Abstract
Lymphangiogenesis is induced by various growth factors, including VEGF-C. Although TGF-β plays crucial roles in angiogenesis, the roles of TGF-β signaling in lymphangiogenesis are unknown. We show here that TGF-β transduced signals in human dermal lymphatic microvascular endothelial cells (HDLECs) and inhibited the proliferation, cord formation, and migration toward VEGF-C of HDLECs. Expression of lymphatic endothelial cell (LEC) markers, including LYVE-1 and Prox1 in HDLECs, as well as early lymph vessel development in mouse embryonic stem cells in the presence of VEGF-A and C, were repressed by TGF-β but were induced by TGF-β type I receptor (TβR-I) inhibitor. Moreover, inhibition of endogenous TGF-β signaling by TβR-I inhibitor accelerated lymphangiogenesis in a mouse model of chronic peritonitis. Lymphangiogenesis was also induced by TβR-I inhibitor in the presence of VEGF-C in pancreatic adenocarcinoma xenograft models inoculated in nude mice. These findings suggest that TGF-β transduces signals in LECs and plays an important role in the regulation of lymphangiogenesis in vivo.
Introduction
Transforming growth factor-β (TGF-β) is a multifunctional cytokine, which regulates the growth, differentiation, migration, adhesion, and apoptosis of various types of cells. TGF-β binds to 2 different types of serine-threonine kinase receptors, known as type II (TβR-II) and type I (TβR-I).1-3 Upon ligand binding, TβR-II transphosphorylates TβR-I, which in turn transmits specific intracellular signals. The type I receptors phosphorylate receptor-activated Smads (R-Smads), and induce complex formation between R-Smads and common-partner Smad (co-Smad). The R-Smad/co-Smad complexes accumulate in the nucleus, where they regulate transcription of target genes, including plasminogen activator inhibitor-1 (PAI-1) and Smad7, through interaction with various transcription factors and transcriptional coactivators. Smad2 and Smad3 are R-Smads activated by TβR-I, whereas Smad4 is the only co-Smad in mammals shared with the TGF-β family signaling pathways. Smad7 is an inhibitory Smad, which interacts with activated TβR-I and interferes with the phosphorylation of R-Smads by TβR-I.
TGF-β inhibits the growth and migration of blood vascular endothelial cells in vitro, whereas it induces angiogenesis in vivo.4 Mice lacking certain components of TGF-β signaling (eg, TGF-β1, TβR-II, or TβR-I) exhibit abnormalities in blood vascular tissues.5-7 We have recently shown that inhibition of TGF-β signaling by low-dose TβR-I inhibitor decreases the coverage of endothelium by pericytes, promoting leakiness of tumor blood vessels.8 In tumor microenvironments, TGF-β signaling has also been reported to inhibit immune function and induce deposition of extracellular matrix proteins, inducing progression of tumors in advanced cancers.9 However, the relationship between TGF-β signaling and lymphangiogenesis has not been determined.
Various growth factors have been reported to be involved in lymphangiogenesis. These include vascular endothelial growth factor (VEGF)-C and D, hepatocyte growth factor, and angiopoetin-1 and -2.10-15 Neo-lymphangiogenesis has also been reported to be induced by receptor tyrosine kinases, for example, fibroblast growth factor receptor 3 (FGFR3) and platelet-derived growth factor receptor (PDGFR)-β.13,16 Among members of the VEGF family, VEGF-A transmits signals through the tyrosine kinase receptors VEGF receptor-2 (VEGFR2) and VEGFR1, which mediate signals that are required for vasculogenesis and hematopoiesis.17,18 VEGF-C and VEGF-D bind to VEGFR3 expressed on lymphatic vessels and mediate signals to LECs, although they also bind to VEGFR2.19 Analysis of Vegfc-null mice has revealed that VEGF-C is essential for normal development of the lymphatic vessels.20 Moreover, VEGF-C has been found to be expressed in various human cancers and to induce tumor metastasis through induction of angiogenesis and lymphangiogenesis.21,22 VEGF-D also promotes metastatic spread of tumors through induction of lymphangiogenesis.11
Among transcription factors, Prox1, a homeobox transcription factor, has been well known as an important regulator of lymphangiogenesis. During embryonic development, LECs arise by sprouting from the cardinal veins and migrate toward VEGF-C to form the primary lymphatic plexus.23 Prox1 is expressed in a subset of endothelial cells of the cardinal vein during embryonic development, and these cells sprout to form the primary lymphatic plexus.24,25 In Prox1-null mice, migration of LECs from the veins is arrested, leading to a complete absence of the lymphatic vasculature. Prox1 induces the expression of various LEC markers, including VEGFR3, LYVE-1, podoplanin, and integrin α9, and represses that of blood vascular endothelial cell markers (eg, VEGFR2 and VE-cadherin) in endothelial cells.26-28
In the present study, we show that TGF-β regulates the growth, migration, and cord formation of human dermal lymphatic microvascular endothelial cells (HDLECs) in vitro. In addition to inhibiting the proliferation and migration of HDLECs, TGF-β signaling suppressed the expression of LEC-related genes, including Prox1 and LYVE-1, in these cells. Moreover, inhibition of endogenous TGF-β signaling induced early lymph vessel development in mouse embryonic stem (ES) cells, and enhanced lymphangiogenesis in a mouse chronic peritonitis model and pancreatic cancer xenograft models. The present findings suggest that endogenous TGF-β signaling negatively regulates lymphangiogenesis in inflammatory tissues as well as in certain tumor tissues.
Methods
Cell culture and reagents
HDLECs were obtained from Cambrex (Walkersville, MD), and cultured in EGM2-MV medium containing endothelial cell growth supplements with 5% fetal bovine serum (FBS; Cambrex). The murine ES cell line R1 (a kind gift of A. Nagy, Mount Sinai Hospital, Toronto, ON)29 was cultured on mitomycin C-arrested mouse embryonic fibroblasts (Chemicon International, Temecula, CA) in stem cell medium (Knock-out DMEM, Invitrogen, Carlsbad, CA) supplemented with 15% FBS, 2 mM of l-glutamine (Invitrogen), 0.1 mM of 2-mercaptoethanol (Invitrogen), 0.1 mM of MEM nonessential amino-acids (Invitrogen), 50 U/mL of penicillin-streptomycin (Invitrogen), and 1000 U/mL of leukemia inhibitory factor (Chemicon International), and passaged every 48 hours. Inflammatory macrophages were collected from ascites fluid in BALB/c mice 4 days after induction of peritonitis by intraperitoneal injection of thioglycollate (enriched thioglycollate medium, 2 mL; BD Biosciences, Franklin Lakes, NJ). BxPC3 and MIA PaCa-2 human pancreatic adenocarcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). BxPC3 cells were grown in RPMI 1640 supplemented with 10% FBS. MIA PaCa-2 cells were grown in DMEM with 10% FBS. Overexpression of VEGF-C in BxPC3 cells and that of TGF-β1 in MIA PaCa-2 cells were done using a lentiviral infection system (a kind gift from H. Miyoshi, RIKEN, Tsukuba, Japan). cDNA encoding VEGF-C,30 active form of TGF-β1,31 or green fluorescent protein (GFP) was inserted into the multicloning site of the lentiviral vector construct, pCSII-CMV-RfA, using pENTR according to standard protocol (Invitrogen). TβR-I inhibitors LY364947 and SB431542 were purchased from Calbiochem (La Jolla, CA) and Sigma-Aldrich (St Louis, MO), respectively. LY364947 was used as a TβR-I inhibitor, unless specifically described. TGF-β1 and TGF-β3 were purchased from R&D Systems (Minneapolis, MN). Cycloheximide was from Sigma-Aldrich. VEGF-A and VEGF-C were purchased from R&D Systems and Calbiochem, respectively.
Antibodies
Antibodies to LYVE-1, Prox1, and podoplanin were purchased from Abcam (Cambridge, United Kingdom), Chemicon International, and Research Diagnostic (Flanders, NJ), respectively. PECAM1 antibody and Mac-1 antibody were from BD PharMingen (Franklin Lakes, NJ). Rat anti–mouse LYVE-1 antibody was a kind gift from Y. Oike and T. Suda (Keio University, Tokyo, Japan). Antibody to phospho-Smad2 was a kind gift from A. Moustakas and C.-H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). Antibodies to Smad2/3 and tubulin were from BD Transduction Laboratories (Franklin Lakes, NJ) and Sigma-Aldrich, respectively. Alexa488- and Alexa594-conjugated secondary antibodies and TOTO-3 were purchased from Invitrogen.
Immunoblotting
Cultured cells were lysed in a buffer containing 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40 (Nacalai Tesque, Kyoto, Japan), 5 mM EDTA, 0.5% deoxycholic acid sodium salt-monohydrate (Nacalai Tesque), 0.1% sodium dodecyl sulfate (SDS, Nacalai Tesque), 1% aprotinin (Mitsubishi Pharma, Osaka, Japan), and 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). The cell lysates were boiled in SDS sample buffer (100 mM Tris-HCl, pH 8.8, 0.01% bromophenol blue, 36% glycerol, 4% SDS, 10 mM dithiothreitol) and subjected to SDS-PAGE. Proteins were electrotransferred to PALL FLUOROTRANS W membranes (PALL, East Hills, NY), immunoblotted with antibodies, and detected using an ECL detection system (GE Healthcare, Little Chalfont, United Kingdom).
Immunostaining
Cultured cells were fixed in iced 1:1 acetone-methanol solution and incubated with antibody to Prox1 overnight at 4°C. Subsequently, cells were incubated with Alexa488-conjugated secondary antibodies (Invitrogen) for 1 hour at room temperature and stained with TOTO-3 for nuclear staining. Frozen sections were briefly fixed with Mildform 10N (WAKO, Osaka, Japan), and incubated with anti-LYVE-1, anti-Prox1, or anti-podoplanin antibodies. Subsequently, samples were incubated with secondary antibodies, and stained with TOTO-3 for nuclear staining. Stained cells and frozen sections were observed with a confocal microscope (Model LSM510 META; Carl Zeiss MicroImaging, Thornwood, NJ). Images were imported into Adobe Photoshop and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Enzyme-linked immunosorbent assay
Expression levels of TGF-β1 protein were determined using TGF-β1 human ELISA kit Quantikine 2nd Generation (R&D Systems), according to manufacturer's protocol. TGF-β3 at 1 ng/mL was used as the exogenous TGF-β ligand to avoid complication in detection of TGF-β1 by ELISA. Induction of TGF-β1 in HDLECs was similar between TGF-β1 and TGF-β3 at 1 ng/mL.
RNA isolation and quantitative RT-PCR
Total RNAs from HDLECs were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNAs were synthesized using the Quantitect Reverse Transcription kit (Qiagen) with random hexamer primers. Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems). The primer sequences used were as follows: human GAPDH: forward 5′-GAAGGTGAAGGTCGGAGTC-3′, reverse 5′-GAAGATGGTGATGGGATTTC-3′, human Prox1: forward 5′-CCCAGGACAGTTTATTGACCG-3′, reverse 5′-GGTTGTAAGGAGTTTGGCCCA-3′, human LYVE-1: forward 5′-AGCCTGGTGTTGCTTCTCACT-3′, reverse 5′-GGTTCGCCTTTTTGCTCACA-3′, human Smad7: forward 5′-CCTTAGCCGACTCTGCGAACTA-3′, reverse 5′-CCAGATAATTCGTTCCCCCTGT-3′, human PAI-1: forward 5′-GGCTGACTTCACGAGTCTTTC-3′, reverse 5′-GCGGGCTGAGACTATGACA-3′, human TGF-β1: forward 5′-AGTGGACATCAACGGGTTCAG-3′, reverse 5′-CATGAGAAGCAGGAAAGGCC-3′, human TGF-β2: forward 5′-CTGTCCCTGCTGCACTTTTGTA-3′, reverse 5′-TGTGGAGGTGCCATCAATACCT-3′, human TGF-β3: forward 5′-TGGAAGTGGGTCCATGAAACCTA-3′, reverse 5′-GATGCTTCAGGGTTAAGAGTGTTG-3′, and human/mouse VEGF-C: forward 5′-TTCCTGCCGATGCATGTCTAA-3′, reverse 5′-TGTTCGCTGCCTGACACTGT-3′.
Cell growth and cord formation assays
HDLECs (2 × 103 cells) were seeded in 96-well plates, and cell growth was quantified for 2 days by WST-1 assay (Nacalai Tesque) according to manufacturer's protocol. Formation of cord-like structures was determined in 3-dimensional gel assays, where 104 cells were mixed with type I-A collagen gel (Nitta Gelatin, Osaka, Japan) and seeded onto culture slide. The formation of cord-like structures was examined using video microscopy for 3 days. At the end of observation, the lengths of the cord-like structures were quantified in the 3-dimensional assay as follows: 4 microscopic fields with 5 Z-axis planes in each condition were photographed, and the total lengths of cord-like structures were quantified as the mean of the sum of the lengths in the 5 planes in each of the 4 microscopic fields. Long-running video microscopic analysis of living cells on the 8-well culture slides (BD Falcon) was performed using a Leica (Deerfield, IL) DM IRB microscope equipped with a hardware-controlled motor stage. The video images were analyzed using ImageJ software. For quantification of images, 8 to 10 fields were evaluated for each mouse.
Cell migration assay
Migration of HDLECs was determined using a Boyden chamber (8 μm pore size, BD Biosciences) with type I collagen coat. HDLECs (5 × 104) were seeded in serum-free medium containing 0.2% BSA in the upper chamber. VEGF-C (50 ng/mL) was added as the chemoattractant to the lower chamber, while TGF-β1 at 1 ng/mL or TβR-I inhibitor at 3 μM was added to the upper chamber. After a 6-hour incubation, cells in the upper chamber were carefully removed using cotton buds and cells at the bottom of the membrane were fixed and stained with 0.5% crystal violet in 20% methanol. Quantification was performed by counting the stained cells in triplicate.
Three-dimensional collagen assays of mouse ES cells
Three-dimensional collagen assays using mouse ES cells were performed as described previously.32,33 Briefly, R1 ES cells were trypsinized, resuspended in stem cell medium without leukemia inhibitory factor supplemented with 30 ng/mL VEGF-A at day 0. The cells were then cultured in drops hanging. After 4 days, when ES cells aggregated to form embryoid bodies, drops were collected and embryoid bodies were seeded on a layer of solidified collagen type I solution (Nitta Gelatin), and a second layer of collagen solution was added on top. After 3 hours, medium with or without 30 ng/mL VEGF-A and 30 ng/mL VEGF-C was added. Medium containing VEGF-A and C was replaced every second day. TβR-I inhibitor at 3 μM or TGF-β1 at 1 ng/mL was added every second day for one week, started from one week after seeding. After culturing for 2 weeks, whole-mount samples were incubated with anti-LYVE-1, anti-PECAM1, anti-Prox1, and Alexa-conjugated secondary antibodies overnight. Samples were examined using a Zeiss LSM510 Meta confocal microscope for immunohistochemistry. Quantification of LYVE-1 stained areas was performed in 5 fields on 3 embryoid bodies.
Chronic peritonitis model
BALB/c mice of 4 to 5 weeks of age were obtained from Sankyo Laboratory (Tokyo, Japan). All animal experimental protocols were performed in accordance with the policies of the Animal Ethics Committee of the University of Tokyo. Chronic peritonitis was induced by 5% thioglycollate. Thioglycollate (2 mL) and TβR-I inhibitor (LY364947, 1 mg/kg) were administered intraperitoneally to BALB/c mice 3 times a week. After injections of these reagents into mice for 2 weeks, the mice were killed and the diaphragms were excised, fixed with Mildform 10N for 1 hour at room temperature and washed with sucrose buffer (dissolved in phosphate-buffered saline [PBS]). Whole-mount samples were subsequently incubated with anti-LYVE-1, anti-Mac-1, and Alexa594-conjugated secondary antibodies overnight. Samples were examined using a Zeiss LSM510 Meta confocal microscope for immunohistochemistry.
Cancer xenograft models
BALB/c nude mice 5 to 6 weeks of age were obtained from CLEA Japan (Tokyo, Japan) and Sankyo Laboratory. Parental, or VEGF-C- or TGF-β1-expressing tumor cells (5 × 106) in 100 μL PBS were implanted subcutaneously into male nude mice and allowed to grow for 2 to 3 weeks to reach proliferative phase, before initiation of TβR-I inhibitor administration. TβR-I inhibitor LY364947, dissolved in 5 mg/mL in DMSO and diluted with 100 μL PBS, or the vehicle control, was injected intraperitoneally at 1 mg/kg, 3 times a week for 3 weeks. Excised samples were directly frozen in dry-iced acetone for immunohistochemistry. Frozen samples were further sectioned at 10-μm thickness in a cryostat and subsequently incubated with primary and secondary antibodies as described above. Samples were observed using a confocal microscope.
Results
TGF-β transduces signals in HDLECs
To study the effects of TGF-β on lymphangiogenesis, we first examined whether TGF-β transduces signals in HDLECs.34 Specific small molecule inhibitors of TGF-β family type I receptor kinases ALK-4, -5, and -7 (TβR-I inhibitors, LY364947 and SB43154235,36 ) were used to suppress endogenous TGF-β family signaling. Immunoblot analysis using phospho-Smad2 antibody revealed weak phosphorylation of Smad2 in untreated HDLECs, and that 1 ng/mL of TGF-β1 enhanced the phosphorylation of Smad2 in these cells (Figure 1A). In contrast, 3 μM of LY364947 and SB431542 decreased the basal and TGF-β1-induced Smad2 phosphorylation.
Transduction of TGF-β signals in HDLECs. (A) Immunoblotting of phospho-Smad2 after TGF-β or TβR-I inhibitor treatment. HDLECs were treated with TGF-β1 or with 2 kinds of TβR-I inhibitors (LY364947 or SB431542, shown as Inhib1 or Inhib2, respectively) in the presence and absence of TGF-β1 for 1 hour, and subjected to immunoblot analysis using phospho-Smad2 antibody (top panel) and Smad2/3 antibody (bottom panel). Ctrl indicates control. (B,C) Real-time PCR of Smad7 and PAI-1. HDLECs were treated as in panel A, and expression of Smad7 and PAI-1 mRNAs was determined at 1 hour and 24 hours after stimulation, respectively (***P < .001). (D) Regulation of growth of HDLECs by TGF-β and TβR-I inhibitor. HDLECs were seeded at a density of 2 × 103 cells/well in 96-well plates, and cells were treated or not with TGF-β1 (1 ng/mL) or LY364947 (3 μM). Photographs of the cells were taken at day 2. Cell numbers were determined by WST assay in triplicate at day 2. Error bars represent standard deviations (*P < .05, **P < .01). (E,F) Autocrine TGF-β signaling in HDLECs. Expression of TGF-β1 mRNA in HDLECs treated with TGF-β1 (1 ng/mL) or TβR-I inhibitors (3 μM) as in panel A was determined by real-time PCR (E). Production of TGF-β1 protein by HDLECs treated as in panel A, but with TGF-β3 (1 ng/mL) as the stimulant, was examined in conditioned medium using an ELISA kit (F). LY364947 was used as TβR-I inhibitor (Inhib). HUVECs were used as a control. Error bars represent standard deviations (*P < .05, **P < .01, ***P < .001).
Transduction of TGF-β signals in HDLECs. (A) Immunoblotting of phospho-Smad2 after TGF-β or TβR-I inhibitor treatment. HDLECs were treated with TGF-β1 or with 2 kinds of TβR-I inhibitors (LY364947 or SB431542, shown as Inhib1 or Inhib2, respectively) in the presence and absence of TGF-β1 for 1 hour, and subjected to immunoblot analysis using phospho-Smad2 antibody (top panel) and Smad2/3 antibody (bottom panel). Ctrl indicates control. (B,C) Real-time PCR of Smad7 and PAI-1. HDLECs were treated as in panel A, and expression of Smad7 and PAI-1 mRNAs was determined at 1 hour and 24 hours after stimulation, respectively (***P < .001). (D) Regulation of growth of HDLECs by TGF-β and TβR-I inhibitor. HDLECs were seeded at a density of 2 × 103 cells/well in 96-well plates, and cells were treated or not with TGF-β1 (1 ng/mL) or LY364947 (3 μM). Photographs of the cells were taken at day 2. Cell numbers were determined by WST assay in triplicate at day 2. Error bars represent standard deviations (*P < .05, **P < .01). (E,F) Autocrine TGF-β signaling in HDLECs. Expression of TGF-β1 mRNA in HDLECs treated with TGF-β1 (1 ng/mL) or TβR-I inhibitors (3 μM) as in panel A was determined by real-time PCR (E). Production of TGF-β1 protein by HDLECs treated as in panel A, but with TGF-β3 (1 ng/mL) as the stimulant, was examined in conditioned medium using an ELISA kit (F). LY364947 was used as TβR-I inhibitor (Inhib). HUVECs were used as a control. Error bars represent standard deviations (*P < .05, **P < .01, ***P < .001).
The transcription induced by TGF-β in HDLECs was further examined by quantitative real-time (RT)-PCR analysis. TGF-β1 induced transcription of SMAD7 and PAI1, and the TβR-I inhibitors strongly suppressed their transcription (Figure 1B,C), suggesting that TGF-β transduces signals in HDLECs and TβR-I inhibitors suppress the signals induced by endogenous TGF-β. We also examined the effects of TGF-β1 (1 ng/mL) and LY364947 and SB431542 (3 μM) on proliferation of HDLECs. TGF-β1 suppressed the growth of HDLECs, whereas TβR-I inhibitors enhanced their proliferation in the presence and absence of TGF-β1 (Figure 1D and data not shown). These findings indicate that HDLECs respond to TGF-β signals similarly to human umbilical vein endothelial cells (HUVECs; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Next, production of TGF-β by HDLECs was examined at mRNA and protein levels. TGF-β induced the expression of TGF-β1 mRNA, whereas the TβR-I inhibitors suppressed it in the presence and absence of TGF-β1 (Figure 1E). Expression of TGF-β2 or TGF-β3 mRNA was not significantly suppressed by the TβR-I inhibitors (Figure S2). Moreover, ELISA revealed that HDLECs produce TGF-β1 protein at a level similar to that produced by HUVECs, and the secretion of TGF-β1 was stimulated and suppressed by exogenous TGF-β and TβR-I inhibitor, respectively (Figure 1F), suggesting the presence of autocrine loop of TGF-β1 signaling in HDLECs.
TGF-β induces expression of LEC markers in HDLECs
We next investigated whether TGF-β signaling regulates the expression of LEC markers in HDLECs. We recently reported that VEGFR3 signaling induces the expression of LYVE-1, but not other LEC markers, in embryonic stem cell (ESC)–derived endothelial cells.30 In contrast, the homeobox transcription factor Prox1 induces expression of most LEC markers in ESC-derived endothelial cells.28
We examined the expression of Prox1 protein in HDLECs by immunocytochemistry and immunoblotting (Figure 2A,B). Prox1 was observed in the nuclei of most of the untreated HDLECs, although expression levels of Prox1 in them varied with some cells displaying very weak or no significant staining by Prox1 antibody (Figure 2A). When the cells were treated with TGF-β1 for 24 hours, expression of Prox1 was strongly suppressed, and the number of cells with weak or no staining of Prox1 had increased. In contrast, TβR-I inhibitor LY364947 induced expression of Prox1 in almost all HDLECs after 24 hours. Immunoblot analysis using anti-Prox1 antibody confirmed the results of immunocytochemistry (Figure 2B).
TGF-β signaling regulates the expression of LEC-related genes in HDLECs. (A) Expression levels of Prox1 determined by immunostaining in HDLECs. HDLECs were untreated (left) or treated with TGF-β1 (1 ng/mL; middle) or TβR-I inhibitor LY364947 (3 μM; right) for 24 hours and subjected to immunocytochemical examination. Bars represent 50 μm. (B) Expression levels of Prox1 in HDLECs treated as described in panel A were determined by immunoblotting. α-Tubulin levels were monitored as a loading control for whole-cell extracts. (C-E) Expression levels of Prox1, LYVE-1, and PAI-1 mRNAs were analyzed by real-time PCR at 24 hours after TGF-β1 or TβR-I inhibitor treatment. In the right 3 columns, cells were treated with 1 μM cycloheximide (CHX) for 24 hours before they were treated with TGF-β1 or TβR-I inhibitor for 24 hours. Values were normalized to amounts of GAPDH mRNA. Error bars represent SD (*P < .05, **P < .01, ***P < .001).
TGF-β signaling regulates the expression of LEC-related genes in HDLECs. (A) Expression levels of Prox1 determined by immunostaining in HDLECs. HDLECs were untreated (left) or treated with TGF-β1 (1 ng/mL; middle) or TβR-I inhibitor LY364947 (3 μM; right) for 24 hours and subjected to immunocytochemical examination. Bars represent 50 μm. (B) Expression levels of Prox1 in HDLECs treated as described in panel A were determined by immunoblotting. α-Tubulin levels were monitored as a loading control for whole-cell extracts. (C-E) Expression levels of Prox1, LYVE-1, and PAI-1 mRNAs were analyzed by real-time PCR at 24 hours after TGF-β1 or TβR-I inhibitor treatment. In the right 3 columns, cells were treated with 1 μM cycloheximide (CHX) for 24 hours before they were treated with TGF-β1 or TβR-I inhibitor for 24 hours. Values were normalized to amounts of GAPDH mRNA. Error bars represent SD (*P < .05, **P < .01, ***P < .001).
Regulation of Prox1 expression as well as that of LYVE-1 by TGF-β signaling was further examined at the mRNA level. Total RNA was isolated from HDLECs treated with or without TGF-β1 or LY364947 for 24 hours, and levels of expression of Prox1 and LYVE-1 mRNAs were determined by quantitative RT-PCR (Figure 2C,D). TGF-β1 suppressed the expression of Prox1 and LYVE-1, whereas LY364947 strongly induced their expression. Similar results were obtained by another TβR-I inhibitor SB431542 (Figure S3A,B). The induction of Prox1 and LYVE-1 by TβR-I inhibitor was abolished by suppression of protein synthesis with cycloheximide treatment of HDLECs, whereas that of PAI-1 was not suppressed (Figure 2C-E). These findings suggest that TGF-β signaling indirectly regulates the expression of Prox1 and LYVE-1 in lymphatic endothelial cells. In contrast, Prox1 was not induced by TβR-I inhibitor in HUVECs, although LYVE-1 was up-regulated (Figure S3C,D).
Induction of cord formation and migration of HDLECs by TGF-β signaling
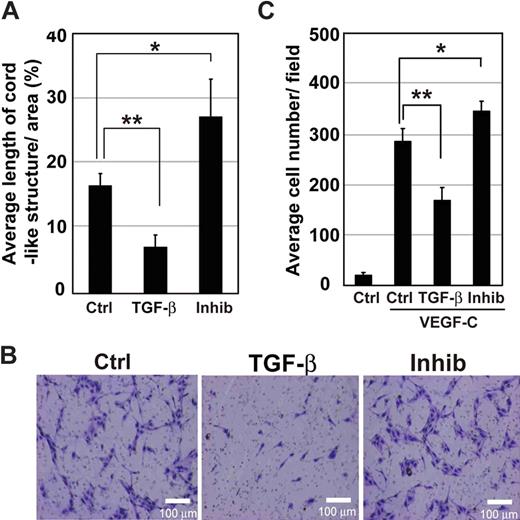
We examined the formation of cord-like structures by HDLECs in three-dimensional type I collagen gels. Quantification of the total length of cord-like structures after 3 days of cultivation confirmed significant decrease and increase in cord formation of HDLECs by treatment with TGF-β1 and TβR-I inhibitor, respectively (Figure 3A).
Formation of cord-like structures and migration of HDLECs are increased by inhibition of TGF-β signaling. (A) Total lengths of cord-like structures of HDLECs in three-dimensional culture were quantified. Cells were mixed with type I collagen gel at a density of 104 cells/well and seeded onto culture-slide wells. HDLECs were treated with TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM). Formation of cord-like structures was observed by video microscopy, and total lengths of cord-like structures of cells were quantified 3 days after cultivation. Error bars represent SD (*P < .05, **P < .01). Effects of TGF-β signaling on migration of HDLECs were determined by Boyden chamber assay. Cells were seeded at 4 × 104 cells/well in the upper chambers coated with type I collagen. (B) Medium containing VEGF-C (50 ng/mL) was placed in the lower chamber, whereas that in the upper chamber did not contain VEGF-C but did contain TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM). Migration of cells was determined after 6 hours. Bars represent 100 μm. (C) Migration of HDLECs was quantified. Cells that had migrated to the lower chambers were counted after 6 hours in triplicate. Error bars represent SD (*P < .05, **P < .01).
Formation of cord-like structures and migration of HDLECs are increased by inhibition of TGF-β signaling. (A) Total lengths of cord-like structures of HDLECs in three-dimensional culture were quantified. Cells were mixed with type I collagen gel at a density of 104 cells/well and seeded onto culture-slide wells. HDLECs were treated with TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM). Formation of cord-like structures was observed by video microscopy, and total lengths of cord-like structures of cells were quantified 3 days after cultivation. Error bars represent SD (*P < .05, **P < .01). Effects of TGF-β signaling on migration of HDLECs were determined by Boyden chamber assay. Cells were seeded at 4 × 104 cells/well in the upper chambers coated with type I collagen. (B) Medium containing VEGF-C (50 ng/mL) was placed in the lower chamber, whereas that in the upper chamber did not contain VEGF-C but did contain TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM). Migration of cells was determined after 6 hours. Bars represent 100 μm. (C) Migration of HDLECs was quantified. Cells that had migrated to the lower chambers were counted after 6 hours in triplicate. Error bars represent SD (*P < .05, **P < .01).
Next, migration of HDLECs was examined by Boyden chamber assay. HDLECs were treated or not with TGF-β1 or TβR-I inhibitor LY364947, and their migration toward VEGF-C (50 ng/mL) was determined after 6-hour incubation. In the absence of VEGF-C, migration of HDLECs was not strongly induced, and there was no significant difference in migration between untreated cells and those treated with TGF-β1 or TβR-I inhibitor (data not shown). VEGF-C induced the migration of HDLECs, which was strongly suppressed by TGF-β1, whereas TβR-I inhibitor weakly enhanced it (Figure 3B,C).
Induction of early lymph vessel development by TβR-I inhibitor in ES cells
To determine whether TGF-β signaling regulates lymphatic vessel development, mouse R1 ES cells were aggregated to form embryoid bodies, and cultured in three-dimensional collagen in the presence of VEGF-A and -C. Although VEGF-A and -C induced formation of lymphatic vessel structures as previously reported,32,33 addition of TβR-I inhibitor LY364947 further induced the production of a network of LYVE-1-positive lymphatic vessel-like structures, whereas addition of TGF-β1 reduced the production of these structures (Figure 4A,B). Immunostaining of Prox1 in PECAM1-positive areas also increased with TβR-I inhibitor and decreased with TGF-β1 (Figure 4C), indicating that the effects of TGF-β signaling on lymphatics are not limited to HDLECs.
Enhancement of early lymph vessel development in ES cells cultured in 3-dimensional collagen by inhibition of TGF-β signaling. Mouse R1 ES cells were cultured in collagen gel with VEGF-A (30 ng/mL) for the first 4 days and subsequently with VEGF-C (30 ng/mL) and VEGF-A (30 ng/mL) for 14 days to form embryoid bodies exhibiting early lymph vessel formation. The embryoid bodies were also treated with TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM) for the last 7 days. (A,B) The embryoid bodies were stained by PECAM1 (red) and LYVE-1 (green) (A). Bars represent 50 μm. Quantification of LYVE-1–stained areas was performed in 5 low-magnification microscopic fields on 3 embryoid bodies (B). Error bars represent SD (***P < .001). (C) Immunostaining for PECAM1 (red) and Prox1 (green) confirmed the effect of modulation of TGF-β signaling on expression of Prox1 in PECAM1-positive structures.
Enhancement of early lymph vessel development in ES cells cultured in 3-dimensional collagen by inhibition of TGF-β signaling. Mouse R1 ES cells were cultured in collagen gel with VEGF-A (30 ng/mL) for the first 4 days and subsequently with VEGF-C (30 ng/mL) and VEGF-A (30 ng/mL) for 14 days to form embryoid bodies exhibiting early lymph vessel formation. The embryoid bodies were also treated with TGF-β1 (1 ng/mL) or TβR-I inhibitor (3 μM) for the last 7 days. (A,B) The embryoid bodies were stained by PECAM1 (red) and LYVE-1 (green) (A). Bars represent 50 μm. Quantification of LYVE-1–stained areas was performed in 5 low-magnification microscopic fields on 3 embryoid bodies (B). Error bars represent SD (***P < .001). (C) Immunostaining for PECAM1 (red) and Prox1 (green) confirmed the effect of modulation of TGF-β signaling on expression of Prox1 in PECAM1-positive structures.
Inhibition of endogenous TGF-β signaling accelerates lymphangiogenesis in a mouse model of chronic peritonitis
We next examined whether inhibition of TGF-β signaling regulates lymphangiogenesis in vivo. Because chronic inflammation is reported to induce lymphangiogenesis, possibly through production of VEGF-C by F4/80-positive macrophages,37 we induced chronic peritonitis in mice as a model of lymphangiogenesis. Formation of inflammatory plaques containing lymphatic vessels and macrophages was induced on the peritoneal side of the diaphragm by intraperitoneal injection of thioglycollate in BALB/c mice. Mice were injected intraperitoneally with 2 mL of 5% thioglycollate and TβR-I inhibitor LY364947 (1 mg/kg) 3 times a week for 2 weeks. Lymphangiogenesis in the plaques of the diaphragms was then examined by immunostaining using LYVE-1 antibody. Formation of lymphatic plaques was observed after 2 weeks in the mice treated with thioglycollate, and TβR-I inhibitor significantly increased the LYVE-1-positive areas in the plaques (Figure 5A,B).
Enhancement of lymphangiogenesis by TβR-I inhibitor in a mouse model of chronic peritonitis. Mice were treated with 5% thioglycollate (2 mL) and TβR-I inhibitor (LY364947, 1 mg/kg) 3 times a week for 2 weeks. Their diaphragms were then examined for lymphangiogenesis in plaques. (A) LYVE-1 immunostaining (shown in red) of diaphragms treated without (control, left panels) or with TβR-I inhibitor (right panels). Sections were also stained for Mac1 (green) (bottom panels). Bars represent 50 μm. (B) Quantification of LYVE-1-positive area in plaques of the diaphragms treated without (control) or with TβR-I inhibitor (n = 3 for each group). Error bars represent SE (***P < .001). (C) Expression of VEGF-C in inflammatory macrophages in the presence and absence of TβR-I inhibitor. Inflammatory macrophages were harvested from ascites fluid of mice 4 days after induction of peritonitis by intraperitoneal injection of thioglycollate, seeded at 106, and treated with or without TβR-I inhibitor for 24 hours. Error bars represent SD (*P < .05).
Enhancement of lymphangiogenesis by TβR-I inhibitor in a mouse model of chronic peritonitis. Mice were treated with 5% thioglycollate (2 mL) and TβR-I inhibitor (LY364947, 1 mg/kg) 3 times a week for 2 weeks. Their diaphragms were then examined for lymphangiogenesis in plaques. (A) LYVE-1 immunostaining (shown in red) of diaphragms treated without (control, left panels) or with TβR-I inhibitor (right panels). Sections were also stained for Mac1 (green) (bottom panels). Bars represent 50 μm. (B) Quantification of LYVE-1-positive area in plaques of the diaphragms treated without (control) or with TβR-I inhibitor (n = 3 for each group). Error bars represent SE (***P < .001). (C) Expression of VEGF-C in inflammatory macrophages in the presence and absence of TβR-I inhibitor. Inflammatory macrophages were harvested from ascites fluid of mice 4 days after induction of peritonitis by intraperitoneal injection of thioglycollate, seeded at 106, and treated with or without TβR-I inhibitor for 24 hours. Error bars represent SD (*P < .05).
Because macrophages have been suggested to be the major sources of lymphangiogenic growth factors, including VEGF-C, we obtained peritoneal macrophages from thioglycollate-treated mice and determined the production of VEGF-C by quantitative RT-PCR. As shown in Figure 5C, production of VEGF-C was not induced by TβR-I inhibitor, suggesting that the observed effect of TβR-I inhibitor on lymphangiogenesis may be primarily induced by its direct action on LECs.
Induction of lymphangiogenesis by TβR-I inhibitor in animal models of cancer
Because TβR-I inhibitor induced growth, migration, and cord formation of LECs in vitro and lymphangiogenesis in a mouse model of chronic peritonitis in vivo, we examined whether it induces lymphangiogenesis in tumor xenograft models using BxPC3 and MIA PaCa-2 pancreatic adenocarcinoma cells. Pancreatic adenocarcinoma cells were inoculated subcutaneously into BALB/c nude mice. The mice were then injected with 1 mg/kg TβR-I inhibitor LY364947 3 times a week for 3 weeks.
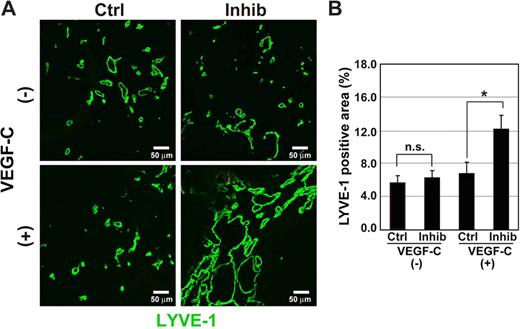
When the BxPC3 cells were mixed with or without 1 μg/mL VEGF-C and inoculated into nude mice, we found that the number of LECs stained by LYVE-1 antibody was slightly increased in the presence of VEGF-C. Interestingly, TβR-I inhibitor significantly increased the LYVE-1-positive areas in tumor tissues in the presence of VEGF-C (Figure 6A,B). Z-stack analysis of tumor tissues by a confocal microscope revealed that the LYVE-1-positive cells tended to form tube-like structures in the tumor tissues (data not shown). To confirm that the LYVE-1-positive cells were indeed LECs, tumor tissues treated with VEGF-C and TβR-I inhibitor were stained with Prox1 and podoplanin antibodies. The LYVE-1–postive cells were costained by Prox1 and podoplanin antibodies (Figure 6C), whereas most PECAM1-positive cells were not stained by LYVE-1 or Prox1 antibodies (Figure 6D). The LYVE-1–positive cells in the BxPC3 xenografts were thus LECs.
Lymphangiogenesis is increased by TβR-I inhibitor in pancreatic adenocarcinoma BxPC3 xenograft models. (A-D) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using a human pancreatic cancer cell line, BxPC3. BxPC3 cells mixed with or without VEGF-C (1 μg/mL) were subcutaneously inoculated in BALB/c nude mice. After tumors had formed, the mice were injected intraperitoneally with TβR-I inhibitor (LY364947, 1 mg/kg) 3 times a week for 3 weeks. They were killed at the end of the experiment, and excised tumors were examined histologically. (A) Immunostaining of BxPC3 xenograft sections by LYVE-1 antibody (shown in green). Bars represent 50 μm. (B) LYVE-1–positive areas in the BxPC3 xenograft sections were determined in the presence and absence of VEGF-C and TβR-I inhibitor (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (*P < .05). (C) Immunostaining of BxPC3 xenograft sections for Prox1 (left panel, red), podoplanin (right panel, red), and LYVE-1 (green). Bars represent 20 μm. (D) Immunostaining of BxPC3 xenograft sections for PECAM1 (red), LYVE-1 (left panel, green), Prox1 (right panel, green), and TOTO-3 (blue). Bars represent 100 μm. (E,F) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using BxPC3 cells overexpressing VEGF-C by infection of the VEGF-C-lentivirus. BxPC3 cells infected with a lentivirus containing GFP were used as a control. (E) Up-regulation of VEGF-C mRNA in BxPC3 cells after infection of the VEGF-C-lentivirus was determined by real-time PCR. (F) LYVE-1-positive areas in the GFP- and VEGF-C–expressing BxPC3 xenograft sections treated with or without TβR-I inhibitor were determined (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (***P < .001).
Lymphangiogenesis is increased by TβR-I inhibitor in pancreatic adenocarcinoma BxPC3 xenograft models. (A-D) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using a human pancreatic cancer cell line, BxPC3. BxPC3 cells mixed with or without VEGF-C (1 μg/mL) were subcutaneously inoculated in BALB/c nude mice. After tumors had formed, the mice were injected intraperitoneally with TβR-I inhibitor (LY364947, 1 mg/kg) 3 times a week for 3 weeks. They were killed at the end of the experiment, and excised tumors were examined histologically. (A) Immunostaining of BxPC3 xenograft sections by LYVE-1 antibody (shown in green). Bars represent 50 μm. (B) LYVE-1–positive areas in the BxPC3 xenograft sections were determined in the presence and absence of VEGF-C and TβR-I inhibitor (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (*P < .05). (C) Immunostaining of BxPC3 xenograft sections for Prox1 (left panel, red), podoplanin (right panel, red), and LYVE-1 (green). Bars represent 20 μm. (D) Immunostaining of BxPC3 xenograft sections for PECAM1 (red), LYVE-1 (left panel, green), Prox1 (right panel, green), and TOTO-3 (blue). Bars represent 100 μm. (E,F) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using BxPC3 cells overexpressing VEGF-C by infection of the VEGF-C-lentivirus. BxPC3 cells infected with a lentivirus containing GFP were used as a control. (E) Up-regulation of VEGF-C mRNA in BxPC3 cells after infection of the VEGF-C-lentivirus was determined by real-time PCR. (F) LYVE-1-positive areas in the GFP- and VEGF-C–expressing BxPC3 xenograft sections treated with or without TβR-I inhibitor were determined (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (***P < .001).
Similar experiments were conducted using BxPC3 cells overexpressing VEGF-C. BxPC3 cells were infected with a lentivirus containing Vegfc, and an increase in the production of VEGF-C mRNA by the VEGF-C-lentivirus-infected cells was confirmed by quantitative RT-PCR (Figure 6E). Similar to the addition of VEGF-C protein, TβR-I inhibitor significantly increased the LYVE-1–positive areas in BxPC3 tumor tissues expressing VEGF-C (Figure 6F).
The effects of TβR-I inhibitor on lymphangiogenesis were also examined using MIA PaCa-2 cells with or without 1 μg/mL VEGF-C protein. As shown in Figure 7, TβR-I inhibitor significantly increased the LYVE-1-positive areas in tumor tissues in the presence of VEGF-C. In addition, we have tested the effect of overexpression of TGF-β1 ligand in tumor cells using a lentivirus expression system in the MIA PaCa-2 model, which resulted in reduction of lymphangiogenesis (Figure S4).
Induction of lymphangiogenesis by TβR-I inhibitor in MIA PaCa-2 xenograft models. (A,B) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using a TGF-β–nonresponsive human pancreatic cancer cell line, MIA PaCa-2. MIA PaCa-2 cells mixed with or without VEGF-C (1 μg/mL) were subcutaneously inoculated in BALB/c nude mice and treated with TβR-I inhibitor (1 mg/kg) as described in Figure 7. Bars represent 50 μm. (A) Immunostaining of MIA PaCa-2 xenograft sections by LYVE-1 (shown in green). (B) LYVE-1–positive areas in the MIA PaCa-2 xenograft sections in the presence or absence of VEGF-C and TβR-I inhibitor were determined (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (*P < .05).
Induction of lymphangiogenesis by TβR-I inhibitor in MIA PaCa-2 xenograft models. (A,B) Effects of TβR-I inhibitor on lymphangiogenesis were examined in a xenograft model using a TGF-β–nonresponsive human pancreatic cancer cell line, MIA PaCa-2. MIA PaCa-2 cells mixed with or without VEGF-C (1 μg/mL) were subcutaneously inoculated in BALB/c nude mice and treated with TβR-I inhibitor (1 mg/kg) as described in Figure 7. Bars represent 50 μm. (A) Immunostaining of MIA PaCa-2 xenograft sections by LYVE-1 (shown in green). (B) LYVE-1–positive areas in the MIA PaCa-2 xenograft sections in the presence or absence of VEGF-C and TβR-I inhibitor were determined (n = 3 for each group). Error bars represent SE. n.s. indicates not significant (*P < .05).
Discussion
TGF-β transduces signals in HDLECs and regulates the expression of LEC markers in vitro
TGF-β is a potent growth inhibitor on vascular endothelial cells and also inhibits their migration and cord formation in vitro. In the presence of TβR-II, TGF-β binds to TβR-I (ALK-5) and an endothelial-specific type I receptor ALK-1 in vascular endothelial cells, and activates Smad2/3 and Smad1/5, respectively. ALK-5 has been reported to be responsible for inhibition of growth and migration of endothelial cells.38 We have found that phosphorylation of Smad2 is induced by TGF-β and suppressed by TβR-I inhibitor in HDLECs. Migration of HDLECs toward VEGF-C and formation of cord-like structures by these cells were thus negatively regulated by TGF-β treatment.
TGF-β also plays pivotal roles in regulating the differentiation of blood endothelial cells. Inhibition of endogenous TGF-β signaling by the TβR-I kinase inhibitor SB431542 results in the proliferation and formation of sheet-like structures of mouse ESC-derived endothelial cells.39 In the present study, we found that TβR-I inhibitor up-regulated the expression of some LEC-related genes, including Prox1 and LYVE-1. We also found that TβR-I inhibitor induced early lymph vessel development in mouse ES cells. Thus, the effects of TβR-I inhibitor on LECs are not limited to HDLECs.
LYVE-1 is a hyaluronan receptor specifically expressed in LECs. However, LYVE-1–deficient mice do not exhibit abnormalities in lymphatic vessels, and the function of LYVE-1 in LECs is unknown.40 In contrast, Prox1 functions as a key transcriptional factor in the differentiation of LECs. Prox1 up-regulates the expression of various LEC-specific genes and induces a shift in the transcriptional program from blood endothelial cells to that of LECs. During embryonic development, Prox1 is expressed in a subset of vein endothelial cells, which begin to express LEC markers and form primary lymphatic sacs.24,25 Elucidation of the signals that induce expression of Prox1 in blood endothelial cells is thus important to understand the mechanisms of lymphangiogenesis. VEGFR3 signaling does not induce the expression of Prox1,30 whereas interleukin-3 signaling has been reported to induce expression of Prox1 in dermal blood endothelial cells.41 Infection by Kaposi sarcoma-associated herpes virus also induces Prox1 expression in human dermal microvascular endothelial cells.42 However, signaling pathways that regulate the expression of Prox1 in LECs have not been fully determined. The finding that TβR-I inhibitor induces the expression of Prox1 in HDLECs suggests that TGF-β signaling is a novel pathway that regulates Prox1 expression.
Induction of lymphangiogenesis by TβR-I inhibitor in vivo
We have shown that TβR-I inhibitor induces lymphangiogenesis in a chronic peritonitis model and in pancreatic carcinoma xenograft models. We used a low dose of TβR-I inhibitor for in vivo treatment, which has been shown to decrease the coverage of endothelium by pericytes and promote efficient accumulation of macromolecules to tumors through leakiness of tumor blood vessels. The low-dose TβR-I inhibitor acted on blood cells and vascular cells and suppressed the phosphorylation of Smad2 in these cells but not in tumor cells.8 It will thus be of interest to examine whether the low-dose TβR-I inhibitor also acts on LECs and regulates lymphangiogenesis.
In the chronic peritonitis model we examined, lymphangiogenesis was induced in the diaphragm of immunocompetent mice through induction of chronic inflammation by repeated injection of thioglycollate. Accumulation of inflammatory cells (eg, macrophages) and LECs could be observed in the plaques.43 Some LECs were positive for Ki-67, suggesting that these LECs were actively proliferating. Under these conditions, TβR-I inhibitor was able to induce lymphangiogenesis without addition of exogenous growth factors. Macrophages may produce VEGF-C37 and other cytokines in sites of chronic inflammation; however, TβR-I inhibitor did not enhance the secretion of VEGF-C from inflammatory macrophages in the present study, suggesting that it may primarily induce lymphangiogenesis through direct action on LECs.
TβR-I inhibitor induced lymphangiogenesis in both xenografts of BxPC3 and those of MIA PaCa-2 cells in the presence of VEGF-C, suggesting that TβR-I inhibitor may induce proliferation of LECs once lymphatic vessels have been formed in the tumors by VEGF-C. An important question is whether TβR-I inhibitor induces lymphatic metastasis of tumors. The present findings suggest that TβR-I inhibitor may induce lymphangiogenesis in tumors that express VEGF-C or -D. However, Laakkonen et al reported that only certain types of cancers secrete VEGF-C.21 We have also found, in an orthotopic transplantation model of diffuse-type gastric carcinoma OCUM-2MLN, that treatment with low-dose TβR-I inhibitor did not affect the extent of lymph node metastasis 16 days later.8,43 Moreover, Ge et al reported that metastasis of certain breast tumors was prevented by TβR-I inhibitor through activation of immune function.44
Dendritic cells, cytotoxic T cells, and natural killer cells, which could be involved in antitumor immune responses, are known to be functionally inhibited by TGF-β signaling.45 Dendritic cells are reported to migrate from sites of inflammation to regional lymph nodes through lymphatic vessels for presenting antigens to initiate further immune responses.46,47 Therefore, it is possible that the use of TβR-I inhibitor may enhance antitumor immune responses via providing more routes for dendritic cells to migrate from tumors to regional lymph nodes and recovering functions of various immune cells, including dendritic cells inhibited by TGF-β ligands. These issues, however, remain for further investigation.
In conclusion, we have shown that inhibition of endogenous TGF-β signaling results in induction of lymphangiogenesis. Although TβR-I inhibitor induces lymphangiogenesis in the presence of VEGF-C and possibly via other lymphangiogenic cytokines, it remains to be determined whether it can induce the spread of tumors through lymphatic vessels or suppress it through activation of immune function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Department of Molecular Pathology of the University of Tokyo for discussion.
This work was supported by KAKENHI (Grant-in-Aid for Scientific Research) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authorship
Contribution: M.O. performed the research and wrote the manuscript; C.I. collected data, contributed vital new reagents or analytical tools, and analyzed and interpreted the data; H.I.S. performed the research and collected the data; K.K. collected the data and contributed vital new reagents or analytical tools; Y.M. collected the data; T.W. contributed vital new reagents or analytical tools; A.K. collected the data; M.R.K. designed and performed the research, analyzed and interpreted the data, performed statistical analysis, and wrote the manuscript; K.M. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kohei Miyazono, Department of Molecular Pathology, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; e-mail: miyazono-ind@umin.ac.jp.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal