Abstract
Engagement of the adhesion receptor glycoprotein (GP) Ib-IX-V by von Willebrand factor (VWF) mediates platelet adhesion to damaged vessels and triggers platelet activation and thrombus formation in heart attack and stroke. GPIb-IX-V contains distinct 14-3-3ζ–binding sites at the GPIbα C-terminus involving phosphorylation of Ser609, an upstream site involving phosphorylated Ser587/Ser590, and a protein kinase A (PKA)–dependent site on GPIbβ involving Ser166. 14-3-3ζ regulates the VWF-binding affinity of GPIb-IX-V and inhibiting 14-3-3ζ association blocks receptor signaling, suggesting a key functional role for 14-3-3ζ. We used deletion mutants of GPIbα expressed in Chinese hamster ovary (CHO) cells to define the relationship of 14-3-3ζ binding to another GPIb-IX-V–associated signaling protein, phosphoinositide 3-kinase (PI3-kinase). Pull-down experiments involving glutathione S-transferase (GST)–PI3-kinase/p85-subunit and GST–14-3-3ζ indicated that both proteins interacted with contiguous GPIbα sequences 580 to 590/591 to 610. Deleting these, but not upstream sequences of GPIbα expressed in CHO cells, inhibited VWF/ristocetin-dependent Akt phosphorylation, relative to wild-type receptor, confirming this region encompassed a functional PI3-kinase–binding site. Pull-down experiments with GST-p85 truncates indicated the GPIbα-binding region involved the p85 breakpoint cluster region (BCR) domain, containing RSXSXP. However, pull-down of GPIb-IX was unaltered by mutation/deletion/phosphorylation of this potential 14-3-3ζ–binding sequence in mutant constructs of GST-p85, suggesting PI3-kinase bound GPIbα independently of 14-3-3ζ; 14-3-3ζ inhibitor peptide R18 also blocked pull-down of receptor by GST-14-3-3ζ but not GST-p85, and GST-p85 pull-downs were unaffected by excess 14-3-3ζ. Together, these data suggest the GPIbα C-terminus regulates signaling through independent association of 14-3-3ζ and PI3-kinase.
Introduction
The platelet adhesion receptor, the glycoprotein (GP) Ib-IX-V complex, plays a pivotal role in regulating platelet adhesion to damaged blood vessels at high shear stress, leading to thrombus formation in heart attack and stroke.1-3 This primarily involves binding of von Willebrand factor (VWF) to GPIbα, the major ligand-binding subunit of the GPIb-IX-V complex, which triggers signal transduction, platelet activation, and activation of the integrin, GPIIb-IIIa (αIIbβ3), that binds VWF or fibrinogen and mediates platelet aggregation. However, GPIbα also binds to counterreceptors such as P-selectin4 on activated platelets or activated endothelial cells, and the leukocyte integrin, Mac-1 (αMβ2)5 to regulate platelet-leukocyte–endothelial cell interactions in vascular biology, as well as controlling platelet procoagulant activity through binding ligands, including α-thrombin, factor XI, factor XII, and/or kininogen (reviewed by Andrews et al3 ). A critical question, however, is how engagement of GPIb-IX-V by VWF (or other ligands) leads to activation of αIIbβ3, and other downstream events. Recent evidence suggests the signaling proteins, 14-3-3ζ and phosphoinositide 3-kinase (PI3-kinase), associated with the cytoplasmic domain of the receptor are intricately involved in regulating GPIb-IX-V–dependent platelet function.6-16 The ultimate aim of the present work was to investigate the functional relationships between binding of 14-3-3ζ and that of PI3-kinase
We and others have previously demonstrated that the cytoplasmic domain of GPIb-IX-V, comprised of the cytoplasmic C-terminal domains of GPIbα (approximately 100 residues), GPIbβ (approximately 34 residues), GPIX (approximately 5 residues), and GPV (approximately 15 residues), contains 3 distinct 14-3-3ζ–binding sites.6-13 One of these is at the extreme C-terminus of GPIbα involving phosphorylation of the penultimate residue, Ser609, and there is a second site N-terminal to this involving phosphorylation of Ser587 and Ser590. There is a further protein kinase A (PKA)–dependent binding site for 14-3-3ζ on GPIbβ involving Ser166. A toggle-switch model involving 14-3-3ζ has been suggested to regulate the affinity of the receptor for VWF, and inhibition of 14-3-3ζ association with the receptor blocks GPIb-IX-V–dependent signaling to αIIbβ3, suggesting a key role for 14-3-3ζ in regulating receptor function.13 In this model, 14-3-3ζ dimer anchored at the constitutively phosphorylated site involving Ser609 of GPIbα may simultaneously bind either (1) the upstream GPIbα site involving Ser587/Ser590 or (2) the PKA-dependent site involving Ser166 of GPIbβ, with these different configurations equating to different functional states of the receptor.13
We have also previously demonstrated that PI3-kinase associates with the GPIb-IX-V complex,14 although whether this interaction involves 14-3-3ζ is unknown. The regulatory p85 subunit of PI3-kinase is coprecipitated with GPIb-IX in platelet lysates (under conditions where GPV is dissociated from GPIb-IX), and PI3-kinase becomes activated in response to VWF.14-18 Du and coworkers recently reported that the PI3-kinase substrate, Akt, is activated following ligation of GPIbα, and that human platelets in the presence of the Akt inhibitor, SH-6, or mouse platelets deficient in Akt-1/2, were defective in GPIb-dependent platelet aggregation or stable adhesion under flow conditions.19 On the other hand, inhibiting PI3-kinase prevents αIIbβ3 activation downstream of GPIb-IX-V.17,18,20 Other published evidence supports a role for the GPIbα C-terminal region in megakaryocyte maturation and thrombopoiesis involving PI3-kinase–dependent pathways,21 and p85 was also implicated in regulating the association of Src kinase with GPIb-IX-V.18
In this study, we have used deletion mutants of GPIbα expressed as a GPIb-IX complex in Chinese hamster ovary (CHO) cells to define the relationship of PI3-kinase association to that of 14-3-3ζ, and identified 2 contiguous sequences (580-590 and 591-610) involved in binding both proteins. In conjunction with pull-down experiments involving glutathione S-transferase (GST) fusion constructs of 14-3-3ζ and PI3-kinase/p85-subunit, the combined data suggest that the C-terminus of GPIbα is an important region regulating receptor-mediated signaling events through an independent association with 14-3-3ζ and PI3-kinase.
Methods
Antibodies
Rabbit polyclonal antibodies against the soluble extracellular portion of GPIbα (glycocalicin) and 14-3-3ζ were raised and affinity-purified as previously described.22,23 A phosphorylation-specific antibody against the C-terminal sequence of GPIbα (RYSGHpSL; pS indicates phosphoserine) was made by immunizing New Zealand White rabbits with a synthetic peptide based on this sequence (Mimotopes, Victoria, Australia) conjugated to keyhole limpet hemocyanin via an N-terminal Cys residue, using previously described methods.9,23-26 The anti–phospho-Ser609 GPIbα antibody was affinity-purified from serum using a combination of phosphorylated and unphosphorylated forms of the immunizing peptide, as described elsewhere.9,23-26 Anti-Akt and anti–phospho-Ser308 Akt antibodies were purchased from Cell Signaling Technology (Beverly, MA). A mouse anti-GPIbα monoclonal antibody, WM23, against the extracellular domain has been reported elsewhere.22,27 Anti-human filamin monoclonal antibody was obtained from Chemicon International (Temecula, CA). Horseradish peroxidase–labeled or fluorescein-5-isothiocyanate (FITC)–labeled anti-rabbit or anti-mouse IgG secondary antibodies were purchased from Silenus (Hawthorn, Australia).
GST-14-3-3ζ and GST-p85 fusion proteins
A fusion protein of GST-14-3-3ζ in a pGEX-2T vector was expressed in Escherichia coli and affinity-purified on glutathione-Sepharose (Pharmacia, Uppsala, Sweden) as previously described.23-26 Similar methods were used to make GST-p85 constructs, containing human p85 residues 1 to 718, the p85 N-terminal domain (p85-N; residues 1-330), and the p85 C-terminal domain (p85-C; residues 325-718). Additional constructs were prepared by site-directed mutagenesis using the QuikChange method according to the manufacturer's instructions (Stratagene, La Jolla, CA), including Ser231/Ala and 221 to 232 deletion mutants of p85-N, and p85-N truncates involving introduction of a stop codon into the pGEX-p85-N vector at nucleotide positions 238, 334, or 904, respectively. Additional constructs were made using pGEX-p85 constructs as templates and a polymerase chain reaction (PCR) amplification method27 involving primers that incorporated unique 5′ BamH1 or 3′ EcoR1 restriction sites. The resulting PCR products were purified, digested with BamH1 and EcoR1, and subcloned back into pGEX-2T. The following GST-fusion proteins were prepared: p85, residues 1 to 718; p85-N, residues 1 to 330; p85-C, residues 325 to 718; SH3, residues 1 to 80 (N-terminal SH3 domain); SH3-P1, residues 1 to 112 (SH3 and PRD-1 domains); SH3–breakpoint cluster region (BCR), residues 1 to 302 (SH3, PRD-1, and BCR domains); BCR, residues 113 to 301 (BCR domain); BCR-N, residues 113 to 213 (N-terminal region of the BCR domain); and BCR-C, residues 211 to 301 (C-terminal region of the BCR domain); BCR-P2, residues 113 to 330 (BCR and PRD-2 domains); BCR S231A, residues 113 to 301 (BCR domain) containing a Ser231/Ala point mutation; and BCR Δ221–232, BCR domain containing a deletion of a putative 14-3-3ζ–binding site. Fusion proteins were expressed in E coli and extracted in 0.05 M Tris (pH 8.0) containing final concentrations of 10 mM phenylmethylsulphonylfluoride (PMSF), 2 mM EDTA, and 1 mM N-ethylmaleimide (NEM), and were purified on glutathione-Sepharose 4B (Pharmacia) essentially as previously described for GST-14-3-3ζ.23-27
Cell lines
CHO cells expressing either wild-type GPIbα or mutant GPIbα containing deletions of cytoplasmic sequences have been described elsewhere.27-30 GPIbα is stably transfected in CHO cells already stably transfected with GPIbβ and GPIX (CHOβIX); CHO cells contain no endogenous GPIbα, GPIbβ, or GPIX (GPV is not required for functional GPIb-IX expression). Equivalent expression of wild-type and mutant GPIb-IX was confirmed by flow cytometry using the anti-GPIbα monoclonal antibody, WM23, and FITC-conjugated secondary antibody also as previously described.27-30 Binding of FITC-labeled VWF (10 μg/mL, final concentration) in the absence or presence of ristocetin (1 mg/mL, final concentration; Helena Laboratories, Beaumont, TX) to CHOβIX cells, or CHOβIX cells expressing wild-type or mutant GPIbα (106/mL) in 0.01 M Tris, 0.15 M sodium chloride (pH 7.4; TS buffer) for 30 minutes at 22°C was measured by flow cytometry using a method similar to that previously reported for VWF binding to CHO cells30 or platelets.31 Cell lysates from approximately 5 × 106 cells were prepared by addition of a 0.5-mL aliquot of TS buffer containing 1% (vol/vol) Triton X-100, EDTA (5 mM, final concentration) and Complete protease inhibitor (Roche, Mannheim, Germany). Lysates were rocked for 1 hour at 4°C, and centrifuged at 15 400g for 30 seconds at 4°C.
Platelet preparation
Washed human platelets, prepared as previously described14 (5 × 108/mL), were either unstimulated or treated with α-thrombin (1 U/mL, final concentration) for 5 minutes at 22°C. Platelets were lysed by the addition of one-tenth volume of 10 × lysis buffer containing 0.2 M Tris, 1.5 M sodium chloride, 10% (vol/vol) Triton X-100, 50 mM EDTA (pH 7.4), and Complete protease inhibitor; rocked for 1 hour at 4°C; and centrifuged at 15 400g for 20 minutes at 4°C. Where indicated, either platelets were pretreated with PGE1 (3 nM, final concentration) for 10 minutes at 22°C, or NEM (10 mM, final concentration) was included in the lysis buffer. Also, where indicated, dephosphorylation of platelet lysates was performed by adding protein calf intestinal phosphatase (CIP; 20 U/mg protein; New England Biolabs, Ipswich, MA) for 1 hour at 37°C.
Pull-down experiments
CHO cell or platelet lysates prepared as described were incubated with GST alone,23-26 GST-14-3-3ζ, or GST-p85 fusion proteins (20 μg bait protein per 2 mg total protein) and 100 μL of a 50% (vol/vol) suspension of glutathione-Sepharose 4B beads in a total volume of 1 mL, and rocked for 2 hours at 22°C. Beads were centrifuged (500g for 5 minutes at 22°C), washed 3 times with 1-mL aliquots of TS buffer, resuspended in 100 μL SDS–polyacrylamide gel electrophoresis sample buffer, and boiled for 3 minutes. Samples were western blotted with antiglycocalicin IgG as previously described.14 In some experiments, GST-p85-N was phosphorylated by PKA/casein kinase II (CKII) according to the manufacturer's instructions (New England Biolabs) for 2 hours at 30°C, prior to performing the pull-down assay. In these assays, sodium orthovanadate (1 mM, final concentration) was included in the assay buffer to minimize dephosphorylation. In competition experiments, either the 14-3-3ζ inhibitor peptide, R18 (100 μM, final concentration); a peptide based on the phosphorylated C-terminus of GPIbα, RYSGHpSL (100 μg); or purified 14-3-3ζ7 (20 μg) were preincubated with bait proteins for 15 minutes at 22°C prior to performing the pull-down assays. Immunoprecipitation from platelet lysates was performed by adding 10 μg polyclonal antiglycocalicin or nonimmune rabbit IgG to precleared lysates incubated at 4°C overnight, as previously described.32 Immunoprecipitated proteins were resolved on SDS 5% to 20% polyacrylamide gels under reducing conditions, and analyzed by Western blotting.32
Akt activity assay
CHO cells expressing wild-type or mutant GPIbα were analyzed for Akt activity in response to VWF using an assay system essentially as previously described.33 Briefly, cells were washed twice with TS buffer, and treated with 1.0 mL of either TS buffer alone, or TS buffer containing purified human VWF27,34 (10 μg/mL, final concentration) plus ristocetin (1.0 mg/mL, final concentration) for 5, 15, or 30 minutes at 37°C. Cells were then lysed by addition of 0.5 mL TS buffer containing 1% (vol/vol) Triton X-100, EDTA (5 mM, final concentration), and Complete protease inhibitor. Lysates were rocked for 1 hour at 4°C and centrifuged at 15 400g for 30 seconds, and supernatants were analyzed by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions and Western blotted with anti-Akt or anti–phospho-Ser308 Akt antibodies as previously described.33
Results
The C-terminal region of GPIbα binds 14-3-3ζ and PI3-kinase
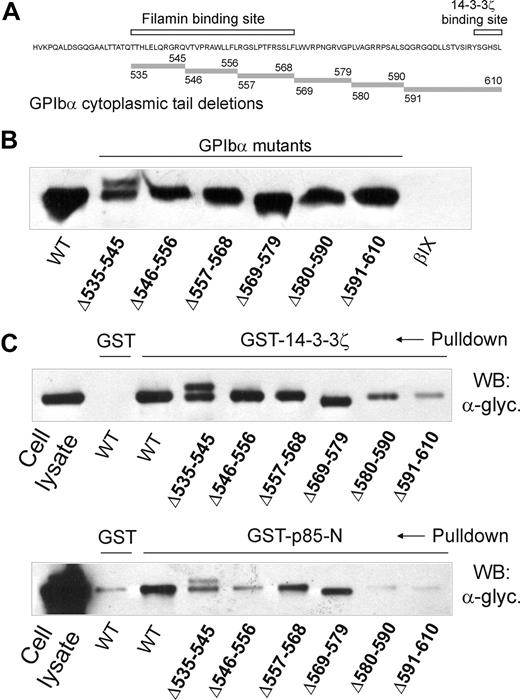
To investigate the relationship between binding sites for 14-3-3ζ and PI3-kinase within the cytoplasmic domain of GPIbα of the GPIb-IX complex, GST-fusion proteins involving the p85-subunit of PI3-kinase or 14-3-3ζ were used in pull-down experiments using CHO cells expressing wild-type or mutant GPIbα containing deletion mutations within the C-terminal tail. For p85, these pull-down experiments were performed using the N-terminal half of the protein spanning residues 1 to 330 (GST-p85-N) rather than full-length p85, because initial experiments in GPIb-IX–expressing CHO cells (data not shown) and platelets (see “The GPIbα-binding region of p85 involves the BCR domain”) showed that this region contained the GPIb-IX–binding site. The deletion mutations (Figure 1A) were previously expressed in CHO cells and used to map the binding site for filamin.29 Figure 1B shows that there was equivalent expression of wild-type and mutant receptor when the CHO cell lysates were Western blotted with an anti-GPIbα antibody (antiglycocalicin). Wild-type receptor and GPIbα containing deletions at 535 to 545, 546 to 556, 557 to 568, or 569 to 579 were precipitated by GST-14-3-3ζ and GST-p85-N, compared with a GST specificity control, when precipitates were western blotted with antiglycocalicin antibody (Figure 1C). However, there was relatively less precipitation of GPIbα deletion mutants, 580 to 590 and 591 to 610, by GST-p85-N (Figure 1C). This may be compared with GST-14-3-3ζ, where there was decreased coprecipitated 580 to 590 and much less 591 to 610 in parallel experiments (Figure 1C), consistent with the presence of known 14-3-3ζ–binding sites centered on phosphorylated residues at Ser587/Ser590 and Ser609 at the GPIbα C-terminus.10,12,13 Together, these results indicated that both 14-3-3ζ and p85 interacted with contiguous GPIbα sequences 580 to 590 and 591 to 610.
Analysis of deletion mutants of GPIbα expressed in CHO cells for binding to 14-3-3ζ and p85. (A) The sequence of the cytoplasmic domain of wild-type GPIbα showing the location of the central region that binds filamin28,29 and the C-terminal 14-3-3ζ–binding sequence6-9 (□). The location of sequences deleted in mutant GPIbα used in pull-down experiments is indicated by the shaded bars. (B) Western blot with antiglycocalicin of lysates of CHO cells expressing wild-type GPIbα or GPIbα deletion mutants or CHOβIX cells lacking GPIbα. (C) Pull-downs of CHO cell lysates with GST alone, GST-14-3-3ζ (top panel), or GST-p85-N (bottom panel). Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody (α-glyc.) as described in “Methods.” The reason for the doublet in the Δ535 to 545 GPIbα-expressing cells was not investigated. The results are representative of 3 separate experiments. WT indicates wild-type GPIbα.
Analysis of deletion mutants of GPIbα expressed in CHO cells for binding to 14-3-3ζ and p85. (A) The sequence of the cytoplasmic domain of wild-type GPIbα showing the location of the central region that binds filamin28,29 and the C-terminal 14-3-3ζ–binding sequence6-9 (□). The location of sequences deleted in mutant GPIbα used in pull-down experiments is indicated by the shaded bars. (B) Western blot with antiglycocalicin of lysates of CHO cells expressing wild-type GPIbα or GPIbα deletion mutants or CHOβIX cells lacking GPIbα. (C) Pull-downs of CHO cell lysates with GST alone, GST-14-3-3ζ (top panel), or GST-p85-N (bottom panel). Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody (α-glyc.) as described in “Methods.” The reason for the doublet in the Δ535 to 545 GPIbα-expressing cells was not investigated. The results are representative of 3 separate experiments. WT indicates wild-type GPIbα.
Compared with pull-downs with GST-14-3-3ζ, there is also decreased pull-down by GST-p85-N of the 535 to 545 and 546 to 556 GPIbα mutants containing deletions in the central filamin-binding region of the receptor, albeit a less dramatic effect than seen with the C-terminal mutants, 580 to 590 and 591 to 610 (Figure 1C). Our subsequent analysis focused on the C-terminal region, however, because these result in essentially complete abrogation of p85 binding, whereas the upstream mutants still allow reduced but persistent binding. Additional studies show that the C-terminal region is required for GPIbα-dependent PI3-kinase activity, whereas the 535 to 545 and 546 to 556 mutants still allow ristocetin/VWF-dependent, PI3-kinase–mediated Akt phosphorylation (results in “PI3-kinase binding to the C-terminal region of GPIbα is functional”). Published evidence also suggests the C-terminal region is critical for other GPIbα-dependent functions, including PI3-kinase–dependent functions in megakaryocytes.10,21,35 It is also possible that reduced binding to mutants 535 to 545 and 546 to 556 may be influenced by association of GPIb-IX with filamin, which binds to adjacent sequences (557-568 and 569-579), and is critically dependent on 567 to 571, within the central region of the GPIbα cytoplasmic domain,28,29 although treating platelets with NEM that dissociates filamin from GPIbα did not appreciably decrease p85 binding (results in “Association of PI3-kinase with GPIbα is independent of 14-3-3ζ”).
PI3-kinase binding to the C-terminal region of GPIbα is functional
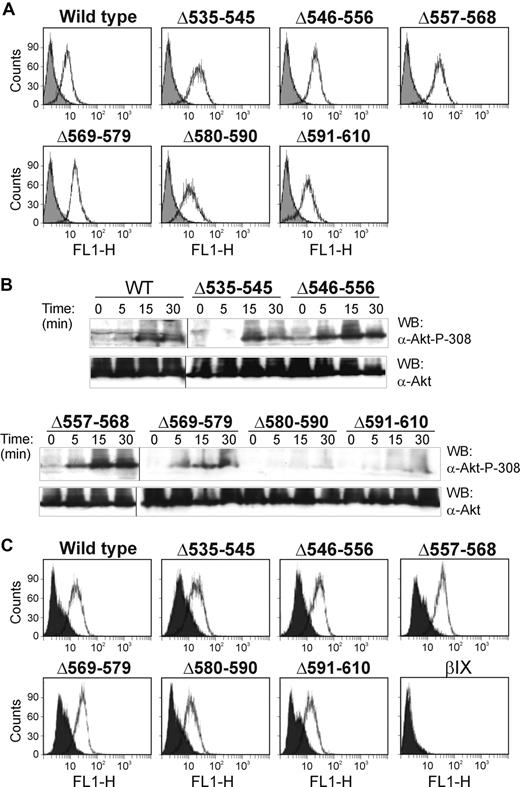
To address whether intact binding sites for 14-3-3ζ/PI3-kinase involving the sequences 580 to 590 and 591 to 610 identified using the GPIbα deletion mutants were required for functional PI3-kinase activity, we used an assay detecting phosphorylated Akt (anti–phospho-Ser308 Akt) as a read-out for GPIb-IX–dependent PI3-kinase activity.33 As shown in Figure 2B, deleting these but not upstream sequences inhibited VWF/ristocetin-dependent Akt phosphorylation, relative to wild-type receptor. Blotting with anti-Akt showed there was no significant difference in the total amount of Akt in these cell lines, and flow cytometry of the cell lines with anti-GPIbα monoclonal antibody, WM23, against an extracellular epitope27,30 showed equivalent levels of receptor expression (Figure 2A). We also demonstrated that the level of ristocetin-dependent binding of FITC-labeled VWF was essentially invariable between cell lines, so that differences in phosphorylation of Akt could not be explained by variable levels of stimulation (Figure 2C). Together, these experiments suggest that the C-terminal region of GPIbα encompassed a functional PI3-kinase–binding site.
VWF/ristocetin activates PI3-kinase in GPIbα-expressing cells. (A) Flow cytometry for the surface expression of GPIbα on CHO cells expressing either wild-type GPIbα or GPIbα deletion mutants using the anti-GPIbα monoclonal antibody, WM23, and a FITC-labeled secondary antibody.26 The solid histogram is the secondary antibody alone. (B) CHO cells expressing either wild-type GPIbα or mutant GPIbα were incubated with VWF/ristocetin for the indicated times. Cells were then lysed, blotted with either anti-Akt or anti–phospho-Ser308 Akt antibodies, and visualized as described in “Methods.” The results are representative of 3 separate experiments. Vertical lines indicate repositioned lanes from the same experiment and Western blot. (C) Binding of FITC-labeled VWF (10 μg/mL, final concentration) in the absence (solid histogram) or presence (open histogram) of ristocetin (1 mg/mL, final concentration) to CHO cells expressing wild-type GPIbα or GPIbα containing deletion mutations. CHOβIX cells (βIX) lacking GPIbα were used as a negative control.
VWF/ristocetin activates PI3-kinase in GPIbα-expressing cells. (A) Flow cytometry for the surface expression of GPIbα on CHO cells expressing either wild-type GPIbα or GPIbα deletion mutants using the anti-GPIbα monoclonal antibody, WM23, and a FITC-labeled secondary antibody.26 The solid histogram is the secondary antibody alone. (B) CHO cells expressing either wild-type GPIbα or mutant GPIbα were incubated with VWF/ristocetin for the indicated times. Cells were then lysed, blotted with either anti-Akt or anti–phospho-Ser308 Akt antibodies, and visualized as described in “Methods.” The results are representative of 3 separate experiments. Vertical lines indicate repositioned lanes from the same experiment and Western blot. (C) Binding of FITC-labeled VWF (10 μg/mL, final concentration) in the absence (solid histogram) or presence (open histogram) of ristocetin (1 mg/mL, final concentration) to CHO cells expressing wild-type GPIbα or GPIbα containing deletion mutations. CHOβIX cells (βIX) lacking GPIbα were used as a negative control.
The GPIbα-binding region of p85 involves the BCR domain
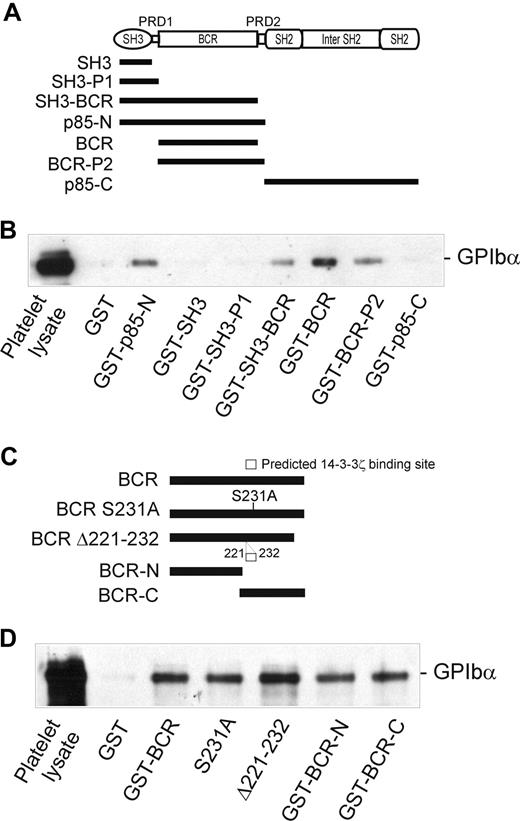
The 85-kDa regulatory subunit (p85) of PI3-kinase (which also consists of an approximately 110-kDa catalytic subunit, p110) is composed of multiple domains (Figure 3A): an N-terminal SH3 domain, a proline-rich domain (PRD-1), a BCR domain, a second PRD (PRD-2), an SH2 domain, an inter-SH2 region (which binds p110), and a second SH2 domain. Individual domains or combinations of domains were expressed as GST-fusion proteins (Figure 3A) in order to determine which of these regions is involved in binding to GPIbα. As shown in Figure 3B, 3 of these constructs, GST-SH3-BCR, GST-BCR, and GST-BCR-P2, were able to coprecipitate GPIb-IX from platelet lysates to a similar extent as GST-p85-N, whereas 3 other constructs, GST-SH3, GST-SH3-P1, and GST-p85-C, were ineffective and similar to GST alone. These results pointed to a critical functional role for the BCR region (residues 113-301), which was present in all of the GPIbα-binding fusion proteins. Furthermore, both the N-terminal and C-terminal portions of the BCR domain (113-213 and 211-301, respectively) associated with GPIbα, indicating that both regions may contribute to GPIbα binding (Figure 3C,D).
Identification of the GPIbα-binding region of p85. (A) The p85 subunit of PI3-kinase and the regions expressed as GST-fusion proteins (■). (B) Pull-down of GPIb-IX from human platelet lysates by GST alone, or by GST-p85 fragment fusion proteins: p85-N, residues 1 to 330; SH3, residues 1 to 80 (N-terminal SH3 domain); SH3-P1, residues 1 to 112 (SH3 and PRD-1 domains); SH3-BCR, residues 1 to 302 (SH3, PRD-1, and BCR domains); BCR, residues 113 to 301 (BCR domain); BCR-P2, residues 113 to 330 (BCR and PRD-2 domains); and p85-C, residues 325 to 718. (C) The BCR domain of p85 and the regions expressed as GST-fusion proteins: BCR, residues 113 to 301 (BCR domain); S231A, residues 113 to 301 (BCR domain containing a Ser231/Ala point mutation); Δ221 to 232, BCR domain containing a deletion of a putative 14-3-3ζ–binding site; BCR-N, residues 113 to 213; and BCR-C, residues 211 to 301. (D) Pull-down of GPIb-IX from human platelet lysates (PL) by GST alone, or by the GST-BCR fragment fusion proteins shown in panel C. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” The results are representative of 3 separate experiments.
Identification of the GPIbα-binding region of p85. (A) The p85 subunit of PI3-kinase and the regions expressed as GST-fusion proteins (■). (B) Pull-down of GPIb-IX from human platelet lysates by GST alone, or by GST-p85 fragment fusion proteins: p85-N, residues 1 to 330; SH3, residues 1 to 80 (N-terminal SH3 domain); SH3-P1, residues 1 to 112 (SH3 and PRD-1 domains); SH3-BCR, residues 1 to 302 (SH3, PRD-1, and BCR domains); BCR, residues 113 to 301 (BCR domain); BCR-P2, residues 113 to 330 (BCR and PRD-2 domains); and p85-C, residues 325 to 718. (C) The BCR domain of p85 and the regions expressed as GST-fusion proteins: BCR, residues 113 to 301 (BCR domain); S231A, residues 113 to 301 (BCR domain containing a Ser231/Ala point mutation); Δ221 to 232, BCR domain containing a deletion of a putative 14-3-3ζ–binding site; BCR-N, residues 113 to 213; and BCR-C, residues 211 to 301. (D) Pull-down of GPIb-IX from human platelet lysates (PL) by GST alone, or by the GST-BCR fragment fusion proteins shown in panel C. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” The results are representative of 3 separate experiments.
Association of PI3-kinase with GPIbα is independent of 14-3-3ζ
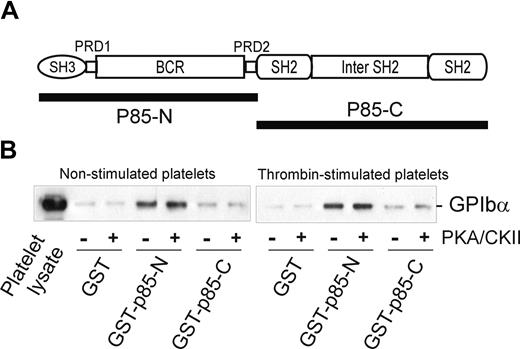
Interestingly, the BCR domain of p85 (residues 113-301), identified as an important region for binding to GPIbα, also contains a potential 14-3-3ζ–binding sequence, RSXSXP, with Ser231 (italic) a consensus site for phosphorylation by PKA. To address the functional role of this region in receptor binding, and/or whether 14-3-3ζ association at this site regulates binding to GPIb-IX, mutations targeting this site were tested for the ability to bind platelet GPIb-IX. However, neither Ser231/Ala mutation nor deletion of the sequence 221 to 232 spanning this site impaired the ability of BCR-containing GST-fusion proteins to pull-down GPIb-IX (Figure 3D). Furthermore, phosphorylation of the GST-p85-N fusion protein (compared with GST-p85-C, used here as a control) with PKA/CKII prior to performing pull-down experiments did not significantly alter the amount of GPIb-IX coprecipitated with GST-p85-N from lysates of either resting or thrombin-stimulated platelets (Figure 4). Activation by thrombin dissociates endogenous p85 from GPIb-IX.14 The combined results suggested that the interaction between p85 and GPIb-IX is independent of phosphorylation, and presumably independent of 14-3-3ζ, and this supposition was also supported by other approaches.
The effect of phosphorylation on the association of p85 and GPIb-IX. (A) The p85 subunit of PI3-kinase. The N-terminal half of p85 (GST-p85-N, residues 1-330) and the C-terminal half of p85 (GST-p85-C, residues 325-718) were used for the pull-down experiments in panel B. (B) Pull-down of GPIb-IX from lysates of human platelets, either nonstimulated or thrombin-stimulated (1 U/mL for 5 minutes at 22°C), by GST alone, GST-p85-N, or GST-p85-C. The bait proteins were either unphosphorylated or phosphorylated with PKA/CKII prior to performing the pull-down experiments in the presence of the phosphatase inhibitor sodium orthovanadate. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” The results are representative of 3 separate experiments.
The effect of phosphorylation on the association of p85 and GPIb-IX. (A) The p85 subunit of PI3-kinase. The N-terminal half of p85 (GST-p85-N, residues 1-330) and the C-terminal half of p85 (GST-p85-C, residues 325-718) were used for the pull-down experiments in panel B. (B) Pull-down of GPIb-IX from lysates of human platelets, either nonstimulated or thrombin-stimulated (1 U/mL for 5 minutes at 22°C), by GST alone, GST-p85-N, or GST-p85-C. The bait proteins were either unphosphorylated or phosphorylated with PKA/CKII prior to performing the pull-down experiments in the presence of the phosphatase inhibitor sodium orthovanadate. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” The results are representative of 3 separate experiments.
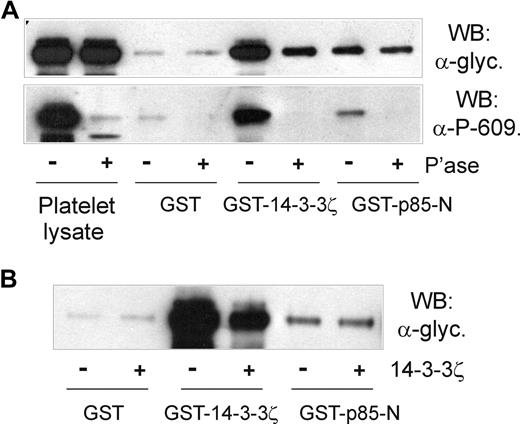
First, the association of p85 and GPIb-IX was shown to be independent of cytoskeleton-GPIb-IX attachment, since NEM treatment did not affect pull-down of GPIb-IX from platelet lysates by GST-p85-N (Figure 5A,B). NEM treatment has previously been shown to dissociate filamin from the cytoplasmic tail of GPIbα,32,36 ruling out any indirect link between GPIb-IX, 14-3-3ζ, and p85. Second, platelets were treated with prostaglandin E1 (PGE1), which increases cAMP-dependent PKA-induced phosphorylation of GPIbβ at Ser166, and binding of 14-3-3ζ.11,13,36 PGE1 treatment, however, also had no effect on the ability of GST-p85-N to pull down GPIb-IX (Figure 5B), suggesting that phosphorylation of GPIbβ/14-3-3ζ binding does not affect association of p85 with GPIbα. Third, the inhibitory 14-3-3ζ-binding peptide R18,13 which dramatically blocked pull-down of GPIb-IX from platelet lysates by GST-14-3-3ζ, had minimal effect on pull-down by GST-p85-N or GST-p85-BCR (Figure 5C). Similarly, a peptide based on the phosphorylated C-terminus of GPIbα, RYSGHpSL, blocked association of 14-3-3ζ but not p85-N or p85-BCR with GPIb-IX (Figure 5C). Last, pull-down of GPIb-IX by GST-14-3-3ζ, but not GST-p85-N, was inhibited by either phosphatase treatment of human platelet lysate (Figure 6A) or by the presence of excess added 14-3-3ζ (Figure 6B). In the case of phosphatase treatment, blotting samples with the anti–phospho-Ser609 GPIbα antibody confirmed both the activity of the phosphatase, and the dependence on Ser609 phosphorylation for efficient binding of 14-3-3ζ,13 but not p85 (Figure 6A).
The effect of cytoskeletal disruption or GPIbβ phosphorylation on the interaction of 14-3-3ζ or p85 with GPIbα. (A) Lysates of human platelets that were untreated, pretreated with PGE1 prior to lysis, or lysed in the presence of NEM, were immunoprecipitated (I.P.) with rabbit antiglycocalicin (Anti-glyc.) or nonimmune control (Cont.) IgG and Western blotted with antifilamin monoclonal antibody; then, the same filters were reprobed with anti-GPIbα (WM23) as indicated (P.L. indicates platelet lysate). (B) Pull-down of GPIb-IX from lysates of human platelets that were untreated, pretreated with PGE1 prior to lysis (that increases phosphorylation of GPIbβ), or lysed in the presence of NEM (that dissociates filamin from GPIbα), by GST alone, or GST-p85-N. Results are representative of 5 separate experiments. (C) Pull-down of GPIb-IX from human platelet lysates in the absence or presence of either a 14-3-3ζ inhibitor peptide (R18; 100 μM, final concentration) or a peptide based on the phosphorylated C-terminus of GPIbα, RYSGHpSL (α604-610; 100 μg), by GST alone, GST-14-3-3ζ, GST-p85-N, or GST-BCR. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” Results are representative of 3 separate experiments.
The effect of cytoskeletal disruption or GPIbβ phosphorylation on the interaction of 14-3-3ζ or p85 with GPIbα. (A) Lysates of human platelets that were untreated, pretreated with PGE1 prior to lysis, or lysed in the presence of NEM, were immunoprecipitated (I.P.) with rabbit antiglycocalicin (Anti-glyc.) or nonimmune control (Cont.) IgG and Western blotted with antifilamin monoclonal antibody; then, the same filters were reprobed with anti-GPIbα (WM23) as indicated (P.L. indicates platelet lysate). (B) Pull-down of GPIb-IX from lysates of human platelets that were untreated, pretreated with PGE1 prior to lysis (that increases phosphorylation of GPIbβ), or lysed in the presence of NEM (that dissociates filamin from GPIbα), by GST alone, or GST-p85-N. Results are representative of 5 separate experiments. (C) Pull-down of GPIb-IX from human platelet lysates in the absence or presence of either a 14-3-3ζ inhibitor peptide (R18; 100 μM, final concentration) or a peptide based on the phosphorylated C-terminus of GPIbα, RYSGHpSL (α604-610; 100 μg), by GST alone, GST-14-3-3ζ, GST-p85-N, or GST-BCR. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody as described in “Methods.” Results are representative of 3 separate experiments.
The association of p85 with GPIbα is independent of phosphorylation or 14-3-3ζ binding. (A) Pull-down of GPIb-IX from human platelet lysates that were either untreated or pretreated with CIP phosphatase for 1 hour at 37°C by GST alone, GST-14-3-3ζ, or GST-p85-N. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with either antiglycocalicin antibody (top panel) or anti–phospho-Ser609 GPIbα antibody (bottom panel). (B) Pull-down of GPIb-IX from human platelet lysates without or with the addition of 14-3-3ζ (20 μg/mL, final concentration) by GST alone, GST-14-3-3ζ, or GST-p85-N. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody. The results are representative of 3 separate experiments.
The association of p85 with GPIbα is independent of phosphorylation or 14-3-3ζ binding. (A) Pull-down of GPIb-IX from human platelet lysates that were either untreated or pretreated with CIP phosphatase for 1 hour at 37°C by GST alone, GST-14-3-3ζ, or GST-p85-N. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with either antiglycocalicin antibody (top panel) or anti–phospho-Ser609 GPIbα antibody (bottom panel). (B) Pull-down of GPIb-IX from human platelet lysates without or with the addition of 14-3-3ζ (20 μg/mL, final concentration) by GST alone, GST-14-3-3ζ, or GST-p85-N. Samples were resolved by SDS 5% to 20% polyacrylamide gel electrophoresis under reducing conditions, and visualized by Western blotting with antiglycocalicin antibody. The results are representative of 3 separate experiments.
Discussion
In this study, we have investigated the interaction of the cytoplasmic domain of GPIbα, the major ligand-binding subunit of the platelet GPIb-IX-V complex, with 2 signaling proteins, 14-3-3ζ and PI3-kinase. Both 14-3-3ζ (the predominant 14-3-3 isoform in platelets) and PI3-kinase have been implicated in playing a critical role in the adhesive and signaling functions of GPIb-IX-V, which are relevant to thrombus formation in heart attack and stroke.2,3 Binding of VWF to GPIb-IX-V triggers signaling, with a major outcome being the activation of platelet integrins, in particular αIIbβ3, that binds fibrinogen or VWF and mediates platelet aggregation.1-3 The assembly and regulation of the adheso-signaling complex involving GPIb-IX-V includes multiple interactions with the cytoplasmic domain of the receptor consisting of the cytoplasmic tails of GPIbα, GPIbβ, GPIX, and GPV. Within these domains, binding sites have been mapped not only for 14-3-3ζ (at least 3 distinct sites centered on phosphorylated residues at Ser166 of GPIbβ and Ser587/Ser590 and Ser609 of GPIbα),6-13 but also calmodulin (juxtamembrane sequences of GPIbβ and GPV)36,37 and filamin (the central region of the GPIbα tail centered on Trp570 and Phe568),28,29 and an association of the receptor with the p85 subunit of PI3-kinase and Src kinase has been demonstrated in resting platelets.14,18 However, the C-terminal sequence of GPIbα is of particular interest, because (1) studies in which wild-type or C-terminally truncated GPIbα (as GPIb-IX) was cotransfected with αIIbβ3 in CHO cells suggested that an intact 14-3-3ζ–binding site encompassing the C-terminal 5 residues of GPIbα is required for VWF/GPIbα-dependent activation of αIIbβ310 ; (2) deletion of the C-terminal 14-3-3ζ–binding sequence of GPIbα regulates PI3-kinase–dependent signaling and proliferation in megakaryocytes21 ; and (3) deletion of the C-terminal 5 amino acids of GPIbα in mice leads to less stable carotid artery thrombus formation in a FeCl3 injury model.35 What is not clear, however, is the relationship between binding of 14-3-3ζ and PI3-kinase to this sequence, whether 14-3-3ζ is required for PI3-kinase association, and whether binding of these proteins could be selectively blocked.
To address these questions, we analyzed a series of GPIbα deletion mutants expressed in CHO cells (as a GPIb-IX complex)29 and GST-fusion proteins of 14-3-3ζ and p85 in pull-down experiments and showed that the binding of both proteins involved the C-terminal sequences 580 to 590 and 591 to 610. The interaction with p85/PI3-kinase was functional, because cells expressing these deletion mutants showed impaired PI3-kinase–dependent Akt phosphorylation compared with wild-type receptor or mutants with upstream deletions. These C-terminal sequences overlap regions previously shown to be functional 14-3-3ζ–binding sites,9,10,12 involving the sequences 585RPpSALpSQG592 (pS) and 606SGHpSL610. Dai et al13 showed that a membrane-permeable peptide inhibitor of the 14-3-3ζ–GPIbα interaction, MPαC, based on the C-terminal sequence of GPIbα, inhibits VWF binding to platelets and VWF-mediated platelet adhesion under flow conditions, as well as overcoming the enhanced VWF binding to GPIb-IX otherwise seen upon dephosphorylation of GPIbβ.13 Activation of PKA, for example by treating platelets with PGE1, leads to phosphorylation of Ser166 within the 14-3-3ζ–binding motif of GPIbβ, inhibits platelet activation, and controls the ability of GPIbβ to regulate actin polymerization. Constitutive phosphorylation of GPIbα at Ser609 may act to maintain 14-3-3ζ attachment, and depending upon the phosphorylation status of GPIbα Ser587/Ser590 and GPIbβ Ser166 (PKA-dependent), control GPIb-IX-V function (“toggle switch” model).13 But how does this interaction of 14-3-3ζ at the C-terminus of GPIbα affect binding of p85 at the same site? Our results suggest that these interactions are independent.
First, treating platelet lysates with phosphatase that markedly affects binding of GPIb-IX to 14-3-3ζ had little affect on binding to p85. Second, blocking 14-3-3ζ binding with a generic inhibitor peptide (R18) also had minimal effect on p85 binding. Third, competitive inhibition of GST-14-3-3ζ pull-down of GPIb-IX by added 14-3-3ζ was ineffective at blocking pull-down by GST-p85 constructs. Last, PGE1 treatment of platelets, which increases phosphorylation of GPIbβ and 14-3-3ζ association,11,13 did not alter p85 association with GPIb-IX. The association of either 14-3-3ζ or p85 is not dependent on the GPIb-IX-V-cytoskeletal interaction because the interactions are not significantly affected by dissociation of filamin from GPIbα using NEM (this study).14
Additional experiments addressed the region of p85 involved in binding receptor, and whether the interaction of p85 with 14-3-3ζ might affect the interaction with GPIbα. Using GST-fusion proteins containing p85 domains, the GPIbα-binding site was localized to the BCR domain (residues 113-301) within the N-terminal portion of p85. Although we did not directly confirm that both BCR and p85-N bind to the same site of GPIbα, the absence of binding of all 3 GST constructs lacking BCR (SH3, SH3-P1, and p85-C) and presence of binding of all 3 constructs containing BCR (SH3-BCR, BCR, and BCR-P2) would strongly suggest that the BCR domain mediates binding of p85 to GPIbα. Both N- and C-terminal halves of BCR (residues 113-213 and 211-301, respectively) interacted with GPIb-IX. However, disruption of a potential 14-3-3ζ–binding motif within the BCR domain, RSXSXP encompassing the consensus PKA/CKII phosphorylation site at Ser231 (italic), failed to alter the capacity of GST-BCR constructs to pull-down GPIb-IX. Phosphorylation in vitro of GST-BCR with PKA/CKII was similarly without effect. Together, these data strongly suggest that p85/PI3-kinase interacts with GPIbα within the same region but independently of 14-3-3ζ. The identification of the BCR region as containing the binding site for GPIbα implies that GPIbα-bound p85 could interact with other binding partners using other p85 domains (Figure 3A). For example, the N-terminal SH3 domain interacts with proline-rich sequences in tyrosine kinases and other signaling proteins, the PRDs (PRD-1 and PRD-2) flanking the BCR domain interact with SH3 domains in p85 or other proteins such as Cbl, 2 discontiguous SH2 domains interact with the conserved tyrosine-phosphorylated Tyr-X-X-Met motifs found in tyrosine kinases, and an inter-SH2 region binds to the catalytic p110 subunit of PI3-kinase.38 The VWF/GPIbα-dependent phosphorylation of the PI3-kinase substrate, Akt (this study), is consistent with the BCR-mediated association of p85, with GPIbα allowing functional association with p110, and assembly of an active PI3-kinase complex. Interestingly, the BCR domain also mediates binding of p85 to small GTP-ases such as Cdc42, Rac, or Rho that regulate actin cytoskeletal rearrangements (and may also contribute to activation of p110),38 suggesting the p85/BCR-GPIbα interaction could also regulate other BCR-mediated functions.
Finally, the independent association of 14-3-3ζ and p85/PI3-kinase at the C-terminus of GPIbα raises the question about the functional role for these interactions, and the potential benefits of selectively blocking one or both in terms of regulating GPIb-IX-V–dependent functions.10-21 This is presumably feasible because both a generic 14-3-3ζ inhibitor peptide and a peptide based on the phosphorylated C-terminus of GPIbα (RYSGHpSL) selectively abolished 14-3-3ζ but not p85 binding to GPIb-IX (this study). PI3-kinase is known to potentiate activation of αIIbβ3 in response to platelet agonists, including VWF, even though its precise role in GPIb-IX-V signaling pathways is yet to be fully established.2 Recent evidence suggests isoform-selective PI3-kinase inhibitors attenuate formation of occlusive thrombi in vivo mediated by αIIbβ3.20 Further studies are warranted to examine the functional interplay between PI3-kinase, other GPIb-IX-V–associated signaling proteins,39-41 and coassociated GPVI/Fc receptor32 within the platelet adheso-signaling complex.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yang Shen and Dr Elizabeth Gardiner for helpful discussion, and Ms Carmen Llerena and Ms Cheryl Berndt for excellent technical assistance.
This work was supported in part by the National Health and Medical Research Council of Australia and the National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: F.-T.M., F.S., J.F.A., and A.D.M. carried out experiments; F.-T.M., R.K.A., A.D.M., S.L.C., S.P.J., F.S., A.F.L., and M.C.B. contributed to experimental design and interpretation; S.L.C., S.P.J., F.S., and A.F.L. provided intellectual input; and R.K.A., A.D.M., and M.C.B. cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael C. Berndt, Department of Immunology, Monash University, Alfred Medical Research and Education Precinct (AMREP), Commercial Road, Melbourne, Victoria, Australia 3004; e-mail: michael.berndt@med.monash.edu.au.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal