratio of donor-derived osteocytes to osteoblasts seen at 2 weeks after transplantation, which is consistent with the finding that approximately 1 of 5 osteoblasts gives rise to an osteocyte.31 The osteoblasts that do not give rise to osteocytes secrete bone matrix proteins,31 contribute to the hematopoietic stem cell niche,13 or assume a more flattened morphology, becoming quiescent progenitors within a functional reservoir of osteogenic cells residing within the bone lining compartment.32 Importantly, histologic sections taken at serial time points suggest that the donor-derived cells transitioned from the endosteal compartment to bone consistent with the in vivo conversion of osteoblasts to osteocytes. However, the depth of the osteocytes embedded within trabeculae could not be accurately assessed in our 2D images of the 3D bony structures. Nonetheless, by all other criteria, the engrafted donor cells derived from either retrovirally transduced or transgenic donor cells appear to have differentiated along established pathways. This conclusion is supported by the exclusive presence of donor-derived osteocytes at locations also occupied by donor-derived osteoblasts.
It is certainly reasonable to consider that the transplantable marrow osteoprogenitor identified in this study lacks long-term self-renewing capacity and thus was unable to sustain robust engraftment beyond 6 months after transplantation. We reject this possibility on the basis of our secondary transplantation data, which show statistically identical levels of osteopoietic engraftment in primary and secondary recipients. Although the analysis of the secondary recipients at 3 weeks after transplantation was too early to allow a valid assessment of hematopoietic stem cell self-renewal, the data in Figure 3 indicate that this interval provides an ideal time point for evaluating osteopoietic engraftment. Thus, the results of our secondary transplantation experiments lend compelling support to the hypothesis that a long-term self-renewing osteoprogenitor resides in the transplantable population of nonadherent marrow cells. Its identity is most compatible with the bipotent hematopoietic-osteopoietic stem cell that we have previously proposed.5 Hence, after the initial burst of repopulating activity by donor-derived stem cells leading to full hematopoietic regeneration and partial osteopoietic chimerism, intrinsic genetic programs or signals from the microenvironment could modulate the differentiation of transplanted stem cells to osteoblasts so that only a low level of donor-derived osteopoiesis is maintained. If this hypothesis is correct, the future challenge will be to identify the mole-cular and cellular regulatory signals responsible for post-transplantation osteopoiesis and devise methods that could be used to manipulate multipotent stem cells toward the osteopoietic lineage.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge Drs Richard Ashmum and Anne-Marie Hamilton-Easton for the flow cytometric analyses and Mr John Gilbert for editorial review of this manuscript.
This work was supported in part by grant R01 HL077643 from National Heart, Lung, and Blood Institute (NHLBI), Cancer Center Support (CORE) Grant P30 CA21765, the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES), the American Lebanese Syrian Associated Charities (ALSAC), the Ministero Istruzione Università e Ricerca (PRIN 2006), the Regione Emilia Romagna, and the Associazione per il Sostegno dell'Ematologia e dell'Oncologia Pediatrica (ASEOP).
National Institutes of Health
Authorship
Contribution: M.D. designed, performed, and analyzed research, and assisted with preparation of the manuscript; R.M. performed research; V.R., C.S., and P.P. performed research; P.C. analyzed research; T.J.H. designed and analyzed research; and E.M.H. oversaw entire project, designed and analyzed research, and prepared manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin M. Horwitz, The Children's Hospital of Philadelphia, Abramson Research Center, 1116D, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: horwitze@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal