Abstract
We investigated the role of CD40 and CD40L in neointima formation and identified the downstream CD40-signaling intermediates (tumor necrosis factor [TNF]–receptor associated factors [TRAF]) involved. Neointima formation was induced in wild-type, CD40−/−, CD40L−/−, and in CD40−/− mice that contained a CD40 transgene with or without mutations at the CD40-TRAF2,3&5, TRAF6, or TRAF2,3,5&6 binding sites. Compared with wild-type mice, CD40−/− mice showed a significant decrease in neointima formation with increased collagen deposition and decreased inflammatory cell infiltration. Neointima formation was also impaired in wild-type mice reconstituted with CD40−/− bone marrow. In vitro, the capacity of CD40−/− leukocytes to adhere to the endothelium was reduced. Ligated carotid arteries of CD40−/− mice showed a smaller total vessel volume and an impaired remodeling capacity, reflected by decreased gelatinolytic/collagenolytic activity. Comparable results were found in mice with defects in CD40-TRAF6 and CD40-TRAF 2/3/5&6 binding, but not in mice with defects in CD40-TRAF2/3&5 binding. Neointima formation and vascular remodeling in CD40-receptor–deficient mice is impaired, due to a decreased inflammatory cell infiltration and matrix-degrading protease activity, with CD40-TRAF6 signaling as the key regulator. This identifies the CD40-TRAF6 axis as a potential therapeutic target in vascular disease.
Introduction
The CD40/CD40L system is an important pathway in immune responses that has been implicated in several inflammatory diseases, such as rheumatoid arthritis, transplant rejection, multiple sclerosis,1,2 and atherosclerosis.3-5
CD40 is a receptor of the tumor necrosis factor (TNF)–receptor superfamily that by itself has no intrinsic signaling ability. Binding of its ligand (CD40L) induces receptor trimerization and recruitment of adaptor proteins called TNF-receptor associated factors (TRAFs), upon which CD40 signaling is elicited. This results in the production of proinflammatory and proatherogenic cytokines/chemokines, growth factors (eg, VEGF), matrix metalloproteinases (MMPs), and adhesion molecules.6
The cytoplasmic tail of CD40 contains 2 independent TRAF-binding domains: a membrane proximal region binding TRAF6, and a distinct membrane distal domain that binds TRAF2&3 and, indirectly, TRAF5.7 Interestingly, as in other TNF-R members, both TRAF binding sites can initiate different CD40 downstream mediators and effectors. For example, in monocytes and macrophages, CD40-TRAF6 interactions result in activation of Src/ERK1/2 and IKK/NFκB proinflammatory pathways,8 while in endothelial cells and smooth muscle cells, inflammation is predominantly mediated via CD40-TRAF2 interactions.9 This was also confirmed in a recent study, in which the different CD40-TRAF interactions differentially modulate chemokine and cytokine expression.10
Although a pivotal role of CD40L in atherosclerotic plaque development, progression, and stability has been proven extensively,3-5 the role of CD40-CD40L interactions in neointima formation, the process associated with luminal narrowing causing major complications after arterial intervention (eg, balloon angioplasty and stenting) in humans, have shown contradictory results.11-13 Moreover, the involvement of downstream CD40 adaptor molecules (TRAFs) in vascular pathology has not been fully elucidated so far.
The aim of this study was to investigate the role of CD40 and CD40L in neointima formation and vascular remodeling using a mouse model of carotid artery ligation14 and to identify which TRAF members are involved in these processes. Since TRAFs are also involved in signaling of other TNF-receptor family members (eg, Toll-like receptors), we used transgenic mice with mutations in the specific TRAF-binding domains of CD40. These mice contain intact, functional TRAFs, but lack the binding of specific TRAFs to CD40, thereby disrupting CD40 signaling without affecting signaling of other TRAF-dependent receptors. This study demonstrates that the CD40/CD40L system in leukocytes is a major pathway required for neointima formation and arterial remodeling that is mainly mediated by signaling through TRAF6.
Methods
Animals and surgery
In total, 314 male wild-type C57BL6/J mice (wt), CD40−/− mice, and CD40L−/− mice were fed a normal chow throughout the experiment (12-14 weeks of age, n = 7-14 per group for histology and blood pressure measurements; n = 24-35 per group for real-time polymerase chain reaction [PCR], zymography, and gelatinase/collagenase assays, fluorescence-activated cell-sorter [FACS] analysis, and platelet assays). To identify the TRAFs required for CD40 signaling in neointima formation, we used CD40−/− mice that express a human/mouse chimeric CD40 transgene (human CD40 extracellular domain; mouse CD40 transmembrane and cytoplasmic domain) under a major histocompatability complex (MHC)II promoter, containing mutations at the TRAF2,3&5 (CD40-T2/3/5), TRAF6 (CD40-T6), or both the TRAF2/3/5 and TRAF6 (CD40-T2/3/5&6) binding site on CD40 or CD40−/− mice containing the chimeric CD40 construct without any mutations at the TRAF binding sites of CD40 (CD40-T-wt)15 (Figure 1S, available on the Blood website; see the Supplemental Materials link at the top of the online article). Mice were anesthetized with 2.5% isoflurane, and the right common carotid artery was ligated with a silk suture (5-0) near the carotid bifurcation to induce neointima formation as described by Kumar et al.14 Experiments were approved by the animal ethics committee of the Maastricht University and performed according to the institutional guidelines.
Bone marrow transplantation
Female C57Bl6/J mice (n = 30; 9 wks old) were maintained in filtertop cages and given water containing polymyxin B sulfate (60 000 U/L) and neomycin (100 mg/L) starting 1 week before bone marrow transplantation until 4 weeks thereafter. Mice were lethally irradiated (10 Gy, 0.5 Gy/min, Philips MU15F/225 kV; Hamburg, Germany) and intravenously injected with 107 bone marrow cells from male CD40−/− mice or C57Bl6/J mice.
Four weeks after the transplantation, the right carotid artery was ligated as described in “Animals and surgery.”
Tissue harvesting and histologic analysis
Four weeks after carotid artery ligation, mice were killed, and the arterial tree was perfused through a catheter inserted in the left cardiac ventricle with phosphate-buffered saline (PBS) containing 0.1 mg/mL sodium nitroprusside (Sigma-Aldrich, St Louis, MO) and subsequently with 1% paraformaldehyde. The carotid arteries were removed, fixed overnight in 1% paraformaldehyde, and embedded in paraffin. Cross-sections (4 μm thick) were cut at 200-μm or 100-μm (bone marrow transplantation) intervals throughout the common carotid artery. For each level, a cross-section was stained with Elastica-von-Giesson staining (EvG) and hematoxylin and eosin (HE). EvG-stained cross-sections were used for morphometric analysis of the lumen area, intimal area (the area within the internal elastic lamina [IEL] minus the lumen area), medial area (defined as the area within the external elastic lamina [EEL] minus the area within the IEL), and total vessel area (area encompassed by the EEL). Intima volumes were determined by multiplying intimal areas with the distance over which the levels neointimas were present. Luminal and total vessel volumes were determined by multiplying lumen and total vessel areas with the distance over 8 levels.
Sections were stained with anti-CD45 (1:50; Pharmingen, San Diego, CA) for analysis of inflammatory cell infiltration, anti-CD3 (1:50; Lab Vision, Fremont, CA) specifically for T cells, anti-mac3 (1:50; Pharmingen) for macrophages, anti-CD31 (1:100; Cell Signaling, Beverly, MA) for endothelial cells, picrosirius red for collagen or with anti–α-smooth muscle actin (ASMA)-fluorescein isothiocyanate (FITC)-conjugated antibody (1:3000; Sigma Diagnostics), and anti–FITC-horseradish peroxidase as secondary antibody for staining of smooth muscle cells (SMCs)/myofibroblasts. For apoptosis and cell proliferation, sections were stained with anti-cleaved caspase-3 (1:200; Cell Signaling), and ki-67 (1:50; Dako, Carpinteria, CA), respectively. TGFβ was stained using LAP TGFβ1 antibody (1:30; R&D Systems, Minneapolis, MN). Double staining with MMP-2 (Santa Cruz Biotechnology, Santa Cruz, CA, 1:50) or -9 (Santa Cruz Biotechnology; 1:50) and Mac3 was performed according to standard procedures. The expression of the different TRAFs in vessel wall was determined by immunohistochemistry with TRAF2 (1:50; Santa Cruz Biotechnology), TRAF3 (1:50; Santa Cruz Biotechnology), and TRAF6 (1:50; Santa Cruz Biotechnology) antibodies. For MHC-II immunostaining, cryosections were stained with rat anti–mouse MHC-II (1:400; Abcam, Cambridge, MA). Morphometric and morphological analysis were performed by one blinded investigator (intra-observer variability was < 10%).
Blood pressure measurement
A polyethylene catheter (PE 20 heat-stretched) was implanted in the femoral artery under isofluorane anesthesia (1.5%-2.5%) at constant temperature, and its tip was advanced into the abdominal aorta. Blood pressure measurements were performed as described.16 Beat-to-beat values of mean arterial pressure were calculated as the area under the curve of each pressure wave using the end diastolic value to determine the heart rate. Data were recorded and averaged over a 10-minute period 15 to 30 minutes after completion of the surgery when hemodynamics were stabilized.
Fluorescence-activated cell sorting analysis
Blood, spleen, and lymph nodes of wild-type, CD40−/−, CD40-Twt, and CD40-T6 mice (n = 6/group) were collected, processed, and stained with fluorescent antibodies against CD3 (for T cells), CD4 (T-helper cells), CD25 (activated T cells), CD69 (activated T cells), B220 (B cells), Gr1 (granulocytes), and Mac1 (macrophages), and analyzed as described previously.17
Leukocyte adhesion assay
Blood was obtained from CD40−/− mice and wild-type mice, and leukocytes were isolated. Subsequently, leukocytes were coincubated with TNFα (200 ng/mL) stimulated endothelial cells (SVECs). Nonadherent cells were washed away with PBS, and the number of adhering CD40−/− or wild-type leukocytes were counted.
Thrombus formation on collagen under flow
Thrombus formation was measured ex vivo with blood from wild-type, CD40−/−, CD40-Twt, or CD40-T6 mice. Blood was collected in PPACK/heparin and perfused over a type-I collagen coated coverslip at a shear rate of 1000 s−1 for 4 minutes as described previously.18
Gelatinase/collagenase assay
Two pools of 6 carotid arteries of CD40−/− and wild-type mice, respectively, were harvested one week after ligation and snap frozen in liquid nitrogen. Proteins were extracted using a lysis buffer containing 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, and 0.2% sodium azide in PBS. Gelatinolytic/collagenolytic activity in ligated carotid arteries was determined using the EnzChek gelatinase/collagenase assay kit (Molecular Probes, Eugene, OR). Equal concentrations of the protein extracts were used to digest a gelatin substrate, yielding highly fluorescent digestion products proportionally to gelatinase and collagenase activity in the ligated carotid arteries. Fluorescence was measured at 24, 48, and 72 hours using a fluorescence microplate reader at 515-nm emission wavelength, and protease activity was calculated using a standard curve of Clostridium histolyticum type IV collagenase (according to the manufacturer's protocol).
Gelatin zymography
MMP2 and MMP9 activity was determined in carotid arteries of wild-type, CD40−/−, CD40-Twt, CD40-T2/3/5, and CD40-T6 1 week after ligation. Tissue samples were extracted in 150 μL sodium dodecyl sulfate (SDS)–lysis (10% glycerol, 20% SDS, 10% 1.5 M Tris [tris(hydroxymethyl)aminomethane] pH 6.8) buffer by cutting the tissue into small pieces. MMP-2 and MMP-9 activity in extracts were detected as previously described.19 Briefly, 4 μL of tissue extracts was diluted (1:5) with distilled water. Samples were electrophoresed in the presence of nonreducing buffer at 4°C in 7.5% SDS-polyacrylamide gels containing 2 mg/mL gelatin. After the removal of SDS, gelatinase activity was revealed by overnight incubation at 37°C and staining with 0.1% Coomassie brilliant blue. Zymograms were quantified in the linear range by densitometry with Quantity One 1-D Image Analysis software system (Bio-Rad, Hercules, CA).
Real-time polymerase chain reaction
RNA was isolated from mouse carotid arteries 1 week after ligation (pools of 3-4 carotids) using the bead-beater and RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was used as a template to generate cDNA using random primers. Real-time polymerase chain reaction (PCR) reactions (MyiQ Icycler; Bio-Rad) were carried out with cDNA (10 ng RNA template), IQ SYBR Green super mix (Bio-Rad), and 3 mM of forward and reverse primers for MMP-2, MMP-9, MMP-13, MMP-14, TIMP-2, and TIMP-3. PCR conditions were 3 minutes at 95°C and 40 cycles of 10 seconds at 95°C and 45 seconds at 60°C, 1 minute 95°C. Samples were run in duplicate. RNA copy numbers were calculated using a standard curve and normalized to housekeeping gene (cyclophilin A) mRNA expression.
Statistical analysis
Values are expressed as means plus or minus SEM, and a Mann-Whitney nonparametrical test was used to compare individual groups of animals. Probability values of less than .05 were considered significant.
Results
CD40/CD40L signaling is required for neointima formation and vascular remodeling
In response to ligation, neointimal lesions developed in the common carotid artery up to approximately 2 mm from the point of ligation. The first 9 cross-sections (with intervals of 200 μm) were used for analysis. Figures 1A-C show representative cross-sections of the ligated common carotid artery with a neointimal lesion of wild-type, CD40−/−, CD40L−/− mice. We calculated the mean neointimal area and intima/media ratios for each individual level, as well as the mean neointimal volumes. Compared with wild-type mice, neointima formation and intima/media ratios throughout the ligated carotid artery (Figure 1D,H) were considerably reduced in CD40−/− mice, resulting in a significantly reduced neointimal volume (Figure 1E). In CD40L−/− mice, neointimal volume was reduced, although not significantly (Figure 1E,H). Lumen area and total vessel area of CD40−/− mice and total vessel area of CD40L−/− mice were significantly smaller than observed in wild-type mice (Figure 1F,G), revealing an impaired remodeling capacity of ligated arteries of CD40 and CD40L-deficient mice. The percent of maximal stenosis was reduced in both CD40 and CD40L-deficient mice (Figure 1I).
Neointima formation and vascular remodeling in wild-type, CD40−/−, and CD40L−/− mice. Representative cross-sections are shown in panels A-C; n = 10, 11, and 10 mice, respectively. Neointima was measured at individual levels (200-μm intervals) throughout the ligated carotid artery segment (D), and neointimal volume was calculated (E). Furthermore, volumes of lumen (F) and total vessel wall (G) were calculated, as well as intima/media ratios (H) and the maximal stenosis rate (I). *P < .05 compared with wild-type mice. Neointima formation in CD40−/− mice was significantly reduced compared with wild-type mice at all individual levels analyzed throughout the ligated carotid artery.
Neointima formation and vascular remodeling in wild-type, CD40−/−, and CD40L−/− mice. Representative cross-sections are shown in panels A-C; n = 10, 11, and 10 mice, respectively. Neointima was measured at individual levels (200-μm intervals) throughout the ligated carotid artery segment (D), and neointimal volume was calculated (E). Furthermore, volumes of lumen (F) and total vessel wall (G) were calculated, as well as intima/media ratios (H) and the maximal stenosis rate (I). *P < .05 compared with wild-type mice. Neointima formation in CD40−/− mice was significantly reduced compared with wild-type mice at all individual levels analyzed throughout the ligated carotid artery.
The effect on remodeling between both genotypes could not be explained by differences in hemodynamics, since no differences in blood pressure, heart rate, heart weight, body weight, and heart weight/body weight ratio were found between CD40−/− mice and controls (Table 1). In the contralateral left, nonligated carotid artery, no differences in vessel geometry were found between wild-type, CD40−/−, or CD40L−/− mice (Figure S2), indicating that CD40/CD40L only modulates the remodeling response induced by a considerable change in blood flow and the development of neointima after arterial ligation. No differences in baseline geometry or medial SMC content (as an indication of contractile capacity) of the right carotid artery were found between wild-type and CD40−/− mice (Figure S3).
Hemodynamic parameters of CD40−/− mice compared with wild-type controls
| . | Wt . | CD40−/− . |
|---|---|---|
| MAP, mmHg | 87.3 ± 2.0 | 84.5 ± 3.4 |
| HR/min | 539.2 ± 35.3 | 516.5 ± 20.1 |
| Body weight, g | 26.5 ± 0.76 | 26.2 ± 0.87 |
| Heart weight, g | 0.15 ± 0.01 | 0.15 ± 0.01 |
| Heart weight/body weight *10-3 | 5.8 ± 0.3 | 5.6 ± 0.2 |
| . | Wt . | CD40−/− . |
|---|---|---|
| MAP, mmHg | 87.3 ± 2.0 | 84.5 ± 3.4 |
| HR/min | 539.2 ± 35.3 | 516.5 ± 20.1 |
| Body weight, g | 26.5 ± 0.76 | 26.2 ± 0.87 |
| Heart weight, g | 0.15 ± 0.01 | 0.15 ± 0.01 |
| Heart weight/body weight *10-3 | 5.8 ± 0.3 | 5.6 ± 0.2 |
MAP indicates mean arterial pressure; and HR, heart rate.
Gelatinase/collagenase activity is reduced in CD40−/− mice
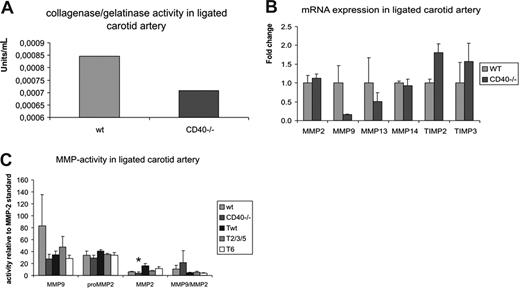
Since matrix-degrading enzymes such as MMPs are required for extracellular matrix turnover and thus vascular remodeling, we measured gelatinolytic/collagenolytic activity in carotid arteries from CD40−/− mice compared with wild-type controls at 1 week after ligation, when MMP activity is reported to be maximal.20 Per group, 2 pools of 6 ligated carotid arteries were used, and protease activity was measured in duplo (total of 4 measurements per group) after 24, 48, and 72 hours of digestion. Gelatinase/collagenase activity in the ligated carotid arteries of CD40−/− mice was 16% decreased compared with wild-type mice (average of the 3 time points, Figure 2A), which explains the impaired remodeling response and increased collagen content of neointimal lesions in CD40−/− mice.
Proteolytic activity is decreased in absence of CD40. (A) Gelatinase/collagenase activity in ligated carotid arteries of CD40−/− mice versus controls. Per group, 2 pools of 6 carotid arteries were used and assayed in duplo (total of 4 measurements per group). Fluorescence was measured at 515 nm after 24, 48, and 72 hours of digestion, and average protease activity was calculated for both groups. (B) Real-time PCR of MMP-2, 9, 13, 14 and TIMP-2 and -3 on ligated carotid arteries of CD40−/− and wild-type mice reveals a decrease in MMP-2 and -9 levels and an increase in TIMP-2 and -3 levels. (C) Zymography for MMP-2 and -9 reveals a decrease in MMP-2 and -9 activity in CD40−/− mice compared with wild-type mice, and in CD40-T6 mice compared with CD40-Twt mice. Error bars represent SEM.
Proteolytic activity is decreased in absence of CD40. (A) Gelatinase/collagenase activity in ligated carotid arteries of CD40−/− mice versus controls. Per group, 2 pools of 6 carotid arteries were used and assayed in duplo (total of 4 measurements per group). Fluorescence was measured at 515 nm after 24, 48, and 72 hours of digestion, and average protease activity was calculated for both groups. (B) Real-time PCR of MMP-2, 9, 13, 14 and TIMP-2 and -3 on ligated carotid arteries of CD40−/− and wild-type mice reveals a decrease in MMP-2 and -9 levels and an increase in TIMP-2 and -3 levels. (C) Zymography for MMP-2 and -9 reveals a decrease in MMP-2 and -9 activity in CD40−/− mice compared with wild-type mice, and in CD40-T6 mice compared with CD40-Twt mice. Error bars represent SEM.
To determine the metalloproteinases responsible for this decrease in gelatinolytic/collagenolytic activity, we investigated the mRNA expression levels of distinct MMPs and their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs) in carotid arteries of wild-type, CD40−/−, 1 week after ligation. MMP-9 mRNA expression levels were markedly reduced in CD40−/− mice compared with wild-type, although this was not significant due to low sample numbers used (Figure 2B). Expression levels of TIMP-2 and -3 tended to be increased. Since mRNA expression does not always reflect the activity of the MMPs, we investigated MMP-2 and MMP-9 activity in carotid arteries of wild-type, CD40−/−, 1 week after ligation using zymography. MMP-2 activity was significantly reduced in CD40−/− compared with wild-type mice, whereas MMP-9 was reduced but not significantly (Figure 2C).
Neointima formation and vascular remodeling is mediated by CD40-signaling through TRAF6
CD40 cannot initiate signal transduction by itself, and CD40-signaling depends on the recruitment of TRAF-adaptor proteins. In neointimal lesions, we found expression of TRAF2, TRAF3, and TRAF6 (Figure S2). For all TRAFs, staining was most prominent in the neointima, while TRAF staining was only modest in the media (M). To determine which CD40-TRAF interactions are required for the development of neointima and remodeling of the arterial wall, we used CD40−/− mice that express a chimeric human/mouse CD40 transgene, under an MHC-II promoter, which contains mutations in the cytoplasmic tail of CD40 that selectively disrupt the binding of specific TRAFs to CD40 (Figure S1A). MHC-II (and therefore also the CD40 transgene) was expressed in the neointimal lesions (Figure S1B).
Figure 3A-D shows representative cross-sections of the ligated common carotid artery with a neointimal lesion of CD40-Twt (with no defects in CD40-TRAF binding), CD40-T2/3/5, CD40-T6, and CD40-T2/3/5&6 (with a defect in binding TRAF2,3,5 and TRAF6) mice. Neointima formation and intima/media ratios were significantly reduced, both at individual levels throughout the ligated artery and neointimal volumes (Figure 3E,F), in mice with defective CD40-T6 binding (levels 1-5) and in CD40-T2/3/5&6 mice (levels 2-7). Neointima in CD40-T2/3/5&6 mice was not further reduced compared with CD40-T6, showing that mainly CD40 signaling through TRAF6 is involved in neointima formation. Furthermore, intima/media ratio and maximal stenosis rates were reduced in both CD40-T6 and CD40T2/3/5&6 mice (Figure 3I,J). Besides CD40-TRAF signaling in neointima formation, we also investigated the involvement of distinct TRAF proteins required for CD40 signaling in vascular remodeling. TRAF6 mediates the effects of CD40 on vascular remodeling, since total vessel area of CD40-T6 and of CD40-T2/3/5&6 mice was significantly reduced compared with CD40-Twt mice (Figure 3H). Like in CD40 or CD40L-deficient mice, no differences in vessel geometry of the contralateral, nonligated artery were found between CD40-Twt, CD40-T2/3/5, CD40-T6, or CD40-T2/3/5&6 mice (Figure S2C,D). MMP-2 and -9 levels were decreased, although not significantly, in CD40-T6 mice (Figure 2). These results reveal that signaling through the CD40-TRAF6 axis is crucial for neointima formation and vascular remodeling in mice.
Neointima formation and carotid artery remodeling in CD40-Twt, CD40-T2/3/5, CD40-T6, and CD40-TRAF2/3/5&6 mice. Representative cross-sections are shown in panels A-D; n = 14, n = 10, n = 7, and n = 14, respectively. Neointima was measured at individual levels (200-μm intervals) throughout the ligated artery segments (E), and neointimal volume was calculated (F). Furthermore, volumes of lumen (G) and total vessel wall (H) were calculated, as well as intima/media ratios (I) and maximal stenosis rates (J). *P < .05 compared with CD40-Twt mice. Neointima formation in CD40-T6 mice was significantly reduced compared with CD40-Twt mice at levels 1-5, whereas reduction in neointima formation in CD40-T2/3/5&6 mice was significant at levels 2-7. Error bars represent SEM.
Neointima formation and carotid artery remodeling in CD40-Twt, CD40-T2/3/5, CD40-T6, and CD40-TRAF2/3/5&6 mice. Representative cross-sections are shown in panels A-D; n = 14, n = 10, n = 7, and n = 14, respectively. Neointima was measured at individual levels (200-μm intervals) throughout the ligated artery segments (E), and neointimal volume was calculated (F). Furthermore, volumes of lumen (G) and total vessel wall (H) were calculated, as well as intima/media ratios (I) and maximal stenosis rates (J). *P < .05 compared with CD40-Twt mice. Neointima formation in CD40-T6 mice was significantly reduced compared with CD40-Twt mice at levels 1-5, whereas reduction in neointima formation in CD40-T2/3/5&6 mice was significant at levels 2-7. Error bars represent SEM.
CD40-TRAF6 signaling is required for inflammatory cell infiltration and collagen turnover in the neointima
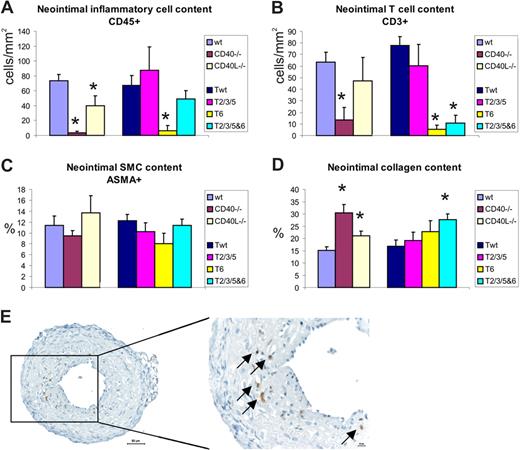
Immunohistochemical staining showed the presence of inflammatory cells in the neointimal layer of the ligated carotid artery (Figure 4E). We found a significant reduction in CD45+ cells relative to neointimal area in CD40 and CD40L-deficient mice compared with wild-type, as well as a significant reduction in relative amount of CD3+ T cells and Mac3+ cells in CD40−/− mice (Figure 4A,B). CD40 signaling through TRAF6 was required for the infiltration of inflammatory cells, since the amount of T cells was reduced in neointimal lesions of mice in which binding of TRAF6 (CD40-T6) and binding of TRAF2/3/5&6 (CD40-T2/3/5&6) to CD40 was disrupted (Figure 4A,B).
Neointimal lesion composition in CD40/CD40L deficiency or in mice with defective CD40-TRAF binding compared with controls. (A) Inflammatory cells stained with CD45-antibody, (B) CD3+ T-cell content of neointima, (C) amount of ASMA-positive SMCs, and (D) collagen content of neointimal lesions. *P < .05 compared with wild-type (for CD40−/− and CD40L−/− mice) or compared with CD40-Twt (for CD40-T2/3/5, CD40-T6, and CD40-T2/3/5&6 mice).
Neointimal lesion composition in CD40/CD40L deficiency or in mice with defective CD40-TRAF binding compared with controls. (A) Inflammatory cells stained with CD45-antibody, (B) CD3+ T-cell content of neointima, (C) amount of ASMA-positive SMCs, and (D) collagen content of neointimal lesions. *P < .05 compared with wild-type (for CD40−/− and CD40L−/− mice) or compared with CD40-Twt (for CD40-T2/3/5, CD40-T6, and CD40-T2/3/5&6 mice).
A reason for the decrease in MMP expression, and the impaired remodeling capacity as observed in ligated carotid arteries of CD40−/− and CD40-Traf6 mice, is due to the decrease in immune cells into the neointima and/or adventitia. Indeed, double immunohistochemistry for MMP2 or MMP9 with the macrophage marker Mac3 revealed that MMP-2 and MMP-9 expression is predominantly found in intimal macrophages, proving that the reduced MMP expression is caused by a reduced recruitment of macrophages (Figure S5).
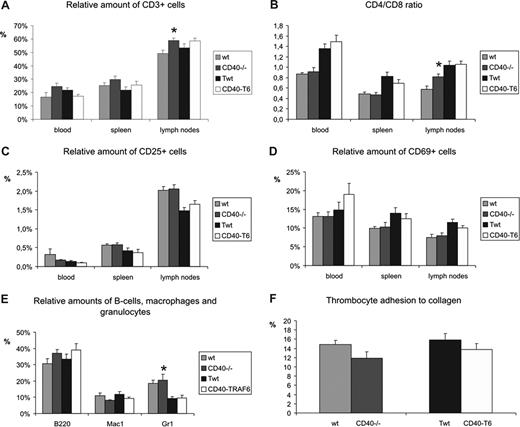
FACS analysis on blood, spleen, and lymph nodes showed that CD40−/− mice have a higher amount of Gr1+ cells in their blood compared with wild-type mice (Figure 5). Furthermore, CD40−/− mice had slightly more CD3+ T cells and an increased ratio of CD4+/CD8+ T cells in lymph nodes, but not in blood or spleen. However, no differences were found in the relative amount of activated (CD25+ or CD69+) T cells in blood, spleen, or lymph nodes. Also, no differences in the amount of (activated) T cells, CD4/CD8 ratio, the amount of B cells, macrophages, or granulocytes were found in blood, spleen, and lymph nodes of CD40-T6 mice compared with the CD40-Twt mice (Figure 5A-E).
FACS analysis and thrombus formation in wild-typeversus CD40−/− and CD40-Twt vs CD40-T6 mice. Relative amount of CD3+ T cells (A), CD4/CD8 ratio (B), and relative amounts of activated T cells (C and D showing CD25+ and CD69+ T cells, respectively) were assessed in blood, spleen and lymph nodes. Furthermore, relative amounts of circulating B cells, macrophages, and granulocytes were determined (E). Thrombocyte adhesion to collagen was measured under flow conditions. Data are percentages of surface covered with platelets after 4 minutes of perfusion (F). *P < .05 compared with wild-type. Error bars represent SEM.
FACS analysis and thrombus formation in wild-typeversus CD40−/− and CD40-Twt vs CD40-T6 mice. Relative amount of CD3+ T cells (A), CD4/CD8 ratio (B), and relative amounts of activated T cells (C and D showing CD25+ and CD69+ T cells, respectively) were assessed in blood, spleen and lymph nodes. Furthermore, relative amounts of circulating B cells, macrophages, and granulocytes were determined (E). Thrombocyte adhesion to collagen was measured under flow conditions. Data are percentages of surface covered with platelets after 4 minutes of perfusion (F). *P < .05 compared with wild-type. Error bars represent SEM.
The amount of ASMA-positive SMCs tended to be reduced in neointima of CD40-T6 mice, but this was not significant (Figure 4C). No significant differences were found in neointimal cell proliferation and apoptosis or angiogenesis (data not shown). An increase in neointimal collagen accumulation was found in CD40−/− and CD40L−/− mice compared with wild-type, which was also observed in CD40-T2/3/5&6 mice compared with the Twt controls (Figure 4D). This increase in collagen content was accompanied by an increase in TGFβ levels in mice with a deficient CD40 signaling or a deficient CD40-T6 signaling (staining intensity: CD40−/− 3.3 ± 0.4 vs wt 1.3 ± 0.1; P < .05; CD40-T6 2.9 ± 0.3 vs CD40-Twt: 1.8 ± 0.3; P = .055).
CD40-expressing leukocytes play a key role in neointima formation and vascular remodeling
Since the decrease of neointima formation in absence of CD40 signaling is associated with a decreased number of inflammatory cells, we first investigated whether CD40−/− leukocytes show an impaired capacity to adhere to the endothelium. Indeed, CD40−/− leukocytes show a 66% decrease in the number of adhering leukocytes compared with their wild-type counterparts (Figure S6).
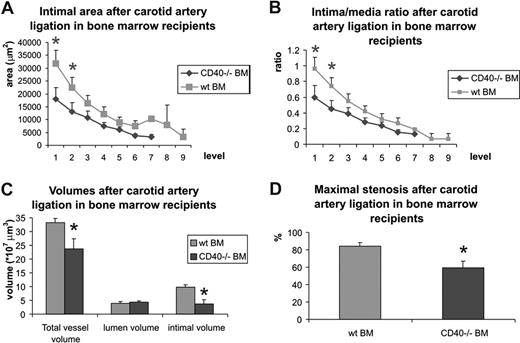
Subsequently, we studied the effect of deficiency of leukocyte CD40 on neointima formation by creating bone marrow chimeras. Wild-type mice reconstituted with CD40−/− bone marrow indeed showed a decrease in neointima formation, reflected by a decrease in neointimal volume and a decrease in maximal stenosis rate (Figure 6A-D). Moreover, deficiency of leukocyte CD40 induced an impaired remodeling capacity, reflected by a decrease in total vessel volume (Figure 6C). These results show that CD40-expressing bone marrow–derived immune cells play a dominant role in the effects of CD40 on neointima formation and remodeling.
Neointima formation and carotid artery remodeling in wild-type mice reconstituted with wild-type bone marrow (wt BM) or CD40−/− BM. Neointima area and intima-media/ratio was measured at individual levels (100-μm intervals) throughout the ligated artery segments (A and B) and neointimal volume, lumen volume, and total vessel volume were calculated (C), as well as maximal stenosis rate (D). *P < .05 compared with wild-type mice reconstituted with wild-type BM. Neointima formation and stenosis rate was significantly reduced in mice reconstituted with CD40−/− BM. Error bars represent SEM.
Neointima formation and carotid artery remodeling in wild-type mice reconstituted with wild-type bone marrow (wt BM) or CD40−/− BM. Neointima area and intima-media/ratio was measured at individual levels (100-μm intervals) throughout the ligated artery segments (A and B) and neointimal volume, lumen volume, and total vessel volume were calculated (C), as well as maximal stenosis rate (D). *P < .05 compared with wild-type mice reconstituted with wild-type BM. Neointima formation and stenosis rate was significantly reduced in mice reconstituted with CD40−/− BM. Error bars represent SEM.
Thrombosis
Since the model of carotid artery ligation is partly based on thrombosis formation and remodeling of the thrombus, we investigated platelet deposition and thrombus formation on collagen under high-shear flow conditions. No differences were found in the rate and extent of thrombus formation between blood from CD40−/− or CD40-T6 mice compared with wild-type or CD40-Twt, respectively (Figure 5F).
Discussion
The pathogenesis of neointima formation and arterial remodeling involves SMC proliferation and extracellular matrix remodeling, but also inflammation and immunity.21 The CD40/CD40L system is widely known to be involved in chronic inflammatory diseases such as atherosclerosis. However, its role in neointima formation remains unclear. In the present study, we show that deficiency of CD40, and to a much lesser extent CD40L, reduces neointima formation after carotid artery ligation.
Deficiency of CD40-CD40L signaling is known to reduce the production of chemokines, cytokines, and adhesion molecules, but also to decrease proteolytic activity and to inhibit angiogenesis.22,23 In mice deficient in CD40, neointimas were smaller, which was indeed accompanied by a decrease in inflammatory cell influx, an increase in collagen and TGFβ expression, and a decreased protease activity, but not with impaired angiogenesis. Moreover, stimulated endothelial cells were less capable to bind to CD40−/− leukocytes than CD40+/+ leukocytes, showing that CD40 indeed mediates the infiltration of leukocytes into the arterial wall. Since neointimas of irradiated mice reconstituted with CD40−/− bone marrow cells showed a similar phenotype as the neointimas of CD40−/− mice, we conclude that bone marrow–derived leukocyte CD40 plays a dominant role in the pathogenesis of neointima formation.
Our studies in transgenic mice with targeted mutations in the CD40-TRAF binding domains allowed us to further dissect the molecular mechanisms and to identify the role of the different CD40-TRAF interactions in neointima formation. We clearly showed that CD40 signaling through TRAF6 is the main pathway that mediates both neointima formation and infiltration of inflammatory cells in the neointima. As a role for CD40-TRAF6 in the production of proinflammatory cytokines (ie, TNFα) by macrophages in vitro has been described in literature,8 and as ligated carotid arteries of CD40-TRAF6 mice showed decreased levels of MMPs, we conclude that the reduction in neointima formation observed in our CD40−/− and CD40-TRAF6 mice is due to a reduction in inflammatory cell influx, a reduction in the production of inflammatory mediators, and a reduction in proteolytic activity that is mediated by CD40-TRAF6 interactions.
Although we identified TRAF6 as the key player in CD40-signaling in neointima formation, a role for other TRAF proteins cannot be fully excluded since TRAF 2, 3, and 5 have overlapping binding sites, which makes it difficult to differentiate individual functions of these distinct TRAF proteins. TRAF3 has been reported to counter-regulate the effects of TRAF2 on NFkB activation.24 Since we disrupted binding of both TRAFs simultaneously, we cannot differentiate between functions of TRAF2 and 3. Recently, a new binding site of TRAF2 has been discovered in the cytoplasmic tail of CD40,25,26 which was not disrupted in the CD40-T2/3/5 or CD40-T2/3/5&6 mice. Although this CD40-TRAF2 binding site could contribute to neointima formation and carotid artery remodeling, we showed a dramatic reduction in neointimal volume after disruption of the TRAF6 binding site of CD40, indicating that binding of TRAF6 to CD40 is essential for the development of neointimal lesions.
Besides its role in neointima formation, CD40 was shown to mediate arterial remodeling of the ligated artery. In CD40−/− and CD40-T6 and CD40-T2/3/5&6 mice, total vessel volume was smaller compared with controls. This could not be explained by differences in hemodynamics or baseline vessel geometry/morphology between both genotypes. In response to flow cessation in the ligated artery, blood flow in the contralateral carotid artery has been shown to be increased and to induce outward remodeling.27 In our study, we did not find any differences in vascular geometry in the contralateral nonligated artery between CD40−/− and wild-type mice, indicating that only outward remodeling in response to the neointima formation was impaired in CD40−/− mice. Activation of CD40 is known to induce the expression of matrix-degrading enzymes such as MMPs, which are required for arterial remodeling.28 Indeed, the impaired remodeling response in CD40−/− and CD40-T6 and CD40-T2/3/5&6 mice was caused by the observed reduction in activity of matrix-degrading enzymes due to an impaired recruitment of MMP-expressing inflammatory cells into the neointima, and simply because there is no need for a substantial outward remodeling since the neointimal lesions are so small. This was especially true for the CD40−/− mouse, in which even the lumen volume is significantly smaller, which can be explained by total lack of outward remodeling when small neointimas develop.
In contrast to the prominent effects of CD40 deficiency, effects of CD40L deficiency were limited. In previous studies using inhibition of CD40L in transplantation models, several laboratories have shown a significant reduction in transplant arteriosclerosis.12,13 In contrast, Remskar et al found an increase in intimal thickening after acute collar-induced arterial injury,11 which was attributed to the decrease in T-lymphocyte activation in absence of CD40L. In our study, the effects of CD40L inhibition were limited. Only the maximal stenosis rate was significantly reduced in CD40L−/− mice compared with wild-type mice, while neointimal volumes and intima/media ratios did not differ. In a separate experiment, we ligated the carotid artery of CD40L−/−/ApoE−/− mice and ApoE−/− mice to induce a foam cell–rich and VSMC-rich neointima and obtained similar results (data not shown). Possible explanations for these discrepancies on the role of CD40L on neointima formation comprise the pathogenesis of the models that are used. Transplant arteriosclerosis is a slow process, in which CD40L-mediated T-lymphocyte activation seems to play a major role,13 while in acute vascular injury, CD40L-mediated T-lymphocyte activation apparently is protective,11 whereas in our flow cessation model, the role of CD40L is limited. It is not clear what causes the difference between CD40−/− and CD40L−/− mice. A possible explanation could be the existence of another ligand for CD40, however, to date no other ligand for CD40 has been identified.
Previous studies have shown that inhibition of CD40L in mice attenuated the development of atherosclerosis and increased atherosclerotic plaque stability.3-5 The associated immune suppression makes complete inhibition of CD40/CD40L signaling not an attractive therapy for vascular diseases. Identification of specific mediators of CD40 signaling in vascular disease may provide valuable information for the development of more specific interventions. So far, little is known about the downstream signaling pathways of CD40 in vascular pathologies. However, in a recent paper by Zirlik et al, the different TRAF proteins were found to be expressed in both human and mouse atherosclerotic plaques and in human aneurysms.10 Moreover, inhibition of the different CD40-TRAF interactions in cultured endothelial cells revealed a tight modulation of the outcome of inflammation, depending on the TRAF that was bound to CD40.10 However, animal studies of the role of distinct TRAF proteins in CD40 effector functions have been hampered by the poor viability of most TRAF−/− mice and the fact that TRAFs are also involved in signaling of other TNF superfamily members. In this study we used transgenic mice with targeted mutations in the CD40-TRAF binding domains, allowing us to study the role of specific TRAFs in CD40 signaling in neointima formation and carotid arterial remodeling.
In conclusion, this study shows that deficiency in CD40 reduces neointima formation and remodeling after carotid artery ligation by inhibiting inflammatory cell infiltration and matrix-degrading protease activity. Moreover, we showed that leukocyte CD40 plays a key role in neointima formation. We identified TRAF6 as the key regulator of CD40 signaling in these processes, as specific disruption of CD40-TRAF6 binding markedly reduced neointima formation, leukocyte infiltration, and matrix-degrading activity. Since complete disruption of CD40 suppresses the immune system and is therefore not suitable as a therapy, the present identification of specific targets downstream of CD40 signaling may provide valuable information for the development of new therapeutic interventions in vascular disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Dutch Heart Foundation (grants 2000T41 and 2003B226), and Dutch Organization for Scientific Research (NWO; VIDI 016.086.236). The authors (A.N., M.D.A., C.W., A.Z., E.L.) participate in the European Vascular Genomics Network (http://www.evgn.org), a Network of Excellence supported by the European Community's Sixth Framework Program for Research Priority 1 (Life Sciences, Genomics, and Biotechnology for Health; contract LSHM-CT-2003-503254).
Authorship
Contribution: M.D. analyzed the data and wrote the paper; L.B., D.L., and E.W. performed experiments; I.M. performed the thrombus experiments; J.H. was responsible for the thrombus experiments; B.J. was responsible for the hemodynamics experiments; J.C. was responsible for morphometry program; A.Z. performed the leukocyte adhesion assay; C.W. and M.D.A. supervised experiments; C.A. generated the mice; U.B. performed the MMP zymographies; A.N. was responsible for the MMP zymographies; R.N. was responsible for the CD40-TRAF mice; and E.L. designed and supervised the experiment and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Esther Lutgens, Department of Patho-logy, Cardiovascular Research Institute Maastricht (CARIM), University of Maastricht, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: e.lutgens@path.unimaas.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal