Abstract
The trial ALL-BFM 95 for treatment of childhood acute lymphoblastic leukemia was designed to reduce acute and long-term toxicity in selected patient groups with favorable prognosis and to improve outcome in poor-risk groups by treatment intensification. These aims were pursued through a stratification strategy using white blood cell count, age, immunophenotype, treatment response, and unfavorable genetic aberrations providing an excellent discrimination of risk groups. Estimated 6-year event-free survival (6y-pEFS) for all 2169 patients was 79.6% (± 0.9%). The large standard-risk (SR) group (35% of patients) achieved an excellent 6y-EFS of 89.5% (± 1.1%) despite significant reduction of anthracyclines. In the medium-risk (MR) group (53% of patients), 6y-pEFS was 79.7% (± 1.2%); no improvement was accomplished by the randomized use of additional intermediate-dose cytarabine after consolidation. Omission of preventive cranial irradiation in non–T-ALL MR patients was possible without significant reduction of EFS, although the incidence of central nervous system relapses increased. In the high-risk (HR) group (12% of patients), intensification of consolidation/reinduction treatment led to considerable improvement over the previous ALL-BFM trials yielding a 6y-pEFS of 49.2% (± 3.2%). Compared without previous trial ALL-BFM 90, consistently favorable results in non-HR patients were achieved with significant treatment reduction in the majority of these patients.
Introduction
Impressive improvements of survival rates in pediatric acute lymphoblastic leukemia (ALL) have been achieved during the last decades.1-21 Today, a long-term cure can be attained for approximately 75% of patients. The identification of biologic and clinical prognostic factors allowed the definition of patient subgroups with distinct relapse risks and the realization of risk-adapted treatment strategies.
In ALL-Berlin-Frankfurt-Münster (BFM) trials, the so-called prednisone response (PR, for definition see “Response and relapse criteria”) evolved as one of the strongest prognostic factors.22,23 Patients with “prednisone good-response” (PGR) comprised 90% of all patients with a cure rate of more than 80%, whereas patients with inadequate PR (“prednisone poor-response,” PPR) had an unfavorable outcome with a probability of event-free survival (pEFS) of less than 50%. Thus, a small but relevant target group for treatment modification was clearly identified.
Because of the excellent outcome of a large patient subset, treatment toxicity had gained in importance. In consequence, a major focus of study ALL-BFM 90, the study immediately before ALL-BFM 95, was the reduction of treatment in favorable patient groups to reduce acute and long-term toxicity.15 Despite the 25% anthracycline dose reduction in induction, a significantly lower relapse rate could be achieved in the medium-risk group, most likely because of a more condensed application of induction therapy. Enabled by the introduction of high-dose methotrexate (HD-MTX), presymptomatic cranial radiotherapy (pCRT) was eliminated in low-risk ALL and stepwise reduced to 12 Gy in medium- and high-risk ALL.15,23
After introduction of the PR as stratification criterion, the formerly used BFM risk factor (BFM-RF), an estimator of the initial leukemic cell mass, turned out to be an insufficient parameter to separate different risk groups within PGR patients.15 Therefore, a new stratification strategy was introduced in trial ALL-BFM 95, which used age, white blood cell count (WBC) at diagnosis, and immunophenotype in addition to PR, response to induction treatment, and the unfavorable translocations t(9;22) and t(4;11). This enabled a better separation of subgroups with distinct relapse risks: in trial ALL-BFM 90, stratification by the new criteria would have resulted in a large “standard-risk” (SR) group of approximately 35% of all patients with a pEFS of approximately 90%, a “medium-risk” (MR) group comprising approximately 50% of the patients with a pEFS of 80%, and a “high-risk” (HR) group with a pEFS of less than 50%.
Subsequent to trial ALL-BFM 90, the new trial ALL-BFM 95 aimed at further reduction of treatment burden while improving outcome in selected subgroups. Objectives of trial ALL-BFM 95 were (1) reduction of the daunorubicin dose in induction treatment by 50% in the SR group; (2) the extension of the maintenance therapy by 12 months in SR boys to prevent the late relapses observed in this patient group; (3) randomized intensification of the extracompartment/consolidation phase with intermediate-dose (ID) cytarabine in addition to high-dose methotrexate in the MR group; (4) omission of pCRT in all MR patients with non-T-ALL; and (5) modification of consolidation/reinduction in HR patients by intensification in the block elements and reintroduction of protocol II. In addition, intensification of maintenance therapy by pulses of dexamethasone and vincristine was evaluated in the MR group in a randomized intergroup trial of the International BFM Study Group, demonstrating no benefit from the intensification.24 The use of allogeneic stem cell transplantation (alloSCT) was not a primary study objective but has been analyzed in detail and published earlier indicating a benefit from alloSCT primarily for patients with T-ALL.25,26
Methods
Patients
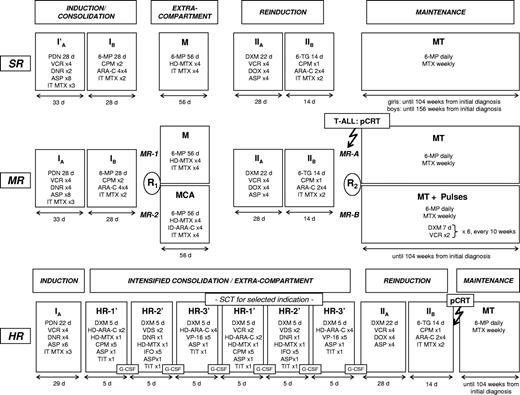
From April 1, 1995, until June 30, 2000, a total of 2283 patients younger than 18 years with ALL were enrolled into the trial ALL-BFM 95. Patients were treated in 82 participating study centers in Austria, Switzerland, and Germany. Randomization of protocol M versus MCA in risk group MR (Figure 1) started on October 1, 1995. Informed consent was obtained from the guardians of each patient in accordance with the Declaration of Helsinki. The trial was approved by the ethical committee of the principal investigator's institution.
Treatment outline ALL-BFM 95. Details of treatment elements are listed in Table 1. Results of the randomization R2 and data on stem cell transplantation have been published before.24-26 Dose of presymptomatic cranial radiotherapy was 12 Gy for patients aged 1 year or older. Therapeutic irradiation dose for patients with initial involvement of the central nervous system was 12 Gy for patients 1 to 2 years and 18 Gy for patients more than 2 years of age. Infants younger than 1 year were neither preventively nor therapeutically irradiated. SR indicates standard risk; MR, medium risk; HR, high risk; PDN, prednisone; VCR, vincristine; DNR, daunorubicin; ASP, E coli L-asparaginase; MTX, methotrexate; 6-MP, 6-mercaptopurine; ARA-C, cytarabine; CPM, cyclophosphamide; DXM, dexamethasone; DOX, doxorubicin; 6-TG, 6-thioguanine; HD, high dose; ID, intermediate dose; IT, intrathecal; TIT, triple intrathecal therapy; G-CSF, granulocyte colony-stimulating factor; MT, maintenance therapy; SCT, stem cell transplantation; pCRT, presymptomatic cranial radiotherapy.
Treatment outline ALL-BFM 95. Details of treatment elements are listed in Table 1. Results of the randomization R2 and data on stem cell transplantation have been published before.24-26 Dose of presymptomatic cranial radiotherapy was 12 Gy for patients aged 1 year or older. Therapeutic irradiation dose for patients with initial involvement of the central nervous system was 12 Gy for patients 1 to 2 years and 18 Gy for patients more than 2 years of age. Infants younger than 1 year were neither preventively nor therapeutically irradiated. SR indicates standard risk; MR, medium risk; HR, high risk; PDN, prednisone; VCR, vincristine; DNR, daunorubicin; ASP, E coli L-asparaginase; MTX, methotrexate; 6-MP, 6-mercaptopurine; ARA-C, cytarabine; CPM, cyclophosphamide; DXM, dexamethasone; DOX, doxorubicin; 6-TG, 6-thioguanine; HD, high dose; ID, intermediate dose; IT, intrathecal; TIT, triple intrathecal therapy; G-CSF, granulocyte colony-stimulating factor; MT, maintenance therapy; SCT, stem cell transplantation; pCRT, presymptomatic cranial radiotherapy.
The median follow-up period for the analyzed patients was 7.2 years. Thirty-seven patients were considered lost to follow-up after a median follow-up time of 3.0 years.
Diagnosis
The diagnosis of ALL was established if at least 25% lymphoblasts were present in the bone marrow (BM). BM and peripheral blood (PB) smears as well as cerebrospinal fluid (CSF) cytospin preparations were reviewed in the study center.
Central nervous system (CNS) involvement was diagnosed if more than 5 cells/μL were counted in CSF and lymphoblasts were identified or if intracerebral infiltrates were detected on cranial computed tomography (CNS3). If blasts were identified in CSF cytospin preparations although CSF cell count was less than or equal to 5 cells/μL, CNS status was classified as CNS2; in the case of traumatic lumbar puncture with identification of blasts, CNS status was categorized as TLP+, and as TLP− if no blasts were identified.27
Immunophenotyping, DNA index, and cytogenetic and molecular genetic analysis
Immunophenotyping, determination of cellular DNA content using flow cytometry, and definition of DNA index was performed as previously described.28,29 Cytogenetic studies were carried out using standard techniques.30 RT-PCR-based screening for BCR/ABL and MLL/AF431,32 was performed since the start of study; screening for TEL/AML133 was initiated in May 1996.
Response and relapse criteria
PR was determined after 7 days of monotherapy with prednisone and one intrathecal dose of methotrexate on day 1 and was centrally reviewed in the study center. The presence of 1 × 109 blasts/L or more in PB on day 8 was defined as PPR, fewer than 1 × 109 blasts/L as PGR.22 BM response was evaluated in aspiration smears on day 33 of induction treatment. Complete remission (CR) was defined as less than 5% blasts in the regenerating BM, the absence of leukemic blasts in blood and CSF, and no evidence of localized disease. Failure to achieve CR after induction was not considered an event. Resistance to therapy (nonresponse) was defined as not having achieved CR by the start of the fourth pulsatile high-dose block. Relapse was defined as recurrence of 25% or more lymphoblasts in BM or localized leukemic infiltrates at any site.
Stratification
Patients were stratified into 3 risk groups according to the following criteria:
HR: PPR, and/or no CR on day 33, and/or evidence of t(9;22) (or BCR/ABL), and/or evidence of t(4;11) (or MLL/AF4).
MR: No HR criteria, and initial WBC 20 × 109/L or more and/or age at diagnosis less than 1 or 6 years or older, and/or T-ALL.
SR: No HR criteria, and initial WBC less than 20 × 109/L, and age at diagnosis between 1 and 6 years, and no T-ALL.
CNS status was no stratification criterion.
Treatment
The treatment strategy is shown in Figure 1, and the details of treatment elements are provided in Table 1. In SR patients, the number of daunorubicin applications in induction was halved to 2 doses of 30 mg/m2 (protocol I′). In protocol M, HD-MTX at 5 g/m2 per 24 hours with late leucovorin rescue was administered as described before.15
Treatment protocol
| Treatment element/drug . | Single or daily dose . | Days of application per elementa . |
|---|---|---|
| Induction/consolidation, protocol I | ||
| Phase A | ||
| Prednisone (PO) | 60 mg/m2 per day | 1-28fg |
| Vincristine (IV) | 1.5 mg/m2 per dose (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (PI over 1 hour) | 30 mg/m2 per dose | 8, 15, 22,h 29 h |
| L-asparaginase (PI over 1 hour) | 5000 IU/m2 per dose | 12, 15, 18, 21, 24, 27, 30,f 33f |
| Methotrexate (IT) | 12 mg/dosei | 1, 12, 33j |
| Phase B (only SR/MR) | ||
| Cyclophosphamide (PI over 1 hour) | 1000 mg/m2 per dose | 36, 64 |
| Cytarabine (IV) | 75 mg/m2 per dose | 38-41, 45-48, 52-55, 59-62 |
| 6-mercaptopurine (PO) | 60 mg/m2 per day | 36-63 |
| Methotrexate (IT) | 12 mg/dosei | 45, 59 |
| Extracompartment therapy (only SR/MR) | ||
| Protocol M | ||
| 6-mercaptopurine (PO) | 25 mg/m2 per day | 1-56 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 8, 22, 36, 50 |
| Methotrexate (IT) | 12 mg/dosei | 8, 22, 36, 50 |
| Protocol MCA (only MR patients randomized into MR-2) | ||
| 6-mercaptopurine (PO) | 25 mg/m2 per day | 1-56 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 8, 22, 36, 50 |
| Methotrexate (IT) | 12 mg/dosei | 8, 22, 36, 50 |
| Cytarabine (PI over 24 hours) | 200 mg/m2 per dose | 9, 23, 37, 51 |
| Intensive consolidation (only HR) (HR-1′/HR-2′/HR-3′) × 2 | ||
| Element HR-1′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,k 6k |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 1 |
| Cyclophosphamide (PI over 1 hour) | 200 mg/m2 per dose | 2-4 (5 doses, 12-hour intervals) |
| Cytarabine (PI over 3 hours) | 2 g/m2 per dose | 5 (2 doses, 12 h interval) |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 1 |
| Element HR-2′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Vindesine (IV) | 3 mg/m2 per dose (max 5 mg) | 1, 6 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 1 |
| Ifosfamide (PI over 1 hour) | 800 mg/m2 per dose | 2-4 (5 doses, 12-hour intervals) |
| Daunorubicin (PI over 24 hours) | 30 mg/m2 per dose | 5 |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 1l |
| Element HR-3′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Cytarabine (PI over 3 hours) | 2 g/m2 per dose | 1-2 (4 doses, 12-hour intervals) |
| Etoposide (PI over 1 hour) | 100 mg/m2 per dose | 3-5 (5 doses, 12-hour intervals) |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 5 |
| Reinduction, protocol II | ||
| Phase A | ||
| Dexamethasone (PO) | 60 mg/m2 per day | 1-21g |
| Vincristine (IV) | 1.5 mg/m2 per dose (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (PI over 1 hour) | 30 mg/m2 per dose | 8, 15, 22, 29 |
| L-asparaginase (PI over 1 hour) | 10 000 IU/m2 per dose | 8, 11, 15, 18 |
| Phase B | ||
| Cyclophosphamide (PI over 1 hour) | 1000 mg/m2 per dose | 36 |
| Cytarabine (IV) | 75 mg/m2 per dose | 38-41, 45-48 |
| 6-thioguanine (PO) | 60 mg/m2 per day | 36-49 |
| Methotrexate (IT) | 12 mg/dosei | 45, 59m |
| Maintenance therapyd | ||
| Methotrexate (PO) | 20 mg/m2 per dosen | Once a week |
| 6-mercaptopurine (PO) | 50 mg/m2 per dayn | Daily |
| Only MR patients randomized into MR-B | ||
| Methotrexate (PO) | 20 mg/m2 per dosen | Once a week |
| 6-mercaptopurine (PO) | 50 mg/m2 per dayn | Daily |
| Dexamethasone (PO)e | 6 mg/m2 per day | 1-7 per pulse |
| Vincristine (IV)e | 1.5 mg/m2 per dose (max 2 mg) | 1, 7 per pulse |
| Treatment element/drug . | Single or daily dose . | Days of application per elementa . |
|---|---|---|
| Induction/consolidation, protocol I | ||
| Phase A | ||
| Prednisone (PO) | 60 mg/m2 per day | 1-28fg |
| Vincristine (IV) | 1.5 mg/m2 per dose (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (PI over 1 hour) | 30 mg/m2 per dose | 8, 15, 22,h 29 h |
| L-asparaginase (PI over 1 hour) | 5000 IU/m2 per dose | 12, 15, 18, 21, 24, 27, 30,f 33f |
| Methotrexate (IT) | 12 mg/dosei | 1, 12, 33j |
| Phase B (only SR/MR) | ||
| Cyclophosphamide (PI over 1 hour) | 1000 mg/m2 per dose | 36, 64 |
| Cytarabine (IV) | 75 mg/m2 per dose | 38-41, 45-48, 52-55, 59-62 |
| 6-mercaptopurine (PO) | 60 mg/m2 per day | 36-63 |
| Methotrexate (IT) | 12 mg/dosei | 45, 59 |
| Extracompartment therapy (only SR/MR) | ||
| Protocol M | ||
| 6-mercaptopurine (PO) | 25 mg/m2 per day | 1-56 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 8, 22, 36, 50 |
| Methotrexate (IT) | 12 mg/dosei | 8, 22, 36, 50 |
| Protocol MCA (only MR patients randomized into MR-2) | ||
| 6-mercaptopurine (PO) | 25 mg/m2 per day | 1-56 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 8, 22, 36, 50 |
| Methotrexate (IT) | 12 mg/dosei | 8, 22, 36, 50 |
| Cytarabine (PI over 24 hours) | 200 mg/m2 per dose | 9, 23, 37, 51 |
| Intensive consolidation (only HR) (HR-1′/HR-2′/HR-3′) × 2 | ||
| Element HR-1′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 1,k 6k |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 1 |
| Cyclophosphamide (PI over 1 hour) | 200 mg/m2 per dose | 2-4 (5 doses, 12-hour intervals) |
| Cytarabine (PI over 3 hours) | 2 g/m2 per dose | 5 (2 doses, 12 h interval) |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 1 |
| Element HR-2′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Vindesine (IV) | 3 mg/m2 per dose (max 5 mg) | 1, 6 |
| Methotrexate (PI over 24 hours)b | 5000 mg/m2 per dose | 1 |
| Ifosfamide (PI over 1 hour) | 800 mg/m2 per dose | 2-4 (5 doses, 12-hour intervals) |
| Daunorubicin (PI over 24 hours) | 30 mg/m2 per dose | 5 |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 1l |
| Element HR-3′c | ||
| Dexamethasone (PO) | 20 mg/m2 per day | 1-5 |
| Cytarabine (PI over 3 hours) | 2 g/m2 per dose | 1-2 (4 doses, 12-hour intervals) |
| Etoposide (PI over 1 hour) | 100 mg/m2 per dose | 3-5 (5 doses, 12-hour intervals) |
| L-asparaginase (PI over 6 hours) | 25 000 IU/m2 per dose | 6 |
| Methotrexate/cytarabine/prednisolone (IT) | 12/30/10 mg/dosei | 5 |
| Reinduction, protocol II | ||
| Phase A | ||
| Dexamethasone (PO) | 60 mg/m2 per day | 1-21g |
| Vincristine (IV) | 1.5 mg/m2 per dose (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (PI over 1 hour) | 30 mg/m2 per dose | 8, 15, 22, 29 |
| L-asparaginase (PI over 1 hour) | 10 000 IU/m2 per dose | 8, 11, 15, 18 |
| Phase B | ||
| Cyclophosphamide (PI over 1 hour) | 1000 mg/m2 per dose | 36 |
| Cytarabine (IV) | 75 mg/m2 per dose | 38-41, 45-48 |
| 6-thioguanine (PO) | 60 mg/m2 per day | 36-49 |
| Methotrexate (IT) | 12 mg/dosei | 45, 59m |
| Maintenance therapyd | ||
| Methotrexate (PO) | 20 mg/m2 per dosen | Once a week |
| 6-mercaptopurine (PO) | 50 mg/m2 per dayn | Daily |
| Only MR patients randomized into MR-B | ||
| Methotrexate (PO) | 20 mg/m2 per dosen | Once a week |
| 6-mercaptopurine (PO) | 50 mg/m2 per dayn | Daily |
| Dexamethasone (PO)e | 6 mg/m2 per day | 1-7 per pulse |
| Vincristine (IV)e | 1.5 mg/m2 per dose (max 2 mg) | 1, 7 per pulse |
PO indicates orally; IV, intravenous push; PI, intravenous infusion; IT, intrathecally.
Adjustments of time schedule were allowed if clinical condition and bone marrow recovery were inadequate (according to protocol guidelines).
A loading dose of 10% was infused over 30 minutes, the remaining 90% over 23.5 hours. Leucovorin rescue was given at hours 42, 48, and 54 (each 15 mg/m2). Increased leucovorin doses was given, when MTX levels at hour 42 or later were >1.0 μmol/L. If the MTX level at hour 54 was >0.25 μmol/L, rescue was continued at 6-hour intervals until MTX levels were ≤0.25 μmol/L.
Each HR′ block was given twice (Figure 1).
Maintenance was given from end of intensive chemotherapy until 104 weeks after diagnosis, for boys in SR until 156 weeks.
Six pulses were given every 10 weeks.
In HR, protocol I/phase A was given with only 21 days of prednisone and 6 doses of L-asparaginase.
Steroids were tapered over 9 days.
In SR, the daunorubicin doses on days 22 and 29 were omitted.
Doses were adjusted for children younger than 3 years.
The day 33 IT methotrexate dose was scheduled on day 27 in HR patients. Patients with CNS status CNS2, TLP+, or CNS3 received additional IT methotrexate on days 18 and 27 (SR or MR) or on day 18 (HR).
Vincristine was omitted in the first HR-1′ course.
Patients with CNS status CNS 3 received additional IT methotrexate on day 5.
Patients with CNS status CNS 3 received additional IT methotrexate on days 1 and 18.
Doses were adjusted to WBC (target range, 2000-3000/μL).
All HR patients received prophylactic granulocyte colony-stimulating factor after each high-dose block.34 alloSCT was recommended for a subset of HR patients if a matched sibling donor was available. Eligibility criteria for alloSCT have been published before.25,26
Escherichia coli L-asparaginase from Medac (Wedel, Germany) was used as first-line asparaginase preparation. In protocol I, dose was reduced at 5000 IU/m2 because of the higher activity and toxicity compared with the formerly applied preparation (Crasnitin).35,36 In case of allergic reactions, Erwinia L-asparaginase (ERWINASE; Speywood, London, United Kingdom) or PEG-asparaginase (ONCASPAR, Medac) were recommended as a substitute.
T-ALL and HR patients 1 year of age or older received pCRT with 12 Gy, scheduled after the end of reinduction (Figure 1). CNS3 patients 2 years of age or older received 18 Gy therapeutic CRT (tCRT) (12 Gy if age was 2 years) and 2 additional intrathecal MTX doses in protocols I and II each and if qualified for HR, one additional intrathecal triple drug application in each HR-2′ course. CNS2 or TLP+ patients received 2 additional intrathecal MTX doses in protocol I.
Maintenance therapy with daily 6-mercaptopurine and weekly methotrexate was adjusted according to WBC (target range, 2 to 3 × 109/L).
Two randomizations were performed in the MR branch. The standard protocol M (6-mercaptopurine and 4 cycles HD-MTX) was randomized against the experimental arm protocol MCA, in which ID-cytarabine (200 mg/m2 per 24 hours) was infused subsequently to each HD-MTX infusion. The second randomization provided 6 pulses with dexamethasone and vincristine applied every 10 weeks in addition to the standard maintenance treatment and was performed within the framework of an international collaboration.24
Historical control group
To evaluate the impact of treatment modifications that were not tested through randomization, patients were retrospectively compared with the matching subsets of patients from the previous trial ALL-BFM 90.15 Risk stratification and treatment in ALL-BFM 90 have been described before15 ; differences to ALL-BFM 95 are summarized in Table 2. For historical comparison of the matching subgroups among the non-HR patients, patients of ALL-BFM 90 were reclassified by the ALL-BFM 95 risk criteria (“SR-95,” “MR-95”) and vice versa ALL-BFM 95 patients by the ALL-BFM 90 risk criteria (“SR-90,” “MR-90”). The reclassification led to an extensive redistribution of patients. Of the reclassified “SR-95” patients from ALL-BFM 90, who constituted the historical control group for the analysis of the impact of the daunorubicin reduction in the SR group in ALL-BFM 95, 52% were treated in risk group “MR-90.” For evaluating the omission of pCRT, ALL-BFM 90 patients of risk group “MR-90” 1 year of age or older with pB-ALL and without initial CNS involvement served as historical control group. In the corresponding group from ALL-BFM 95 which did not receive pCRT, 36% were treated in risk group “SR-95.” Treatment in SR and MR in trial ALL-BFM 90 was basically comparable. It differed in the indication for cranial irradiation and in the additional asparaginase in protocol M given in the context of the randomized question in the MR group in ALL-BFM 90 (Table 2). No significant impact on outcome has been shown with respect to the additional asparaginase.15
Comparison of study ALL-BFM 90 and 95: differences in risk groups definitions and treatment
| . | ALL-BFM 90 . | ALL-BFM 95 . |
|---|---|---|
| All risk groups | ||
| Asparaginase preparation | Crasnitin | L-asparaginase Medac |
| Asparaginase dosage in protocol I | 10 000 IU/m2 per dose | 5000 IU/m2 per dose |
| SR* | ||
| SR criteria | No HR-90 criteria and BFM-RF <0.8 and no T-ALL and CNS-negative | No HR-95 criteria and age 1 to <6 years and WBC <20 × 109/L and no T-ALL |
| Chemotherapy protocol IA | 4 doses daunorubicin | 2 doses daunorubicin |
| Duration of maintenance for boys | 24 months from diagnosis | 36 months from diagnosis |
| MR* | ||
| MR criteria | No HR-90 criteria and BFM-RF >0.8 and/or T-ALL and/or CNS-positive | No HR-95 criteria and age <1 or >6 years and/or WBC >20 × 109/L and/or T-ALL |
| Presymptomatic cranial irradiation | 12 Gy | 0 Gy (T-ALL: 12 Gy) |
| Randomization protocol M | ± asparaginase | ± cytarabine |
| Randomization maintenance | − | Vincristine/dexamethasone pulses |
| HR* | ||
| HR criteria | PPR and/or no CR d33 and/or t(9;22) (or BCR/ABL) | PPR and/or no CR d33 and/or t(9;22) (or BCR/ABL) or t(4;11) (or MLL/AF4) |
| Consolidation/reinduction | 9 HR courses | 6 HR′ courses + protocol II |
| . | ALL-BFM 90 . | ALL-BFM 95 . |
|---|---|---|
| All risk groups | ||
| Asparaginase preparation | Crasnitin | L-asparaginase Medac |
| Asparaginase dosage in protocol I | 10 000 IU/m2 per dose | 5000 IU/m2 per dose |
| SR* | ||
| SR criteria | No HR-90 criteria and BFM-RF <0.8 and no T-ALL and CNS-negative | No HR-95 criteria and age 1 to <6 years and WBC <20 × 109/L and no T-ALL |
| Chemotherapy protocol IA | 4 doses daunorubicin | 2 doses daunorubicin |
| Duration of maintenance for boys | 24 months from diagnosis | 36 months from diagnosis |
| MR* | ||
| MR criteria | No HR-90 criteria and BFM-RF >0.8 and/or T-ALL and/or CNS-positive | No HR-95 criteria and age <1 or >6 years and/or WBC >20 × 109/L and/or T-ALL |
| Presymptomatic cranial irradiation | 12 Gy | 0 Gy (T-ALL: 12 Gy) |
| Randomization protocol M | ± asparaginase | ± cytarabine |
| Randomization maintenance | − | Vincristine/dexamethasone pulses |
| HR* | ||
| HR criteria | PPR and/or no CR d33 and/or t(9;22) (or BCR/ABL) | PPR and/or no CR d33 and/or t(9;22) (or BCR/ABL) or t(4;11) (or MLL/AF4) |
| Consolidation/reinduction | 9 HR courses | 6 HR′ courses + protocol II |
Risk groups as defined in ALL-BFM 90 and 95, respectively.
Statistical analysis
EFS was defined as the time from diagnosis to the date of last follow-up in complete remission or first event. Events were resistance to therapy (nonresponse), relapse, secondary neoplasm (SN) or death from any cause. Failure to achieve remission due to early death or nonresponse was considered as events at time 0. Survival was defined as the time of diagnosis to death from any cause or last follow-up. For analysis of randomized patient subsets, disease-free survival (DFS) was calculated from time of randomization to the first event or the last follow-up date. Patients lost to follow-up were censored at the last contact. The Kaplan-Meier method was used to estimate survival rates37 ; differences were compared with the 2-sided log-rank test.38 Cox proportional hazards model was used for univariate and multivariate analyses.39 Cumulative incidence (CI) functions for competing events were constructed by the method of Kalbfleisch and Prentice40 and were compared with the Gray test.41 Comparison of randomized groups was performed as intent-to-treat and per-protocol analysis. Differences in the distribution of individual parameters among patient subsets were analyzed using the χ2 test for categorized variables and the Mann-Whitney U test for continuous variables.
At the end of protocol I, MR patients were randomly assigned to either receive additional cytarabine or not in protocol M. The sample size was derived based on the primary endpoint of DFS. According to the results of the preceded studies, the probability of long-term DFS of MR patients treated with protocol M was estimated to be 73%. To detect an increase of 9% (8%, 7%), a total of 680 (872, 1154) patients were required to be randomized (alpha = 0.05, beta = 0.2).42
Statistical analyses were conducted using the SAS program (SAS-PC, version 9.1; SAS Institute, Cary, NC).
All patient data were updated in June 2006.
Results
Patients' characteristics
Of the 2283 patients enrolled in ALL-BFM 95, 39 patients were excluded because of participation in pilot trials for feasibility of protocol MCA (n = 20) and for the infant ALL trial INTERFANT 9943 (n = 19). Seventy-five patients were not eligible according to the protocol criteria for the following reasons: significant pretreatment (n = 44), ALL was a SN (n = 9), major medical ailment preventing protocol therapy (patients with Down syndrome were only excluded if premorbidity prevented protocol therapy; n = 4), lack of essential data for establishing the diagnosis (n = 5), incorrect diagnosis (n = 3), treatment in a different protocol (n = 2), death before start of protocol treatment or treatment in a nonparticipating center (n = 2), and age at diagnosis older than 18 years (n = 6). Eventually, 2169 patients were evaluable for this study.
Patient characteristics and early treatment response of the total evaluable population and according to risk groups are summarized in Table 3. Basic characteristics (sex, age, WBC, immunophenotype) were comparable in trials ALL-BFM 90 and 95 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Patients' characteristics
| Variable . | All patients (total n = 2169) . | SR, % (n = 758) . | MR, % (n = 1157) . | HR, % (n = 254) . | ||
|---|---|---|---|---|---|---|
| N* . | % . | 6y-pEFS, % (SE) . | ||||
| All | 2169 | 100 | 79.6 (0.9) | 100 | 100 | 100 |
| Sex | ||||||
| Male | 1226 | 56.5 | 78.4 (1.2) | 54.9 | 56.4 | 61.8 |
| Female | 943 | 43.5 | 81.1 (1.3) | 45.1 | 43.6 | 38.2 |
| Age† | ||||||
| Less than 1 y | 34 | 1.6 | 40.4 (8.5) | 0 | 1.5 | 6.7 |
| 1 to less than 6 y | 1255 | 57.9 | 84.3 (1.0) | 100 | 35.1 | 35.8 |
| 6 to less than 10 y | 447 | 20.6 | 80.3 (1.9) | 0 | 33.1 | 25.2 |
| 10 to less than 15 y | 340 | 15.7 | 70.5 (2.5) | 0 | 23.9 | 25.2 |
| 15 y and older | 93 | 4.3 | 58.3 (5.4) | 0 | 6.5 | 7.1 |
| Initial WBC (/μ L) | ||||||
| Less than 10 000 | 1071 | 49.4 | 84.2 (1.1) | 76.8 | 38.8 | 15.7 |
| 10 000 to less than 20 × 109/L | 319 | 14.7 | 83.9 (2.1) | 23.2 | 9.8 | 11.8 |
| 20 000 to less than 50 × 109/L | 362 | 16.7 | 79.4 (2.2) | 0 | 27.5 | 17.3 |
| 50 000 to less than 100 × 109/L | 180 | 8.3 | 74.0 (3.3) | 0 | 12.2 | 15.4 |
| 100 000 to less than 200 × 109/L | 111 | 5.1 | 62.9 (4.6) | 0 | 6.5 | 14.2 |
| 200 000 and over | 126 | 5.8 | 52.6 (4.5) | 0 | 5.3 | 25.6 |
| BFM-RF | ||||||
| Less than 0.8 | 765 | 35.8 | 86.2 (1.3) | 53.6 | 30.1 | 8.7 |
| More than 0.8 | 1372 | 64.2 | 75.8 (1.2) | 46.4 | 69.9 | 91.3 |
| CNS status | ||||||
| CNS1 | 1717 | 79.5 | 81.3 (1.0) | 84.7 | 77.9 | 71.0 |
| CNS2 | 112 | 5.2 | 79.7 (3.9) | 3.3 | 5.9 | 7.5 |
| CNS3 | 64 | 3.0 | 57.7 (6.2) | 1.2 | 2.9 | 8.7 |
| TLP+ | 148 | 6.8 | 69.2 (3.8) | 4.1 | 8.2 | 9.1 |
| TLP− | 119 | 5.5 | 81.0 (3.7) | 6.7 | 5.1 | 3.6 |
| Immunophenotype | ||||||
| Precursor B | 1798 | 86.5 | 80.2 (1.0) | 100 | 82.6 | 65.5 |
| T | 277 | 13.3 | 74.8 (2.6) | 0 | 17.1 | 34.5 |
| Other‡ | 3 | 0.1 | 0 | 0.3 | 0 | |
| DNA index | ||||||
| Less than 1.16 | 1187 | 78.7 | 76.2 (1.3) | 65.4 | 83.2 | 91.9 |
| More than 1.16 | 322 | 21.3 | 88.9 (1.8) | 34.6 | 16.8 | 8.1 |
| TEL/AML1 | ||||||
| Negative | 916 | 78.6 | 75.1 (1.4) | 73.2 | 78.7 | 94.4 |
| Positive | 250 | 21.4 | 91.2 (1.8) | 26.8 | 21.3 | 5.6 |
| BCR/ABL | ||||||
| Negative | 1918 | 97.9 | 80.4 (0.9) | 100 | 100 | 82.4 |
| Positive | 42 | 2.1 | 26.2 (6.8) | 0 | 0 | 17.6 |
| MLL/AF4 | ||||||
| Negative | 1154 | 97.9 | 77.4 (1.2) | 100 | 100 | 86.9 |
| Positive | 25 | 2.1 | 40.0 (9.8) | 0 | 0 | 13.1 |
| Non-T lineage NCI risk criteria‖ | ||||||
| Standard risk | 1256 | 71.0 | 86.5 (1.0) | 100 | 54.1 | 34.9 |
| High risk | 512 | 29.0 | 67.4 (2.1) | 0 | 45.9 | 65.1 |
| T lineage NCI risk criteria‖ | ||||||
| Standard risk | 72 | 26.2 | 90.1 (3.5) | 33.3 | 10.5 | |
| High risk | 203 | 73.8 | 69.2 (3.3) | 66.7 | 89.5 | |
| Prednisone response | ||||||
| Good | 1963 | 91.4 | 82.1 (0.9) | 100 | 100 | 27.2 |
| Poor | 184 | 8.6 | 55.0 (3.7) | 0 | 0 | 72.7 |
| BM day 15 | ||||||
| M1 | 880 | 61.5 | 87.1 (1.1) | 72.2 | 65.0 | 22.3 |
| M2 | 365 | 25.5 | 75.5 (2.3) | 24.1 | 25.3 | 29.9 |
| M3 | 186 | 13.0 | 47.3 (3.7) | 3.8 | 9.7 | 47.7 |
| Nonremission day 33 | ||||||
| No | 2120 | 97.7 | 80.6 (0.9) | 100 | 100 | 80.7 |
| Yes | 49 | 2.3 | 36.3 (6.9) | 0 | 0 | 19.3 |
| Variable . | All patients (total n = 2169) . | SR, % (n = 758) . | MR, % (n = 1157) . | HR, % (n = 254) . | ||
|---|---|---|---|---|---|---|
| N* . | % . | 6y-pEFS, % (SE) . | ||||
| All | 2169 | 100 | 79.6 (0.9) | 100 | 100 | 100 |
| Sex | ||||||
| Male | 1226 | 56.5 | 78.4 (1.2) | 54.9 | 56.4 | 61.8 |
| Female | 943 | 43.5 | 81.1 (1.3) | 45.1 | 43.6 | 38.2 |
| Age† | ||||||
| Less than 1 y | 34 | 1.6 | 40.4 (8.5) | 0 | 1.5 | 6.7 |
| 1 to less than 6 y | 1255 | 57.9 | 84.3 (1.0) | 100 | 35.1 | 35.8 |
| 6 to less than 10 y | 447 | 20.6 | 80.3 (1.9) | 0 | 33.1 | 25.2 |
| 10 to less than 15 y | 340 | 15.7 | 70.5 (2.5) | 0 | 23.9 | 25.2 |
| 15 y and older | 93 | 4.3 | 58.3 (5.4) | 0 | 6.5 | 7.1 |
| Initial WBC (/μ L) | ||||||
| Less than 10 000 | 1071 | 49.4 | 84.2 (1.1) | 76.8 | 38.8 | 15.7 |
| 10 000 to less than 20 × 109/L | 319 | 14.7 | 83.9 (2.1) | 23.2 | 9.8 | 11.8 |
| 20 000 to less than 50 × 109/L | 362 | 16.7 | 79.4 (2.2) | 0 | 27.5 | 17.3 |
| 50 000 to less than 100 × 109/L | 180 | 8.3 | 74.0 (3.3) | 0 | 12.2 | 15.4 |
| 100 000 to less than 200 × 109/L | 111 | 5.1 | 62.9 (4.6) | 0 | 6.5 | 14.2 |
| 200 000 and over | 126 | 5.8 | 52.6 (4.5) | 0 | 5.3 | 25.6 |
| BFM-RF | ||||||
| Less than 0.8 | 765 | 35.8 | 86.2 (1.3) | 53.6 | 30.1 | 8.7 |
| More than 0.8 | 1372 | 64.2 | 75.8 (1.2) | 46.4 | 69.9 | 91.3 |
| CNS status | ||||||
| CNS1 | 1717 | 79.5 | 81.3 (1.0) | 84.7 | 77.9 | 71.0 |
| CNS2 | 112 | 5.2 | 79.7 (3.9) | 3.3 | 5.9 | 7.5 |
| CNS3 | 64 | 3.0 | 57.7 (6.2) | 1.2 | 2.9 | 8.7 |
| TLP+ | 148 | 6.8 | 69.2 (3.8) | 4.1 | 8.2 | 9.1 |
| TLP− | 119 | 5.5 | 81.0 (3.7) | 6.7 | 5.1 | 3.6 |
| Immunophenotype | ||||||
| Precursor B | 1798 | 86.5 | 80.2 (1.0) | 100 | 82.6 | 65.5 |
| T | 277 | 13.3 | 74.8 (2.6) | 0 | 17.1 | 34.5 |
| Other‡ | 3 | 0.1 | 0 | 0.3 | 0 | |
| DNA index | ||||||
| Less than 1.16 | 1187 | 78.7 | 76.2 (1.3) | 65.4 | 83.2 | 91.9 |
| More than 1.16 | 322 | 21.3 | 88.9 (1.8) | 34.6 | 16.8 | 8.1 |
| TEL/AML1 | ||||||
| Negative | 916 | 78.6 | 75.1 (1.4) | 73.2 | 78.7 | 94.4 |
| Positive | 250 | 21.4 | 91.2 (1.8) | 26.8 | 21.3 | 5.6 |
| BCR/ABL | ||||||
| Negative | 1918 | 97.9 | 80.4 (0.9) | 100 | 100 | 82.4 |
| Positive | 42 | 2.1 | 26.2 (6.8) | 0 | 0 | 17.6 |
| MLL/AF4 | ||||||
| Negative | 1154 | 97.9 | 77.4 (1.2) | 100 | 100 | 86.9 |
| Positive | 25 | 2.1 | 40.0 (9.8) | 0 | 0 | 13.1 |
| Non-T lineage NCI risk criteria‖ | ||||||
| Standard risk | 1256 | 71.0 | 86.5 (1.0) | 100 | 54.1 | 34.9 |
| High risk | 512 | 29.0 | 67.4 (2.1) | 0 | 45.9 | 65.1 |
| T lineage NCI risk criteria‖ | ||||||
| Standard risk | 72 | 26.2 | 90.1 (3.5) | 33.3 | 10.5 | |
| High risk | 203 | 73.8 | 69.2 (3.3) | 66.7 | 89.5 | |
| Prednisone response | ||||||
| Good | 1963 | 91.4 | 82.1 (0.9) | 100 | 100 | 27.2 |
| Poor | 184 | 8.6 | 55.0 (3.7) | 0 | 0 | 72.7 |
| BM day 15 | ||||||
| M1 | 880 | 61.5 | 87.1 (1.1) | 72.2 | 65.0 | 22.3 |
| M2 | 365 | 25.5 | 75.5 (2.3) | 24.1 | 25.3 | 29.9 |
| M3 | 186 | 13.0 | 47.3 (3.7) | 3.8 | 9.7 | 47.7 |
| Nonremission day 33 | ||||||
| No | 2120 | 97.7 | 80.6 (0.9) | 100 | 100 | 80.7 |
| Yes | 49 | 2.3 | 36.3 (6.9) | 0 | 0 | 19.3 |
CR indicates complete remission; BM, bone marrow; CNS, central nervous system; SR, standard risk; MR, medium risk; HR, high risk.
Data refer to patients with successful investigation of the respective criteria.
Median age was 5.0 years (range, 0.07-17.92 years). Nineteen additional patients younger than 1 year were treated in the Interfant-99 pilot study43 and were not included in the analyses.
Two patients had the immunophenotype of a mature B-cell leukemia (cytomorphologically FAB L1); one patient had a biphenotypic acute leukemia.
NCI-SR, age 1 or younger and less than 10 years, and WBC less than 50 × 109/L; NCI-HR, age 10 years or older or WBC 50 × 109/L or more. Infants less than 1 year are excluded from the NCI definition.
One patient of the SR group was falsely BCR/ABL-negative at initial diagnosis (BCR/ABL-positive in relapse and in subsequently repeated analysis of the initial material).
Event-free survival
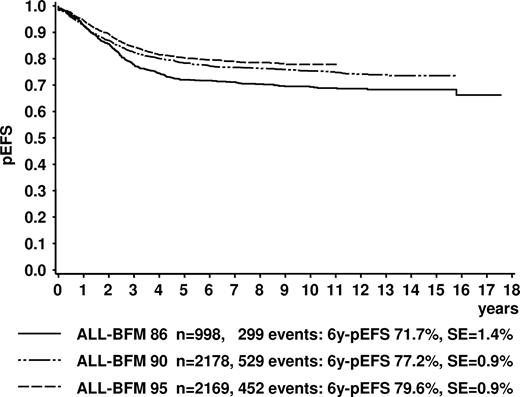
The pEFS at 6 years (6y-pEFS ± SE) for all 2283 study patients of ALL-BFM 95 was 78.6% (± 0.9%); probability of survival at 6 years (6y-SUR ± SE) was 86.3% (± 0.6%). For the 2169 evaluable patients, 6y-pEFS was 79.6% (± 0.9%; Figure 2); 6y-SUR was 87.0% (± 0.7%). CR was achieved by 99.1% of evaluable patients. In comparison to the previous trial ALL-BFM 90, there was a slight improvement of 6y-pEFS (P = .062; Figure 2) and 6y-SUR (6y-SUR ALL-BFM 90: 84.8% ± 0.8%, P = .044).
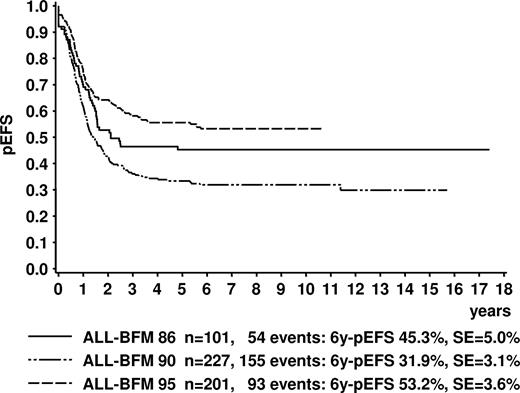
Kaplan-Meier estimate of event-free survival of all evaluable patients in trials ALL-BFM 86, 90, and 95. Log-Rank tests: ALL-BFM 86 versus 90, P = .001; ALL-BFM 86 versus 95, P < .001; ALL-BFM 90 versus 95, P = .062. 6y-pEFS indicates probability of event-free survival at 6 years; SE, standard error.
Kaplan-Meier estimate of event-free survival of all evaluable patients in trials ALL-BFM 86, 90, and 95. Log-Rank tests: ALL-BFM 86 versus 90, P = .001; ALL-BFM 86 versus 95, P < .001; ALL-BFM 90 versus 95, P = .062. 6y-pEFS indicates probability of event-free survival at 6 years; SE, standard error.
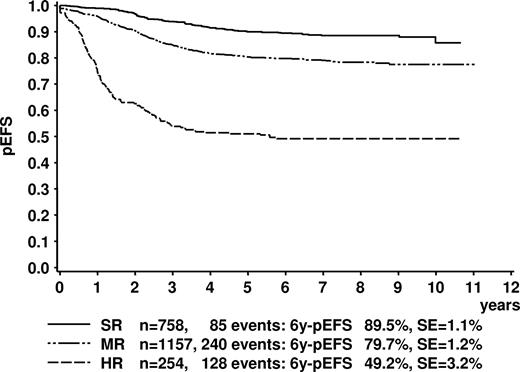
Stratification by the ALL-BFM 95 risk criteria resulted in distinct groups with 6y-pEFS of 89.5% (± 1.1%) for SR, 79.7% (± 1.2%) for MR, and 49.2% (± 3.2%) for HR (Figure 3).
Kaplan-Meier estimate of event-free survival of the trial ALL-BFM 95 according to risk groups. Log-rank test, P < .001. SR indicates standard risk; MR medium risk; HR, high risk; 6y-pEFS, probability of event-free survival at 6 years; SE, standard error.
Kaplan-Meier estimate of event-free survival of the trial ALL-BFM 95 according to risk groups. Log-rank test, P < .001. SR indicates standard risk; MR medium risk; HR, high risk; 6y-pEFS, probability of event-free survival at 6 years; SE, standard error.
Events
Remission failures.
Twenty patients failed to achieve remission because of early death or resistant disease. Sixteen patients died during the 5-week induction (protocol IA); 5 of them died of leukemia-associated complications; in 11 patients, death was suspected to be treatment-related (Table 4). Among 49 patients who were not in remission by protocol day 33, 45 finally reached CR by the start of fourth HR course. Four patients had not achieved CR by this time point (nonresponse).
Treatment results
| . | All, n . | CI,* % (SE) . | Risk group . | |||||
|---|---|---|---|---|---|---|---|---|
| SR, n . | CI,* % (SE) . | MR, n . | CI,* % (SE) . | HR, n . | CI,* % (SE) . | |||
| Overall | 2169 | 758 | 1157 | 254 | ||||
| Death before CR | 16‖ | 0.7 (0.2) | 0 | 13 | 1.1 (0.3) | 3 | 1.2 (0.7) | |
| Resistant disease | 4 | 0.2 (0.1) | 0 | 0 | 4 | 1.6 (0.8) | ||
| Death in first CR | 46 | 2.1 (0.3) | 5 | 0.7 (0.3) | 19 | 1.6 (0.4) | 22 | 8.7 (2.1) |
| During/after chemotherapy | 33¶ | 0.6 (0.2) | 5 | 0.7 (0.3) | 19 | 1.6 (0.4) | 9 | 3.5 (1.2) |
| After stem cell transplantation† | 13 | 1.5 (0.3) | 0 | 0 | 13 | 5.1 (1.8) | ||
| Relapses | 356 | 16.2 (0.8) | 62 | 7.8 (1.0) | 197 | 16.8 (1.2) | 97 | 38.6 (3.6) |
| Isolated BM | 232 | 10.5 (0.7) | 41 | 5.1 (0.8) | 120 | 10.1 (1.0) | 71 | 28.3 (3.6) |
| Isolated CNS | 39 | 1.8 (0.3) | 8 | 1.1 (0.4) | 25 | 2.2 (0.5) | 6 | 2.4 (1.2) |
| Isolated testes | 12 | 0.5 (0.2) | 3 | 0.4 (0.3) | 8 | 0.6 (0.3) | 1 | 0.4 (0.7) |
| Combined CNS/BM involved | 48 | 2.2 (0.4) | 6 | 0.8 (0.3) | 31 | 2.7 (0.5) | 11 | 4.3 (1.7) |
| Combined BM/other (without CNS) | 22 | 1.0 (0.2) | 3 | 0.3 (0.2) | 13 | 1.2 (0.4) | 6 | 2.4 (1.5) |
| Other relapses‡ | 3 | 0.1 (0.1) | 1 | 0.1 (0.1) | 0 | 2 | 0.8 (0.9) | |
| Secondary neoplasms§ | 30 | 2.0 (0.4) | 17 | 2.4 (0.6) | 11 | 1.8 (0.7) | 2 | 2.1 (1.5) |
| . | All, n . | CI,* % (SE) . | Risk group . | |||||
|---|---|---|---|---|---|---|---|---|
| SR, n . | CI,* % (SE) . | MR, n . | CI,* % (SE) . | HR, n . | CI,* % (SE) . | |||
| Overall | 2169 | 758 | 1157 | 254 | ||||
| Death before CR | 16‖ | 0.7 (0.2) | 0 | 13 | 1.1 (0.3) | 3 | 1.2 (0.7) | |
| Resistant disease | 4 | 0.2 (0.1) | 0 | 0 | 4 | 1.6 (0.8) | ||
| Death in first CR | 46 | 2.1 (0.3) | 5 | 0.7 (0.3) | 19 | 1.6 (0.4) | 22 | 8.7 (2.1) |
| During/after chemotherapy | 33¶ | 0.6 (0.2) | 5 | 0.7 (0.3) | 19 | 1.6 (0.4) | 9 | 3.5 (1.2) |
| After stem cell transplantation† | 13 | 1.5 (0.3) | 0 | 0 | 13 | 5.1 (1.8) | ||
| Relapses | 356 | 16.2 (0.8) | 62 | 7.8 (1.0) | 197 | 16.8 (1.2) | 97 | 38.6 (3.6) |
| Isolated BM | 232 | 10.5 (0.7) | 41 | 5.1 (0.8) | 120 | 10.1 (1.0) | 71 | 28.3 (3.6) |
| Isolated CNS | 39 | 1.8 (0.3) | 8 | 1.1 (0.4) | 25 | 2.2 (0.5) | 6 | 2.4 (1.2) |
| Isolated testes | 12 | 0.5 (0.2) | 3 | 0.4 (0.3) | 8 | 0.6 (0.3) | 1 | 0.4 (0.7) |
| Combined CNS/BM involved | 48 | 2.2 (0.4) | 6 | 0.8 (0.3) | 31 | 2.7 (0.5) | 11 | 4.3 (1.7) |
| Combined BM/other (without CNS) | 22 | 1.0 (0.2) | 3 | 0.3 (0.2) | 13 | 1.2 (0.4) | 6 | 2.4 (1.5) |
| Other relapses‡ | 3 | 0.1 (0.1) | 1 | 0.1 (0.1) | 0 | 2 | 0.8 (0.9) | |
| Secondary neoplasms§ | 30 | 2.0 (0.4) | 17 | 2.4 (0.6) | 11 | 1.8 (0.7) | 2 | 2.1 (1.5) |
CR indicates complete remission; BM, bone marrow; CNS, central nervous system; SR, standard risk; MR, medium risk; HR, high risk.
Cumulative incidences are indicated at 6 years except for the secondary neoplasms, which are calculated at 10 years.
A total of 58 patients underwent allogeneic stem cell transplantation in first CR.
Numbers of isolated mediastinal cases (1); isolated lumbosacral epidural (1); relapse site unknown (1; relapse diagnosed abroad).
Four additional secondary neoplasms developed after relapse and relapse treatment.
Numbers of leukemia-associated cases: hypoxia resulting from mediastinal mass (1); cerebral bleeding (2); bacterial sepsis (1); multiple organ failure resulting from tumor lysis syndrome (1); treatment-related: bacterial sepsis (6); cerebral bleeding (1); cerebral thrombosis (2); multiple organ failure (1); disseminated intravascular coagulation (1).
Number of cases of bacterial sepsis (8); fungal infection (9); viral infection (1); pneumocystis carinii pneumonia (1); infection/sepsis with unknown pathogen (7); progressive leukencephalopathy (1); cerebral bleeding (2); cerebral infarction (1); interstitial pneumonia of unknown origin (2); and erroneous intrathecal vincristine application (1).
Deaths in CR.
Forty-six patients (2.1%) died after achievement of CR because of treatment complications. Incidence was highest in the HR group (8.7%, 22 deaths, 13 of them resulting from alloSCT-related complications). Twenty-four patients with death in CR were treated in SR and MR. Nine of them died in protocol I, 11 in protocol II, and 4 during maintenance. Four of the 9 chemotherapy-related deaths in the HR group were associated with the high-dose blocks: 2 patients died in protocol I and 3 patients in protocol II (Table 4).
Relapses.
CI at 6 years (6y-CI) of relapse was 16.2% (± 0.8%). Incidences within risk groups are shown in Table 4. BM was the most frequent site of relapse in all risk groups. Six-year CI of isolated CNS relapse and of all relapses with CNS involvement was 1.8% (± 0.3%) and 4.1% (± 0.5%), respectively.
Secondary neoplasms.
Thirty-four patients developed a SN at a median time of 45.7 months (range, 8.7-105.1 months). Thirty of them occurred after initial treatment in first CR. Among these, acute myeloid leukemia (n = 9) and myelodysplastic syndrome (n = 7) were most frequent. Two patients had an ALL; a relapse of the primary ALL was ruled out in these cases. Other SN were non-Hodgkin lymphoma (n = 4), Hodgkin lymphoma (n = 1), PNET/Ewing sarcoma (n = 2), malignant rhabdoid tumor (n = 1), malignant fibrous histiocytoma (n = 1), Langerhans' cell histiocytosis (n = 2), and granulocytic sarcoma (n = 1). In 4 other patients, the SN developed after relapse and relapse treatment: brain tumor (n = 2), Burkitt leukemia (n = 1), and Epstein-Barr virus-associated lymphoblastic lymphoma (n = 1).
Evaluation of the new stratification strategy
The impact of the new risk stratification in ALL-BFM 95 was evaluated in comparison to the former ALL-BFM 90 risk stratification. Because HR criteria were basically the same in both trials, HR patients were excluded from this analysis. EFS and resulting risk ratios of the subgroups SR and MR by ALL-BFM 90 and ALL-BFM 95 criteria are given in Table 5. Although both risk stratifications resulted in significant differences between SR and MR, discrimination was better when using ALL-BFM 95 criteria.
Impact of risk stratification according to ALL-BFM 90 and ALL-BFM 95 risk criteria in non-HR patients of the 2 trials (univariate Cox regression analysis)
| . | N . | 6y-pEFS, % (SE) . | Risk ratio . | 95% confidence interval . | P (Wald) . |
|---|---|---|---|---|---|
| ALL-BFM 90 | |||||
| SR-90 | 635 | 85.4 (1.4) | 1 | ||
| MR-90 | 1285 | 81.7 (1.1) | 1.40 | 1.11-1.77 | .005 |
| SR-95 | 826 | 88.7 (1.1) | 1 | ||
| MR-95 | 1094 | 78.7 (1.3) | 1.99 | 1.59-2.49 | <.001 |
| ALL-BFM 95 | |||||
| SR-90 | 683 | 88.2 (1.3) | 1 | ||
| MR-90 | 1207 | 81.0 (1.1) | 1.66 | 1.29-2.12 | <.001 |
| SR-95 | 758 | 89.5 (1.1) | 1 | ||
| MR-95 | 1157 | 79.7 (1.2) | 2.05 | 1.60-2.63 | <.001 |
| . | N . | 6y-pEFS, % (SE) . | Risk ratio . | 95% confidence interval . | P (Wald) . |
|---|---|---|---|---|---|
| ALL-BFM 90 | |||||
| SR-90 | 635 | 85.4 (1.4) | 1 | ||
| MR-90 | 1285 | 81.7 (1.1) | 1.40 | 1.11-1.77 | .005 |
| SR-95 | 826 | 88.7 (1.1) | 1 | ||
| MR-95 | 1094 | 78.7 (1.3) | 1.99 | 1.59-2.49 | <.001 |
| ALL-BFM 95 | |||||
| SR-90 | 683 | 88.2 (1.3) | 1 | ||
| MR-90 | 1207 | 81.0 (1.1) | 1.66 | 1.29-2.12 | <.001 |
| SR-95 | 758 | 89.5 (1.1) | 1 | ||
| MR-95 | 1157 | 79.7 (1.2) | 2.05 | 1.60-2.63 | <.001 |
Impact of new or modified treatment elements
Dose reduction of anthracyclines in the SR group.
To evaluate the effect of the reduction of 2 doses of daunorubicin in protocol IA in SR, data were compared with the matching historical control group of ALL-BFM 9015 (see “Historical control group”). The matching subset in ALL-BFM 90, which was SR by 95 criteria (“SR-95”), had received a total of 4 doses of daunorubicin in protocol IA.
The 6y-pEFS of SR patients in ALL-BFM 95 was 89.5% (± 1.1%; n = 758) compared with 88.7% (± 1.1%; n = 826) in “SR-95” patients of ALL-BFM 90 (difference, 0.8%; 95% confidence interval, −1.6% to 3.2%). Six-year CI of relapses was 7.8% (± 1.0%) in ALL-BFM 95 and 9.3% (± 1.0%) in “SR-95” patients of ALL-BFM 90 (difference, 1.5%; 95% confidence interval, −1.3% to 5.5%). As approximately one-half each of the historical control group was treated in risk group SR and MR, respectively, the matching subgroups of the 2 trials were compared after aditional subdivision into “SR-90” and “MR-90.” The results of these analyses likewise showed no disadvantage of the daunorubicin reduction in either subgroup (data not shown).
There were no cases of induction death in the ALL-BFM 95 SR group, whereas 2 treatment-related deaths before CR occurred in the “SR-95” group in ALL-BFM 90.
Extension of maintenance therapy in boys in the SR group.
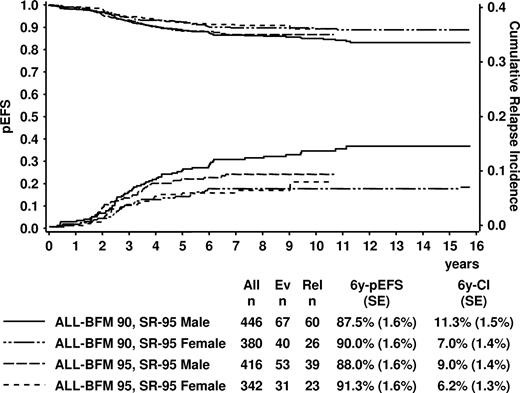
Taking into account that the boys of the ALL-BFM 95 SR group received 1 additional year of maintenance, further analyses of the SR group were performed stratified by gender and once more comparing the ALL-BFM 95 data with the matching subsets from trial ALL-BFM 90. EFS of boys was similar in study ALL-BFM 90 (4 daunorubicin doses; 6y-pEFS, 87.5% ± 1.6%) and ALL-BFM 95 (2 daunorubicin doses + extended maintenance; 88.0% ± 1.6%, P = .71; Figure 4). Likewise, no significant difference could be shown comparing the outcome of girls in study ALL-BFM 90 (4 daunorubicin doses; 6y-pEFS, 90.0% ± 1.6%) to ALL-BFM 95 (2 daunorubicin doses; 6y-pEFS, 91.3% ± 1.6%, P = .79), who all had received 24 months of maintenance therapy (Figure 4). In both trials, there was a slightly worse pEFS in boys compared with girls (ALL-BFM 90, P = .049; ALL-BFM 95, P = .12). Six-year CI of relapse was 9.0% (± 1.4%) in “SR-95” boys of ALL-BFM 95 and 11.3% (± 1.5%) in the matching boys in trial ALL-BFM 90 (P = .154).
Kaplan-Meier estimate of event-free survival and cumulative incidence of relapses of SR patients by ALL-BFM 95 risk criteria (“SR-95”) in trials ALL-BFM 90 and 95 according to sex. Patients of ALL-BFM 95 received 2 doses of daunorubicin in induction, patients of ALL-BFM 90 4 doses. Duration of maintenance was 36 months for boys of ALL-BFM 95 and 24 months for the remaining patients. “SR-95” indicates standard risk by ALL-BFM 95 criteria; Ev, number of events; Rel, number of relapses; 6y-pEFS, probability of event-free survival at 6 years; 6y-CI, cumulative incidence of relapses at 6 years; SE, standard error.
Kaplan-Meier estimate of event-free survival and cumulative incidence of relapses of SR patients by ALL-BFM 95 risk criteria (“SR-95”) in trials ALL-BFM 90 and 95 according to sex. Patients of ALL-BFM 95 received 2 doses of daunorubicin in induction, patients of ALL-BFM 90 4 doses. Duration of maintenance was 36 months for boys of ALL-BFM 95 and 24 months for the remaining patients. “SR-95” indicates standard risk by ALL-BFM 95 criteria; Ev, number of events; Rel, number of relapses; 6y-pEFS, probability of event-free survival at 6 years; 6y-CI, cumulative incidence of relapses at 6 years; SE, standard error.
Omission of preventive cranial radiotherapy in the MR group and dose reduction of irradiation in patients with initial CNS involvement
In study ALL-BFM 90, pCRT with 12 Gy was scheduled for all “MR-90” patients. To evaluate the effect of the omission of pCRT in all non-HR patients with pB-ALL in ALL-BFM 95, the corresponding patient groups, which had an indication for pCRT in trial ALL-BFM 90 but not in ALL-BFM 95, were compared. These groups comprise patients aged 1 year or older with pB-ALL without initial CNS involvement classified as “MR-90” according to the ALL-BFM 90 risk criteria. The pEFS of these patients was similar in ALL-BFM 90 (pCRT intended) and ALL-BFM 95 (pCRT not intended; difference of pEFS at 6y, −1.6%, 95% confidence interval, −4.4% to 1.1%; Figure 5A). Detailed analysis of relapse sites revealed a significantly higher incidence of relapses with CNS involvement in the nonirradiated patients in ALL-BFM 95 (6y-CI, 4.4% ± 0.8%) compared with the corresponding irradiated group in ALL-BFM 90 (6y-CI, 1.9% ± 0.5%; P = .001), mostly because of isolated CNS relapses (Figure 5B). Additional stratification by “SR-95” and “MR-95” showed the same trend in either risk group, whereas the incidence of isolated CNS relapses between ALL-BFM 90 and 95 was similar in the remaining “MR-95” patients who were SR by ALL-BFM 90 criteria and thus in neither trial were irradiated (data not shown).
Kaplan-Meier estimate and cumulative relapse incidences for evaluation of the impact of preventive cranial radiotherapy. The curves compare the matching patients from ALL-BFM 90 and 95 who received presymptomatic cranial radiotherapy in trial ALL-BFM 90 yet were not irradiated in ALL-BFM 95 (pB-ALL, aged 1 year or older, no initial CNS involvement, risk group MR-90). (A) Event-free survival (P[log-rank] = .280). (B) Cumulative incidence of systemic relapses with CNS involvement (P[Gray] = .270) and isolated CNS relapses (P[Gray] < .001). Isol. CNS rel. indicates isolated relapse in the central nervous system; comb. CNS rel., combined relapse involving the central nervous system; 6y-pEFS, probability of event-free survival at 6 years; 6y-CI, cumulative incidence of relapses at 6 years; SE, standard error.
Kaplan-Meier estimate and cumulative relapse incidences for evaluation of the impact of preventive cranial radiotherapy. The curves compare the matching patients from ALL-BFM 90 and 95 who received presymptomatic cranial radiotherapy in trial ALL-BFM 90 yet were not irradiated in ALL-BFM 95 (pB-ALL, aged 1 year or older, no initial CNS involvement, risk group MR-90). (A) Event-free survival (P[log-rank] = .280). (B) Cumulative incidence of systemic relapses with CNS involvement (P[Gray] = .270) and isolated CNS relapses (P[Gray] < .001). Isol. CNS rel. indicates isolated relapse in the central nervous system; comb. CNS rel., combined relapse involving the central nervous system; 6y-pEFS, probability of event-free survival at 6 years; 6y-CI, cumulative incidence of relapses at 6 years; SE, standard error.
CNS3 patients aged one year or older in ALL-BFM 95, who received tCRT with 18 Gy, showed no disadvantage in EFS compared with CNS3 patients of trial ALL-BFM 90 who were irradiated with 24 Gy (ALL-BFM 95, 59.2% ± 6.4%; ALL-BFM 90, 52.8% ± 7.3%; P = .63).
Intensification of protocol M by cytarabine in the MR group
Among the 1032 patients who were randomized to receive or not additional cytarabine in protocol M, 518 patients were randomized into protocol M and 514 into protocol MCA. In 9 patients, randomized treatment was not applicable because of death (n = 7) or treatment withdrawal (n = 2) after randomization but before protocol M/MCA. Thirteen patients randomized to receive protocol M and 69 patients randomized into protocol MCA were eventually treated in the other arm. In 18 patients, the performed treatment was not reported.
The intent-to-treat analysis revealed a 6y-pDFS of 80% (± 2%) for protocol M and 80% (± 2%) for protocol MCA (P = .99). The per-protocol analysis also showed no difference between the treatment arms (6y-pDFS M, 80% ± 2%; MCA, 80% ± 2%; P = .97). Deaths in CCR were similar in patients treated with MCA (5 deaths) or M (3 deaths); none of them occurred concurrently with protocol M/MCA. Documentation of the individual treatment schedule was available from 299 patients treated with M and 233 patients with MCA. Patients receiving MCA needed a median of 72 days (range, 53-139 days) to the subsequent treatment element compared with 71 days (range, 60-119 days) in patients receiving M.
Intensification of the HR treatment by modified high-dose blocks and reinduction with protocol II
The modified treatment approach in ALL-BFM 95 was evaluated compared with the HR treatment regimens of the previous trials ALL-BFM 90 and ALL-BFM 86. To eliminate the bias by the slightly different HR criteria of the 3 trials, only patients with PPR and/or no CR on day 33 (NRd33) were compared. They were eligible for HR treatment in all 3 trials and made up the majority of the HR patients (84% of HR patients according to ALL-BFM 95 criteria). The results are shown in Figure 6. The inferior result of the ALL-BFM 90 HR regimen compared with the HR treatment in ALL-BFM 8615 could also be shown in the updated analysis for the patients with PPR/NRd33 (P = .029). The HR treatment in ALL-BFM 95 produced a 6y-pEFS of 53.2% (± 3.6%), which was higher than in ALL-BFM 90 and 86 (Figure 6). The improved outcome in the HR group of trial ALL-BFM 95 compared with ALL-BFM 90 was due mainly to a lower incidence of nonresponse (6y-CI ALL-BFM 90, 6.6% ± 1.7%; ALL-BFM 95, 2.0% ± 1.0%; P = .020) and systemic relapses (6y-CI ALL-BFM 90, 51.3% ± 4.3%; ALL-BFM 95, 31.3% ± 3.9%; P < .001), whereas the incidence of isolated extramedullary relapses was comparable between the trials (6y-CI ALL-BFM 90, 3.5% ± 2.2%; ALL-BFM 95, 3.0% ± 1.7%; P = .76).
Kaplan-Meier estimate of event-free survival of patients with prednisone poor-response and/or nonremission on day 33 in the trials ALL-BFM 86, 90, and 95. All patients were treated in the HR arm of the respective trial. Log-rank tests: ALL-BFM 86 versus 90, P = .029; ALL-BFM 86 versus 95, P = .14; ALL-BFM 90 versus 95, P < .001. 6y-pEFS indicates probability of event-free survival at 6 years; SE, standard error.
Kaplan-Meier estimate of event-free survival of patients with prednisone poor-response and/or nonremission on day 33 in the trials ALL-BFM 86, 90, and 95. All patients were treated in the HR arm of the respective trial. Log-rank tests: ALL-BFM 86 versus 90, P = .029; ALL-BFM 86 versus 95, P = .14; ALL-BFM 90 versus 95, P < .001. 6y-pEFS indicates probability of event-free survival at 6 years; SE, standard error.
In patients with t(9;22) or BCR/ABL, the impact of the modified ALL-BFM 95 HR treatment was only analyzed compared with ALL-BFM 90 because t(9;22) was no HR criterion in the earlier studies. No benefit from the ALL-BFM 95 HR treatment could be proven in this patient subset; 6 year-pEFS was 33% (± 9%) in ALL-BFM 90 (n = 27) compared with 26% (± 7%) in ALL-BFM 95 (n = 42; P = .91). In addition, for the patients with t(4;11) or MLL/AF4, which per se was not a HR criterion in ALL-BFM 90, pEFS could not be improved by the ALL-BFM 95 HR treatment (6y-pEFS ALL-BFM 90, 36% ± 10%, n = 22; ALL-BFM 95, 40% ± 10%; n = 25; P = .55).
Discussion
The trial ALL-BFM 95 comprised a large unselected population of 2169 evaluable patients. Compared with the previous trial ALL-BFM 90, results could be significantly improved in the HR group through well-directed treatment intensification. On the other hand, it was possible to maintain the excellent results of the former trial in the large SR and MR subgroups despite significant dose reductions with respect to anthracyclines in induction and cranial irradiation. The randomized treatment intensifications in protocol M and maintenance24 in the MR group were of no significant advantage. Overall, ALL-BFM 95 showed a slight, not yet statistically significant, improvement of 6y-pEFS compared with ALL-BFM 90 and an improved probability of survival.
For the newly defined SR group comprising approximately one-third of all patients, an approximated pEFS of 90% was expected. This gave reason to cautiously reduce the daunorubicin dose in this subset to diminish the risk of acute and late toxicity associated with anthracyclines. Anthracycline-induced long-term cardiotoxicity has been shown to be significantly associated with high cumulative anthracycline doses.44-46 However, with longer follow-up, cardiac abnormalities can also become obvious in patients treated with low doses of less than 250 mg/m2.47,48 In the randomized trials, which had been published at the time of the ALL-BFM 95 planning phase, no benefit for the use of anthracyclines in addition to a 3-drug induction with prednisone, vincristine, and L-asparaginase could be proven with respect to EFS (for review, see Messinger et al49 ). However, the treatment schedules in these trials were crucially different from ALL-BFM 95, and results of these early trials were inferior to the results in the BFM studies achieved at that time, which hampered a clear extrapolation from those trials. In ALL-BFM 90, the anthracycline dose in induction was already reduced by 25% without adverse effects on survival, but this modification was combined with a more condensed induction phase.15 Halving the induction daunorubicin dose in the SR group in ALL-BFM 95 yielded an excellent 6y-pEFS of 89.5% and could be safely performed as shown in comparison to the historical control group of the previous trial ALL-BFM 90. This confirmed the prior results of other trials and may encourage a further decrease of anthracyclines in ALL low-risk groups.
Boys have been shown to be at higher risk of relapse than girls, particularly through a higher rate of late relapses after 2 years from diagnosis.50-52 This observation was the rationale for the extension of maintenance for boys in the ALL-BFM 95 SR group. Comparing these patients with the matching subgroup of ALL-BFM 90, no improvement of EFS could be achieved by the longer maintenance treatment, although the data suggest a slight reduction of late relapses (difference of point estimates at 10 years: P = .067). However, it has to be pointed out that treatment of the matching subgroups (“SR-95”) in ALL-BFM 90 and 95 in addition differed in the number of daunorubicin doses in protocol I. This treatment modification was not of disadvantage for “SR-95” girls, but an interaction with the duration of maintenance in the male subset cannot be fully excluded. A meta-analysis on the impact of duration of maintenance performed by the Childhood ALL Collaborative Group in 1996 revealed an overall slight benefit of a 3-year maintenance duration compared with 2 years.53 However, the results of the individual studies were not fully consistent,54-58 suggesting a variable impact in the context of different treatment regimens. Furthermore, the overall effectiveness of the intensive chemotherapy in those earlier trials is not necessarily comparable with the studies conducted approximately 10 years later, and the positive impact of longer maintenance may disappear with a more effective intensive chemotherapy phase.
The omission of pCRT in all MR patients with pB-ALL in ALL-BFM 95 affected approximately 50% of the total patient population. The pCRT was omitted without replacement by intensified intrathecal or systemic chemotherapy. The association of pCRT with secondary brain tumors as well as impairment of endocrinologic and neurocognitive functions has frequently been described in the literature.59-64 However, these trials refer to irradiation doses of 18 Gy and higher, and little is known about the impact of 12 Gy cranial irradiation with respect to CNS-related late effects. The updated results from ALL-BFM 90 showed a CI of brain tumors of 3.4% plus or minus 1.6% after 16 years among the patients who had received 12 Gy pCRT demonstrating that even with reduced doses secondary brain tumors still are a major concern of pCRT. Because of the long latency of these events, which in ALL-BFM 90 in median developed 9.8 years after primary ALL diagnosis, a final statement regarding this issue cannot yet be made for ALL-BFM 95. However, considering the strong association between pCRT and the development of brain tumors in ALL-BFM 90,60 it is likely that the incidence of brain tumors will be reduced through omission of pCRT in pB-ALL MR patients. There was a slightly yet significantly higher incidence of isolated CNS relapses and a trend to more combined CNS relapses in the nonirradiated patients from ALL-BFM 95 compared with the matching patients of ALL-BFM 90. The increase of CNS relapses with the omittance of pCRT either with or without replacement by intensified intrathecal treatment has been reported before by others.65-67 It is a dilemma to counterbalance the increase of (early occurring) CNS relapses in the patients treated without pCRT against the high incidence of secondary brain tumors after pCRT developing typically after a long interval of several years. The survival of the patients with brain tumor in ALL-BFM 90 was 0% after 3.5 years with a median survival of 1.2 years. Survival of the 19 patients with isolated CNS relapse in the analyzed subgroup in ALL-BFM 95 was 58% (SE 11%) at 6 years from relapse diagnosis. Considering the dismal prognosis of the secondary brain tumors and the other potential sequelae of CRT, we would conclude that the moderate increase of the isolated CNS relapses with favorable outcome in a considerable proportion of these patients is worth to avoid the potential burden of pCRT and may be overcome by treatment modifications, such as IT therapy in the maintenance phase.
In the MR group, the combined administration of HD-MTX and ID-cytarabine showed no advantage over HD-MTX alone. These results are in accordance with 2 other randomized studies, which tested the administration of ID-MTX or HD-MTX combined with HD-cytarabine in consolidation and could also show no benefit from additional cytarabine treatment.68,69 In these trials, the drugs were administered simultaneously68 or overlapping starting the cytarabine infusion at hour 12 of each ID-MTX infusion.69 In contrast, a sequential regimen was conducted in ALL-BFM 95 starting ID-cytarabine at the end of the 24-hour HD-MTX infusion. This was based on the results of in vitro studies demonstrating time schedule-dependent antagonistic or synergistic effects of this drug combination.70-72 However, the schedule of sequential administration as conducted in ALL-BFM 95 seems not to increase the cytostatic effectiveness of HD-MTX in vivo. Patients receiving MCA in median needed 1 more day to the subsequent element than the control group. This difference was statistically significant (P = .004) but, nevertheless, is rather unlikely to be clinically relevant with respect to pEFS. However, it may reflect the higher grade of hematologic toxicity and infections in protocol MCA (Table S4).
In trial ALL-BFM 90, the HR treatment with 9 rotational high-dose blocks yielded disappointing results, which were worse than in the previous trial ALL-BFM 86. Major differences between the HR treatments of the 2 trials comprised lower individual and cumulative doses of alkylating agents and the lack of a consolidation/reintensification element providing a continuous drug exposure in trial ALL-BFM 90. Thus, the HR treatment in ALL-BFM 95 was further modified mainly by higher dose intensity of alkylating agents in the blocks and by reintroduction of protocol II for late reintensification. This treatment strategy led to a significant improvement of pEFS compared with ALL-BFM 90 in patients with PPR and/or NRd33. However, no improvement could be achieved for patients with t(9;22). Despite the more intensive chemotherapy regimen in the ALL-BFM 95 HR group, no increase of the chemotherapy-related death rate was observed compared with trial ALL-BFM 90 (6y-CI ALL-BFM 90, 3.7% ± 1.6%, ALL-BFM 95 6y-CI, 3.5% ± 1.2%; P = .94). In addition, in other study groups, intensification of therapy for poor-risk patients have led to impressive improvements of outcome.21,66,73,74 However, the comparability with the ALL-BFM 95 HR therapy is hampered by the variety of risk stratification strategies in these trials leading to published results, which apply to distinct patient subgroups. Trial AIEOP-ALL 95 used similar HR stratification criteria as ALL-BFM 9574 enabling the comparison of the 2 HR therapies. Four-year pEFS was 56.5% plus or minus 3.9% in AIEOP compared with 51.4% plus or minus 3.1% in BFM; the only difference between the treatments was a second protocol II in AIEOP-ALL 95 substituting the fourth to sixth HR course of ALL-BFM 95. Whether the slightly better results in AIEOP are the result of the differences in age distribution (16.1% of the patients were ≥ 10 years in AIEOP vs 32.3% in BFM) or to treatment differences is not fully known.
The SR and MR risk stratification in ALL-BFM 90 was based mainly on the BFM-RF. With the aim to improve the discrimination between SR and MR and to allow an easier comparability with the results of other trials, these criteria were modified in ALL-BFM 95 substituting the BFM-RF by age and WBC. There are basic similarities to the NCI consensus criteria, but categories were defined differently based on the cutoff points that provided the best discrimination between risk groups in the previous studies and to identify a SR group with an pEFS of more than 90%. The new risk stratification resulted in an extensive redistribution of non-HR patients. According to the former risk criteria of ALL-BFM 90, 47% of the ALL-BFM 95 SR patients would have been treated in MR and 25% of MR patients in SR. Applying the 2 stratification strategies of ALL-BFM 90 and ALL-BFM 95 to the ALL-BFM 95 patient population confirmed that the ALL-BFM 95 risk criteria robustly discriminate 3 different risk groups with better separation compared with ALL-BFM 90 risk criteria.
Considerable progress could be achieved over the first 2 decades of running controlled treatment trials on childhood ALL. However, the better the results, the more difficult it becomes to achieve further significant improvement of the overall outcome. In trial ALL-BFM 95, an excellent outcome of nearly 90% pEFS for about one-third and 80% pEFS for about one-half of the total childhood ALL population was achieved. Nevertheless, more than 70% of the events occurred in these subsets. Further efforts will be necessary to establish sufficient methods to evaluate the individual relapse risk and to allow specific risk-adapted treatment intensity for all patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and families who participated in this trial, the physicians and nurses of all hospitals for their input in performing this study, and the study committee for productive discussions during the development and progress of the trial. The authors also thank E. Odenwald for expert cytology, N. Götz, D. Janousek, U. Meyer, I. Krämer, and K. Mischke for data management, B. Burkhardt for her careful reading the manuscript, J. Nordhausen for help in analysis of the randomization data, and the staff of the reference laboratories for continuous excellent cooperation.
This work was supported by grants from the Deutsche Krebshilfe, Bonn, Germany (50-2614-Ri 6; H.R.) and Madeleine-Schickedanz-Kinderkrebsstiftung, Fürth, Germany.
Authorship
Contribution: H.R., A.R., and H.G. helped in designing the study protocol, collecting and analyzing the data, and contributed patients to the study; M.D. helped in writing the study protocol, collecting the data, and contributed patients to the study; L.L., R.B., G.M., F.N., C.N., and T.K. helped in collecting the data and contributed patients to the study; J.B., G.H., K.W., A.F., J.-D.B., U.B., C.U., D.N., H.W., and F.Z. helped in designing the study protocol, collecting the data, and contributed patients to the study; M. Schrappe helped in designing the study protocol, collecting and analyzing the data, writing the manuscript, and contributed patients to the study; M. Stanulla and A.M. helped in collecting and analyzing the data, writing the manuscript, and contributed patients to the study; M.Z. helped in designing the study protocol, collecting and analyzing the data, and writing the manuscript; W.-D.L. was head of the immunologic reference laboratory and responsible for the immunophenotypic analyses; R.R. was responsible for the immunophenotypic analyses; J.H. was head of the oncogenetic reference laboratory and was responsible for the cytogenetic and molecular genetic analyses. All authors approved the final version of the manuscript.
A complete list of ALL-BFM 95 Study Committee, participating centers, and clinicians can be found in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Schrappe, University Hospital Schleswig-Holstein, Campus Kiel, Department of Pediatrics, Schwanenweg 20, 24105 Kiel, Germany; e-mail: m.schrappe@pediatrics.uni-kiel.de.

![Figure 5. Kaplan-Meier estimate and cumulative relapse incidences for evaluation of the impact of preventive cranial radiotherapy. The curves compare the matching patients from ALL-BFM 90 and 95 who received presymptomatic cranial radiotherapy in trial ALL-BFM 90 yet were not irradiated in ALL-BFM 95 (pB-ALL, aged 1 year or older, no initial CNS involvement, risk group MR-90). (A) Event-free survival (P[log-rank] = .280). (B) Cumulative incidence of systemic relapses with CNS involvement (P[Gray] = .270) and isolated CNS relapses (P[Gray] < .001). Isol. CNS rel. indicates isolated relapse in the central nervous system; comb. CNS rel., combined relapse involving the central nervous system; 6y-pEFS, probability of event-free survival at 6 years; 6y-CI, cumulative incidence of relapses at 6 years; SE, standard error.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/9/10.1182_blood-2007-09-112920/4/m_zh80090818580005.jpeg?Expires=1765884885&Signature=IK2ZNIGgS8xkTJ4Ak2MN5T0oQ7r7ZTxcMZfvheXTwpwy6cwEQclsq4djgp9HaptZvPYJ1grCi5mGIpi-B-pchSmeWpj9WcqdIbl4URNQA2jKHdTw4b5O8zc6ZJhkfAc5VTWt~SvKAuw4dfv8EewIjHagQeYG4FH-rKoTk9gIuBB41k103mgBnz9rbj8o6cTBgN~PV4gje4SVKSERGGHU6hwcSBhE6ikccjaZ-~T-tqulesMq-LaW~ZHGo5b~BfKYae~ZdbtGp04rakHrXFCL4WSP5nkBbZshsg~fkq85IfMklK4eS4yFtUwA9oSthqIWZYWkxeq4EcBnpZELHuvBAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal