Abstract
Targeting CD33 or CD45 is currently exploited for immunotherapy of acute myeloid leukemia (AML). Gemtuzumab ozogamicin (GO), an immunoconjugate of an anti-CD33 antibody that facilitates cellular uptake of a toxic calicheamicin-γ1 derivative, induces complete remissions in a subset of patients with AML. We herein tested whether simultaneous targeting of CD45 could improve GO cytotoxicity against AML cell lines and primary AML cells. We found that the anti-CD45 antibody, BC8, dose-dependently increased cytotoxicity induced by GO, and, to a lesser degree, free calicheamicin-γ1. BC8 promoted CD33 endocytosis, suggesting that its effect on GO cytotoxicity may be, at least partly, due to increased uptake and intracellular GO availability. Finally, compared with either agent alone, BC8 combined with GO resulted in marked tumor growth inhibition and superior survival rates of mice bearing human AML xenografts. These data suggest that further study of this antibody combination for clinical use in AML is warranted.
Introduction
CD33 and CD45 are currently exploited for immunotherapy of acute myeloid leukemia (AML).1,2 The recognition that CD33 is found on most AML blasts led to the development and clinical use of gemtuzumab ozogamicin (GO; Mylotarg).2-4 This immunoconjugate uses an anti-CD33 antibody (hP67.6) to facilitate uptake of a calicheamicin-γ1 derivative, after which the toxin is cleaved and DNA damage/cell death is induced.3 GO cytotoxicity thus critically relies on CD33-mediated endocytosis.5 While the exact underlying mechanisms remain elusive, we recently demonstrated the importance of tyrosine phosphorylation in this process.6
CD45 is a tyrosine phosphatase expressed at high density on myeloid and lymphoid cells, as well as most AML blasts, and is not internalized after antibody engagement.7-9 While studies with unconjugated cytolytic anti-CD45 antibodies have been pursued,10 anti-CD45 antibodies have also been explored for targeted delivery of radiation to intensify conditioning before hematopoietic cell transplantation.11 Although encouraging,12,13 this therapy is currently limited to selected patients and is associated with substantial toxicity.
Since previous studies showed that CD45 interacts with CD22, a protein that is related to CD33, and that cross-linking of CD45 induces tyrosine phosphorylation of CD22,14-16 we reasoned that CD45 engagement could impact CD33 function and potentially GO cytotoxicity. In an attempt to develop and evaluate an efficacious and minimally toxic immunotherapeutic approach, we therefore investigated the effects of the combination of an unconjugated anti-CD45 antibody (BC8) with GO on AML cells in vitro and in vivo.
Methods
Human AML cell lines and primary AML blast cell samples
ML-1, HL-60, and NB4 cells were maintained as described.6,17,18 Thawed aliquots of frozen samples of density gradient–isolated mononuclear cells containing leukemic blasts from patients with non-M3 AML were cultured in IMDM (GIBCO-Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 25 ng/mL human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF), and interleukin-3 (IL-3), respectively (PeproTech, Rocky Hill, NJ). All patients signed informed consents in accordance with the Declaration of Helsinki, and the institutional review board of the Fred Hutchinson Cancer Research Center approved all protocols.
Purification of BC8 antibody
The murine BC8 IgG1 antibody was produced as described.19
Assays for drug-induced cytotoxicity
Drug-induced cytotoxicity of GO, N-acetyl gamma calicheamicin dimethyl hydrazine (referred to as calicheamicin-γ1), or hP67.6 (all kindly provided by Wyeth-Ayerst Research, Radnor, PA) was determined in the presence or absence of BC8 and/or a nonbinding murine IgG1 isotype antibody (31A)20 as described.5,18 Cultures with primary AML blasts additionally contained a low, subsaturating concentration (25 μM) of the drug efflux inhibitor, PK11195,5,18 to partially reverse drug resistance.
Determination of CD33 expression and internalization
Mouse model of human AML
Female athymic Balb/c-nu/nu mice (Harlan Sprague Dawley, Indianapolis, IN) aged 6 to 8 weeks were maintained under protocols approved by our Institutional Animal Care and Use Committee. Mice were injected with 6 × 107 HL-60 cells subcutaneously in each flank. Approximately 10 days later, mice with similar tumor sizes (∼ 500 mm3) were selected and randomized to treatment groups. GO was administered intraperitoneally on days +1, +5, and +9 at 5 μg GO/animal per dose with a second round of GO administered on days +22, +26, and +30. BC8 was administered at 10 μg/animal per dose intraperitoneally on a cycle schedule of 5 consecutive days followed by no therapy for the subsequent 2 days. The first cycle of BC8 was delivered on day +1, and cycles continued until day +40. Animals were monitored for general appearance, weight change, and tumor volumes.18,21 Mice were killed if tumors caused discomfort, impaired ambulation, or weight loss of more than 30% of starting weight.
Statistical analysis
Parametric statistical analyses were performed using repeated measures ANOVA with Tukey-Kramer Multiple Comparisons Test (InStat 3.05; GraphPad, San Diego, CA).
Results and discussion
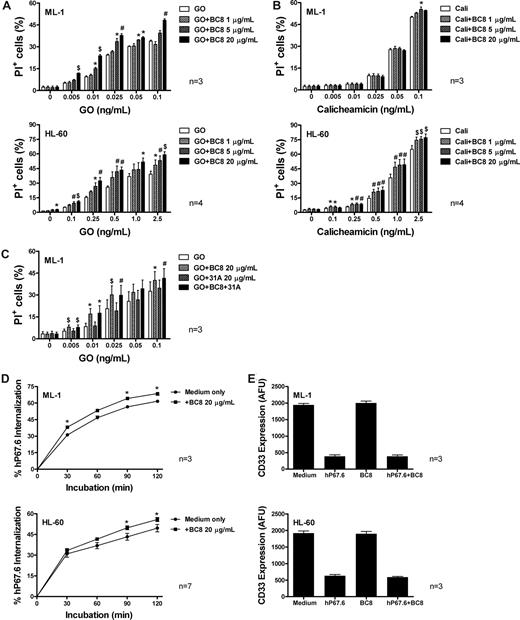
To determine the effect of BC8 (unconjugated anti-CD45 antibody) on cytotoxicity of GO or unbound calicheamicin-γ1, we first examined a panel of human AML cell lines (ML-1, HL-60, and NB4). Consistent with previous studies,17,18 continuous exposure to GO or calicheamicin-γ1 for 3 days resulted in dose-dependent cytotoxicity (Figure 1A,B; Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article). Treatment with BC8 alone (up to 20 μg/mL) exerted only minimal cytotoxic effects; however, BC8 significantly enhanced GO-induced cytotoxicity in all cell lines. By comparison, BC8 failed to enhance calicheamicin-γ1–induced cytotoxicity in ML-1 cells, and increased calicheamicin-γ1–induced cytotoxicity in HL-60 and NB4 cells to a lesser degree than GO. Unlike GO, unconjugated anti-CD33 antibody (hP67.6; up to 25 μg/mL) either alone or combined with BC8 (up to 20 μg/mL) failed to exert any cytotoxic effect on ML-1 or HL-60 cells (Figure S2), demonstrating that CD33 cross-linking by hP67.6, either alone or with BC8, does not elicit significant growth inhibition in the absence of toxin delivery. This contrasts with previous studies, in which an unconjugated murine IgG1 anti-CD33 antibody induced apoptosis of AML blasts.22 The reasons for these different results are unclear and will require further investigation. Finally, 31A, a nonbinding antibody of the same isotype as BC8, neither affected GO- or calicheamicin-γ1–induced cytotoxicity (Figure S3) nor interfered with the effect of BC8 on GO cytotoxicity (Figure 1C).
Effect of BC8 on GO- and calicheamicin-γ1–induced cytotoxicity as well as CD33 internalization and modulation in human AML cell lines in vitro. (A,B) Drug-induced cytotoxicity. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated with various concentrations of (A) GO or (B) calicheamicin-γ1 for 3 days in the presence or absence of increasing concentrations of BC8. Cytotoxicity was assessed using PI staining and expressed as the percentage of PI+ cells. (C) GO-induced cytotoxicity in presence of BC8 and 31A. ML-1 cells were incubated with various concentrations of GO for 3 days in the presence or absence of BC8 (20 μg/mL) and/or 31A (20 μg/mL). (D) CD33 endocytosis. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated for 30 minutes with medium containing 2.5 μg/mL unconjugated, unlabeled hP67.6 in ice water to prevent internalization during the staining procedure. Cells were then washed in ice-cold PBS to remove unbound antibody, resuspended in antibody-free medium, and incubated at 37°C (in 5% CO2 and air) for various periods of time. Subsequently, cells were chilled and incubated with biotin-conjugated mouse anti–human IgG4 monoclonal antibody (5 μg/mL), followed by incubation with streptavidin-PE conjugate (5 μg/mL) to detect remaining hP67.6 on the cell surface. One sample that was kept in ice water was used to determine the starting level of antibody bound to the cell. (E) CD33 modulation. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated overnight in the presence or absence of hP67.6 and/or BC8. Cell surface CD33 was then measured by subsequent staining with hP67.6, a biotin-conjugated mouse anti–human IgG4 antibody, and a streptavidin-PE conjugate. Results are expressed as arbitrary fluorescence units (AFU) and shown as mean plus or minus SEM from 3 to 7 independent experiments. *P < .05; #P < .01; $P < .001.
Effect of BC8 on GO- and calicheamicin-γ1–induced cytotoxicity as well as CD33 internalization and modulation in human AML cell lines in vitro. (A,B) Drug-induced cytotoxicity. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated with various concentrations of (A) GO or (B) calicheamicin-γ1 for 3 days in the presence or absence of increasing concentrations of BC8. Cytotoxicity was assessed using PI staining and expressed as the percentage of PI+ cells. (C) GO-induced cytotoxicity in presence of BC8 and 31A. ML-1 cells were incubated with various concentrations of GO for 3 days in the presence or absence of BC8 (20 μg/mL) and/or 31A (20 μg/mL). (D) CD33 endocytosis. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated for 30 minutes with medium containing 2.5 μg/mL unconjugated, unlabeled hP67.6 in ice water to prevent internalization during the staining procedure. Cells were then washed in ice-cold PBS to remove unbound antibody, resuspended in antibody-free medium, and incubated at 37°C (in 5% CO2 and air) for various periods of time. Subsequently, cells were chilled and incubated with biotin-conjugated mouse anti–human IgG4 monoclonal antibody (5 μg/mL), followed by incubation with streptavidin-PE conjugate (5 μg/mL) to detect remaining hP67.6 on the cell surface. One sample that was kept in ice water was used to determine the starting level of antibody bound to the cell. (E) CD33 modulation. ML-1 and HL-60 cells (top and bottom panels, respectively) were incubated overnight in the presence or absence of hP67.6 and/or BC8. Cell surface CD33 was then measured by subsequent staining with hP67.6, a biotin-conjugated mouse anti–human IgG4 antibody, and a streptavidin-PE conjugate. Results are expressed as arbitrary fluorescence units (AFU) and shown as mean plus or minus SEM from 3 to 7 independent experiments. *P < .05; #P < .01; $P < .001.
The more limited effect of BC8 on calicheamicin-γ1–induced cytotoxicity relative to GO-mediated cytotoxicity suggests an interaction with the antibody portion of GO, rather than a sensitizing effect to the toxic moiety, as an important mechanism of action for increased cytotoxicity in the presence of BC8. Indeed, BC8 significantly enhanced internalization of antibody-bound CD33 in all 3 cell lines assessed, whereas it did not alter the degree of CD33 modulation, that is, the decrease in maximal CD33 binding after antibody engagement23 (Figure 1D,E; Figures S3,S4). These data suggest that the effect of BC8 on GO-induced cytotoxicity is, at least partly, mediated by enhanced uptake and intracellular availability of the immunoconjugate, whereas CD33 abundance does not seem to be affected. Additional mechanistic studies will determine whether the effect of BC8 is through CD45-mediated signaling (eg, with changes in tyrosine phosphorylation), as we initially hypothesized. Alternatively, BC8-mediated activation of Fc receptor signaling could underlie its effect on GO cytotoxicity. It is noteworthy that bispecific antibodies between CD33 and the Fc receptor CD64 could inhibit AML cells much more effectively than anti-CD33 antibodies, suggesting that Fc receptor signaling may significantly alter CD33 function.24 Furthermore, it remains to be investigated whether cross-linking of CD45 may activate cell death pathways in AML cells, similar to lymphocytes,25 a possibility that could explain the effect of BC8 on calicheamicin-γ1–induced cytotoxicity.
We also assessed the effect of BC8 on GO cytotoxicity in primary AML samples. In 4 samples tested with adequate cell viability allowing a 3-day culture assay, BC8 (20 μg/mL) significantly enhanced GO (0.25 ng/mL) cytotoxicity, as expressed by a reduction of the live cell fraction (defined as annexin-V−/PI−; from −14.1 ± 5.87 [mean ± SEM] to −27.17 ± 6.51, P < .02) and an increase in the fraction of d-ad cells (PI+; from 15.09 ± 6.20 to 20.61 ± 6.79, P < .05). These limited results demonstrate that the effect of BC8 on GO cytotoxicity is not restricted to AML cell lines but can also be observed in primary AML cells.
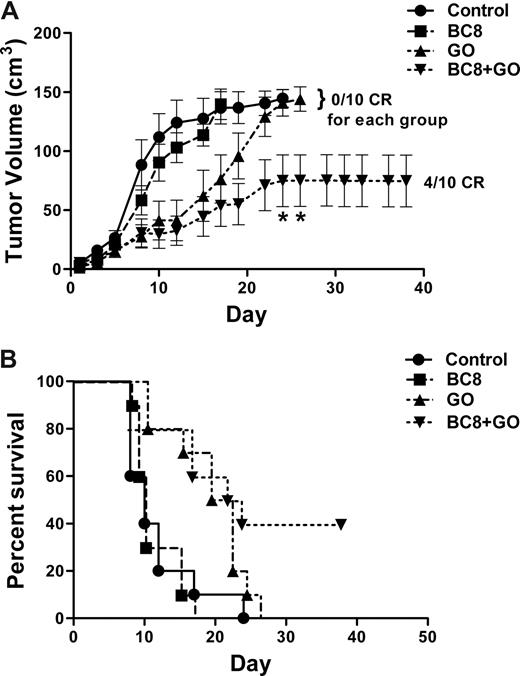
Lastly, we investigated the effect of BC8 on GO cytotoxicity in a human AML xenograft murine model. Mice harboring HL-60 AML xenografts were administered either a regimen of GO or BC8 alone, or the combination of the 2 antibodies. As shown in Figure 2, BC8 significantly enhanced the antitumor effect of GO (P < .05), and prolonged survival of experimental mice (P = .008).
In vivo effect of BC8 on GO-induced cytotoxicity in mice bearing human AML xenografts. (A) Tumor volumes. Athymic mice bearing HL-60 xenografts were randomized and either left untreated (●) or injected intraperitoneally with BC8 (10 μg, ■) on a 5 days on/2 days off schedule for 5 cycles, GO (5 μg, ▴) on days +1, +5, +9, +22, +26, and +30, or BC8 (10 μg) combined with GO (5 μg) (▾) administered on an identical schedule as in the individual treatment group. Tumors were measured in 3 dimensions with calipers 3 times per week. Tumor volumes of dead mice were maintained in the curve until the last mouse of the corresponding group was killed. Results are shown as mean plus or minus SEM. CR denotes complete resolution of measurable tumor. *P < .05 compared with treatment with BC8 alone. (B) Survival. Groups of 10 mice bearing HL-60 tumor xenografts were either left untreated (●) or injected intraperitoneally with BC8 (10 μg, ■), GO (5 μg, ▴), or a combined therapy of BC8 (10 μg) and GO (5 μg) (▾) as described for panel A, and analyzed for survival as a function of time. Proportions of surviving animals in each treatment group are shown. Mice were killed if the tumors caused discomfort, impaired ambulation, or weight loss of more than 30% of starting weight. Four of 10 animals survived after combined therapy with BC8 and GO, whereas none of the animals treated with either BC8 or GO survived (P = .008; Fisher exact test); similarly, all untreated animals died.
In vivo effect of BC8 on GO-induced cytotoxicity in mice bearing human AML xenografts. (A) Tumor volumes. Athymic mice bearing HL-60 xenografts were randomized and either left untreated (●) or injected intraperitoneally with BC8 (10 μg, ■) on a 5 days on/2 days off schedule for 5 cycles, GO (5 μg, ▴) on days +1, +5, +9, +22, +26, and +30, or BC8 (10 μg) combined with GO (5 μg) (▾) administered on an identical schedule as in the individual treatment group. Tumors were measured in 3 dimensions with calipers 3 times per week. Tumor volumes of dead mice were maintained in the curve until the last mouse of the corresponding group was killed. Results are shown as mean plus or minus SEM. CR denotes complete resolution of measurable tumor. *P < .05 compared with treatment with BC8 alone. (B) Survival. Groups of 10 mice bearing HL-60 tumor xenografts were either left untreated (●) or injected intraperitoneally with BC8 (10 μg, ■), GO (5 μg, ▴), or a combined therapy of BC8 (10 μg) and GO (5 μg) (▾) as described for panel A, and analyzed for survival as a function of time. Proportions of surviving animals in each treatment group are shown. Mice were killed if the tumors caused discomfort, impaired ambulation, or weight loss of more than 30% of starting weight. Four of 10 animals survived after combined therapy with BC8 and GO, whereas none of the animals treated with either BC8 or GO survived (P = .008; Fisher exact test); similarly, all untreated animals died.
In conclusion, BC8 significantly increases GO-induced cytotoxicity in human AML cells in vitro as well as in a mouse model in vivo. These data suggest that further investigation of this combination, which may provide improved GO efficacy with relatively low toxicity, is warranted. These findings may also be relevant for other CD33- and possibly CD22-targeting immunoconjugates that depend on cellular uptake for toxic effects.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a Specialized Center of Research grant (no. 7040) from the Leukemia & Lymphoma Society, by National Institutes of Health grants CA091316 and CA095448, and the Frederick Kullman Memorial Fund. R.B.W. is the recipient of a Leukemia & Lymphoma Society Special Fellow Award (no. 3588-07). J.M.P. is supported by Career Development Awards from the Lymphoma Research Foundation and the Damon Runyon Cancer Foundation. I.D.B. is the recipient of an American Cancer Society Clinical Research Professorship (no. CRP-95-129-11).
National Institutes of Health
Authorship
Contribution: R.B.W. designed and performed research, analyzed and interpreted data, and drafted the paper; K.M.B performed research and analyzed data; F.R.A. and I.D.B. interpreted data and drafted the paper; J.M.P. designed research, analyzed and interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland B. Walter, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-373, Seattle, WA 98109-1024; e-mail: rwalter@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal