Abstract
Actin dynamics during T-cell activation are controlled by the coordinate action of multiple actin regulatory proteins, functioning downstream of a complex network of kinases and other signaling molecules. The c-Abl nonreceptor tyrosine kinase regulates actin responses in nonhematopoietic cells, but its function in T cells is poorly understood. Using kinase inhibitors, RNAi, and conditional knockout mice, we investigated the role of c-Abl in controlling the T-cell actin response. We find that c-Abl is required for normal actin polymerization and lamellipodial spreading at the immune synapse, and for downstream events leading to efficient interleukin-2 production. c-Abl also plays a key role in signaling chemokine-induced T-cell migration. c-Abl is required for the appropriate function of 2 proteins known to be important for controlling actin responses to T-cell receptor (TCR) engagement, the actin-stabilizing adapter protein HS1, and the Rac1-dependent actin polymerizing protein WAVE2. c-Abl binds to phospho-HS1 via its SH2 domains and is required for full tyrosine phosphorylation of HS1 during T-cell activation. In addition, c-Abl is required for normal localization of WAVE2 to the immune synapse (IS). These studies identify c-Abl as a key player in the signaling cascade, leading to actin reorganization during T-cell activation.
Introduction
T-cell activation involves regulated reorganization of the actin cytoskeleton. Signaling through chemokine receptors results in loss of microvilli and reorganization of actin filaments to generate a protrusive leading edge, integrin-rich domains associated with adhesion, and a trailing uropod.1-3 Similarly, engagement of the T-cell receptor (TCR) results in the formation of actin-rich clusters of signaling molecules, which converge at the cell-cell contact site to generate the mature immune synapse (IS).1-3 Mutations in actin regulatory proteins are associated with immunodeficiency or autoimmunity,1 emphasizing the importance of appropriate actin dynamics for the immune response.
T-cell actin dynamics are controlled by the coordinate function of several proteins, including WAVE2, WASp, and HS1.1-4 HS1 is a 75-kDa protein expressed specifically in hematopoietic cells. It is related to the more widely expressed protein cortactin (EMS1), an oncogenic actin regulatory protein.5 Like WAVE2 and WASp, HS1 interacts with the Arp2/3 complex to induce actin polymerization.6,7 HS1 also binds F-actin filaments and prolongs their half-life in vitro, and is believed to function largely by stabilizing branched actin filaments generated by WASp and WAVE2. In keeping with this, we have shown that HS1-deficient T cells generate actin-rich structures at sites of TCR engagement, but these structures are disorganized and collapse rapidly, resulting in defective T-cell activation.4
HS1 differs from WAVE2 and WASp in that it is not regulated by Rho GTPases. Instead, HS1 is tyrosine phosphorylated in response to immunoreceptor ligation.4,8-11 Several pieces of evidence indicate that tyrosine phosphorylation of HS1 is important for its function.12-15 Phosphorylation is required for HS1 recruitment to the IS, and for its ability to promote actin polymerization at that site. Phosphorylation also provides binding sites for SH2-containing proteins, including Lck, Vav1, and PLCγ. Interactions with HS1 stabilize Vav1 at the IS,4 but the significance of other SH2 domain interactions is unknown. In B cells and platelets, HS1 phosphorylation involves the synergistic action of Lyn and Syk.10,12 Syk directly phosphorylates tyrosines 378 and 397, an event that is a prerequisite for subsequent phosphorylation at Y222 by Src kinases.10,16 We have shown that Y378 and Y397 are the predominant sites for HS1 phosphorylation in T cells stimulated by TCR cross-linking.4 However, the relevant kinase has not been identified, and other signals may induce modifications at other sites.
Regulation of T-cell actin responses requires upstream signaling through Src, Syk, and Tec family kinases. However, actin responses in other cell types also involve the Abl family of nonreceptor tyrosine kinases.17,18 This family consists of c-Abl and Abl-related gene (Arg). Kinase activity of these proteins is tightly regulated in vivo, and translocation mutants give rise to oncogenic forms such as Bcr-Abl, Tel-Abl, and Tel-Arg.19,20 Abl kinases have a highly conserved N-terminal region containing an SH3 domain, an SH2 domain, and the catalytic domain. The C-terminal portions, which are more divergent, contain actin-binding domains not found in other nonreceptor tyrosine kinases.19,21
c-Abl is broadly expressed, and germ-line deletion leads to increased neonatal mortality.22 Surviving mice exhibit severe defects in lymphocyte development and function, indicating that c-Abl plays an important role in the immune system. Analysis of Arg−/− mice with conditional deletion of Abl in the T-cell lineage demonstrates that the observed defects are cell intrinsic.23 Interestingly, mice expressing a c-Abl truncation mutant that lacks the C-terminal actin-binding domain but retains the kinase domain exhibit similar phenotypes,24 suggesting that actin binding is required for c-Abl function in leukocytes. Arg is expressed most abundantly in the brain,21,25 and Arg-deficient mice exhibit behavioral abnormalities but do not exhibit gross immunologic defects.25 Thus, although there is recent evidence that Arg also functions in T cells,23 it appears that c-Abl is the more important family member in lymphocytes.
Because of their clinical importance, analysis of Abl function in T cells has largely focused on the effects of overexpressing constitutively active Abl and Arg translocation mutants. Relatively little is known about the normal role of c-Abl in T-cell biology. Recently, the Pendergast group has shown that TCR engagement activates Abl kinases, and that Abl kinases promote signaling events leading to IL-2 production and proliferation.23,26 This finding is consistent with recent reports that treatment of chronic myelogenous leukemia (CML) patients with the Abl kinase inhibitor imatinib mesylate (Gleevec, previously STI571; Novartis Pharma, Basel, Switzerland) is associated with immunosuppression in a subset of patients, possibly due to decreased numbers of CD4+ T cells.27,28 The mechanisms by which c-Abl promotes T-cell activation are not well understood. In other cell types, c-Abl has been shown to shuttle between the nucleus and cytoplasm.19,29 In the cytosol, one role of Abl and Arg is to regulate actin cytoskeletal dynamics.19,21 The importance of Abl/Arg as actin-regulatory molecules has not been investigated in T cells.
Boyle et al recently reported that growth factor–induced actin responses in fibroblasts involve tyrosine phosphorylation of the HS1 homolog cortactin by Abl family kinases.30 We therefore asked if HS1 and c-Abl interact in a similar fashion during T-cell activation. Our results show that c-Abl is important for actin responses at the IS and for chemokine-induced T-cell migration. We find that HS1 binds to and can serve as a substrate for Abl family kinases in vitro, and that c-Abl is required for full phosphorylation of HS1 in response to TCR engagement. In addition, we show that c-Abl interacts functionally with other key molecules that work in concert with HS1 to shape the T-cell actin response.
Methods
Details regarding reagents, plasmids, and cell culture can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Luciferase assays and IL-2 ELISAs
Jurkat T cells were transfected with 40 μg of the indicated suppression vectors, together with 5 μg of an interleukin-2 (IL-2) promoter-linked luciferase reporter plasmid (pIL2-luc; gift from Dr David McKean, Mayo Clinic, Rochester, MN), and 50 ng of a TK-renilla reporter plasmid (Promega, Madison, WI). After 48 hours, 106 cells were distributed into 24-well culture plates and stimulated for 6 hours as indicated. Antibody stimulation was performed using 1 μg/mL OKT3 with or without 1 μg/mL α-CD28. Stimulation with antigen-presenting cells (APCs) was performed by adding 5 × 105 Raji B cells/well and SEE (Toxin Technologies, Sarasota, FL) to 1 μg/mL. Samples were assayed on a Berthold LB960 Plate luminometer using the Dual Luciferase Assay kit (Promega), and normalized for Renilla activity.
To assay IL-2 production, APCs were prepared by T-depleting mouse splenocytes by incubation with anti-Thy1 (J1j.10) and rabbit serum complement (Pel-Freez Biologicals, Rogers, AR). Purified mouse primary T cells (2 × 105/well) were incubated with APCs at a ratio of 2:1 with the indicated doses of 2C11 or SEB for 24 hours. IL-2 in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA; eBioscience, San Diego, CA).
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as described previously.4,31 Briefly, CH12 or Raji B cells were stained with CellTracker Blue (Invitrogen, Carlsbad, CA) and pulsed with 10 μM mouse cytochrome c 88-103 peptide or 2 μg/mL SEE, respectively. B cells were conjugated to the appropriate T cells (AND transgenic or Jurkat) for 15 minutes; fixed; labeled with anti-WAVE2, bodipy-phallicidin, or rhodamine-phalloidin (Invitrogen); and mounted using MOWIOL 4-88 (Hoechst Celanese, Charlotte, NC). Cells were imaged on a Axiovert 200M microscope (Zeiss, Thornwood, NY) using a 63×/1.4 NA Plan apo objective. Images were collected using a Coolsnap FX-HQ camera (Roper Scientific, Tucson, AZ), and nearest-neighbor deconvolution was performed using Slidebook version 4.0 software (3I, Denver, CO). Quantitation was performed by randomly selecting conjugates containing a T cell contacting a blue B cell (for shRNA studies, selected conjugates contained a GFP+ T cell) and counting conjugates showing a distinct F-actin band at the contact site. Conjugates scored as polarized typically exhibit average pixel intensities at the IS at least 2 times greater than at other sites along the T-cell surface. All analysis was performed by an individual blinded to experimental conditions. At least 50 conjugates were scored in each of 3 experiments. Data represent the average plus or minus standard deviation; statistical analysis was calculated using a paired Student t test.
Live cell imaging
Time-lapse images of actin dynamics were collected as described previously4 on an inverted Nikon Eclipse TE300, using an UltraVIEW LCI spinning-disk confocal (Perkin-Elmer Life Sciences, Waltham, MA). Delta-T dishes (Bioptechs, Butler, PA) were coated with 20 μg/mL OKT3, blocked with bovine serum albumin (BSA), and maintained at 37°C during imaging. Spreading was initiated by adding a concentrated cell suspension in serum-free RPMI, and images were collected every 5 seconds. Cell areas were determined in pixels using Slidebook. Video sequences were aligned based on initial contact with the coverslip and average cell areas were calculated for the population of cells for each 5-second time point.
Conjugate and chemotaxis assays
Conjugate formation was analyzed as described.32 For chemotaxis assays, T cells were resuspended at 2 × 107/mL in migration buffer (RPMI 1640, 1% fetal bovine serum [FBS], 25 mM HEPES [pH 7.0]) and prewarmed to 37°C with 5% CO2 for 1 hour. ChemoTex chambers (96-well, 5-μm pore size; NeuroProbe, Gaithersburg, MD) were filled with 30 μL migration medium with or without 10 nm CXCL12 (SDF-1α) or CCL21 (SLC; R&D Systems, Minneapolis, MN) in the bottom wells. A total of 5 × 105 cells were applied to the upper wells, and chambers were incubated for 2 hours at 37°C in 5% CO2. Cells in the upper wells were scraped, membranes were washed, and chambers were centrifuged at 500g for 5 minutes to collect cells trapped in the membrane. Migrated cells collected from the bottom wells were counted manually. Migration is shown as the average from triplicate wells, expressed as percentage of input cells.
Determination of cellular F-actin content
Purified mouse primary CD4+ T cells (106 in 50 μL phosphate-buffered saline [PBS]) were incubated with or without 10 μg/mL anti-CD3 (500A2; BD Biosciences, San Jose, CA) for the indicated times at 37°C. The reaction was terminated by adding 400 μL of 3% paraformaldehyde in PBS. Fixed cells were washed once with fluorescence-activated cell sorter (FACS) buffer (PBS, 5% FBS, 1 mM EDTA, and 0.02% sodium azide), once with permeabilization buffer (PBS, 0.1% saponin, and 1% FBS), and resuspended in permeabilization buffer containing bodipy-phallicidin for 30 minutes. After staining, cells were washed twice with permeabilization buffer, resuspended in FACS buffer, and analyzed by flow cytometry.
Cell stimulation, affinity precipitation, and immunoblot analysis
Jurkat lysates were prepared as described previously.33 Cells were treated with or without imatinib mesylate or transfected with vector or shAbl-3, and stimulated with C305 for the indicated times. Alternatively, cells were treated with pervanadate to promote tyrosine phosphorylation. Whole-cell lysates made from 1 to 2 × 107 cells were incubated with HS1 or CrkL antibodies for immunoprecipitations, or with GST-tagged SH2 domains for affinity precipitation. The Rac1/Cdc42 pull-down assay was done as described.34 Proteins were resolved on 4% to 12% NuPage gels (Invitrogen), transferred to nitrocellulose membranes, and probed with primary antibodies followed by secondary antibodies (Alexa Fluor 680 antimouse [Invitrogen] or IR Dye 800 antirabbit [Rockland, Gilbertsville, PA]). Images were acquired and quantified using the Odyssey Imaging system (LI-COR Biosciences, Lincoln, NE).
In vitro kinase assays
A total of 5 to 10 μg recombinant HS1 was incubated with 0.25 to 1 μg recombinant Abl or Arg in 25 mM HEPES (pH 7.2), 100 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, 1 mM DTT, and 100 μM ATP. The reaction was conducted at 30°C for 30 minutes and quenched by adding 4× sodium dodecylsulfate (SDS) buffer and heating for 3 minutes. Proteins were resolved by SDS-polyacrylamide gel electophoresis and immunoblotted with antiphosphotyrosine (4G10) and anti-HS1.
Results
Abl family kinases bind to and phosphorylate HS1
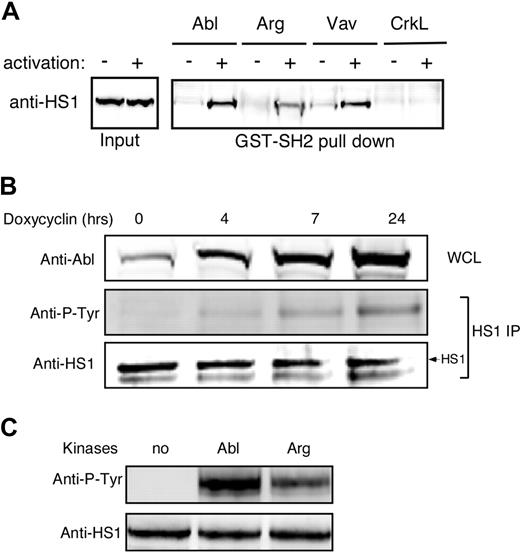
We showed previously that HS1 functions in a phosphorylation-dependent manner to stabilize F-actin at the IS.4 One role for HS1 phosphorylation is to mediate binding to SH2 domain-containing signaling molecules, including the Rho GEF Vav-1. Since the HS1 homolog cortactin was recently identified as a substrate for Abl family kinases in nonhematopoietic cells,30 we asked if phospho-HS1 also interacts with Abl family kinases. As shown in Figure 1A, GST-tagged SH2 domains of both Abl and Arg bound to HS1 from activated T-cell lysates, and the Abl SH2 domain did so with similar efficiency to Vav-1. A control SH2 domain from CrkL failed to associate with HS1, demonstrating specificity of the interaction. Interestingly, the Abl SH2 domain reproducibly bound more HS1 than the Arg SH2 domain (Figures 1A,S1).
Abl family kinases bind and phosphorylate HS1. (A) Jurkat T cells were treated with or without pervanadate for 10 minutes, and lysates were subjected to affinity precipitation with the SH2 domains of indicated proteins. The precipitates were analyzed by immunoblotting with anti-HS1. (B) Ba/F3 pTET-ON p210 Bcr-Abl–inducible cells were treated with 1 μg/mL doxycycline for the indicated times. Bcr-Abl expression was assessed by immunoblotting of whole-cell lysates (WCL). HS1 was immunoprecipitated from cell lysates and phosphorylation was assessed by blotting for phosphotyrosine (Anti-P-Tyr). (C) A total of 10 μg of recombinant HS1 in 30 μL phosphorylation buffer was incubated with or without 0.5 μg of recombinant Arg or Abl kinases at 30°C for 10 minutes and immunoblotted with antiphosphotyrosine and anti-HS1.
Abl family kinases bind and phosphorylate HS1. (A) Jurkat T cells were treated with or without pervanadate for 10 minutes, and lysates were subjected to affinity precipitation with the SH2 domains of indicated proteins. The precipitates were analyzed by immunoblotting with anti-HS1. (B) Ba/F3 pTET-ON p210 Bcr-Abl–inducible cells were treated with 1 μg/mL doxycycline for the indicated times. Bcr-Abl expression was assessed by immunoblotting of whole-cell lysates (WCL). HS1 was immunoprecipitated from cell lysates and phosphorylation was assessed by blotting for phosphotyrosine (Anti-P-Tyr). (C) A total of 10 μg of recombinant HS1 in 30 μL phosphorylation buffer was incubated with or without 0.5 μg of recombinant Arg or Abl kinases at 30°C for 10 minutes and immunoblotted with antiphosphotyrosine and anti-HS1.
As one means of asking if Abl family kinases can interact with HS1 in cells, we used Ba/F3 pTET-ON p210 cells, which inducibly express the oncogenic form of Bcr-Abl.35 Cells were cultured in the presence of doxycycline to induce increasing levels of Bcr-Abl expression, and HS1 phosphorylation was assessed by immunoprecipitation and immunoblotting with antiphosphotyrosine antibody. HS1 phosphorylation increased linearly with Bcr-Abl induction (Figure 1B). To test whether Abl family kinases can phosphorylate HS1 directly, recombinant HS1 was incubated in vitro with recombinant Abl or Arg, and HS1 phosphorylation was assessed. As shown in Figure 1C, both Abl and Arg could phosphorylate HS1 in vitro. Incubation with c-Abl consistently resulted in stronger phosphorylation than incubation with Arg, again suggesting that HS1 preferentially interacts with c-Abl over Arg. Taken together, these results show that Abl family kinases can bind to and directly phosphorylate HS1.
c-Abl is required for full HS1 phosphorylation during T-cell activation
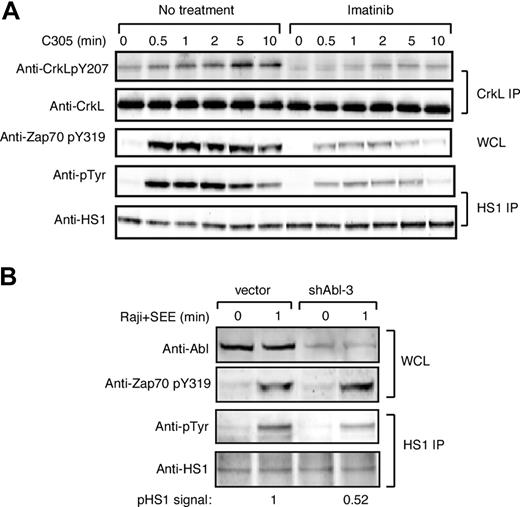
To ask if Abl family kinases contribute to HS1 phosphorylation during T-cell activation, we examined tyrosine phosphorylation of HS1 in Jurkat T cells treated with the Abl family kinase inhibitor imatinib mesylate. To verify inhibition of Abl kinase activity, lysates were immunoprecipitated with anti-CrkL, a well-characterized Abl substrate, and eluates were blotted for phosphotyrosine 207.26,36 As anticipated, CrkL was tyrosine phosphorylated in response to TCR cross-linking, and this was inhibited by treatment with imatinib mesylate (Figure 2A top panels). ZAP-70 phosphorylation at Y319 was also inhibited (Figure 2A middle panel), confirming the findings of Zipfel et al.26 Like CrkL and ZAP-70, HS1 was tyrosine phosphorylated upon TCR cross-linking, and this was inhibited by treatment with imatinib mesylate (Figure 2A bottom panels). Quantitation showed that at the peak of HS1 phosphorylation, imatinib mesylate treatment inhibited HS1 phosphorylation by 4.6-fold.
c-Abl is required for efficient HS1 phosphorylation in response to TCR engagement. (A) Jurkat T cells were pretreated with or without 10 μM imatinib mesylate for 1 hour, and then stimulated with C305 for indicated times. Lysates were immunoprecipitated with anti-CrkL or anti-HS1, and immunoblotted with indicated antibodies. Phosphorylation of ZAP-70 at tyrosine 319 was assessed by immunoblotting of whole-cell lysates. (B) Jurkat T cells were transfected with control vector or shAbl-3 and cultured for 48 hours to suppress c-Abl expression. Cells were stimulated with or without SEE-pulsed Raji B cells for 1 minute, and lysates were immunoprecipitated with anti-HS1 and immunoblotted with the indicated antibodies. Abl expression and ZAP-70 phosphorylation was assessed by immunoblotting of whole-cell lysates.
c-Abl is required for efficient HS1 phosphorylation in response to TCR engagement. (A) Jurkat T cells were pretreated with or without 10 μM imatinib mesylate for 1 hour, and then stimulated with C305 for indicated times. Lysates were immunoprecipitated with anti-CrkL or anti-HS1, and immunoblotted with indicated antibodies. Phosphorylation of ZAP-70 at tyrosine 319 was assessed by immunoblotting of whole-cell lysates. (B) Jurkat T cells were transfected with control vector or shAbl-3 and cultured for 48 hours to suppress c-Abl expression. Cells were stimulated with or without SEE-pulsed Raji B cells for 1 minute, and lysates were immunoprecipitated with anti-HS1 and immunoblotted with the indicated antibodies. Abl expression and ZAP-70 phosphorylation was assessed by immunoblotting of whole-cell lysates.
Although imatinib mesylate is highly specific for Abl family kinases, it was important to control for possible off-target drug effects. Moreover, pharmacologic treatment tests only the effects of kinase activity, and it is well known that Abl kinases engage in important kinase-independent adaptor functions.24,37,38 We therefore developed RNAi-based vectors to suppress protein expression. Because we cannot detect Arg expression in T cells using available antibodies, we focused on suppressing c-Abl. Two independent targeting constructs (shAbl-1 and shAbl-3) were generated. Semiquantitative Western blotting revealed that both constructs suppressed Abl expression by approximately 60% to 70% in a population of cells transfected with an efficiency of approximately 80% (Figure 2B top panel; data not shown). The results using both constructs were similar, however, since shAbl-1 nonspecifically suppressed irrelevant target proteins; only data obtained from shAbl-3 are shown. Jurkat T cells transfected for 48 hours with shAbl-3 were stimulated with SEE-pulsed APCs, and phosphorylation of immunoprecipitated HS1 was assessed. Abl suppression reduced TCR-stimulated tyrosine phosphorylation of HS1 by approximately 50% (Figure 2B), confirming that c-Abl contributes to HS1 phosphorylation. Interestingly, unlike treatment with imatinib mesylate, Abl suppression did not affect ZAP-70 phosphorylation, suggesting that the loss of ZAP-70 pY319 may be due to an off-target effect of imatinib mesylate. Together with the inhibitor studies, these results demonstrate that c-Abl is required for efficient tyrosine phosphorylation of HS1 in response to TCR engagement. Moreover, our data are consistent with the idea that c-Abl and ZAP-70 each contribute to the overall tyrosine phosphorylation of HS1.
c-Abl regulates IL-2 transcription during T-cell activation
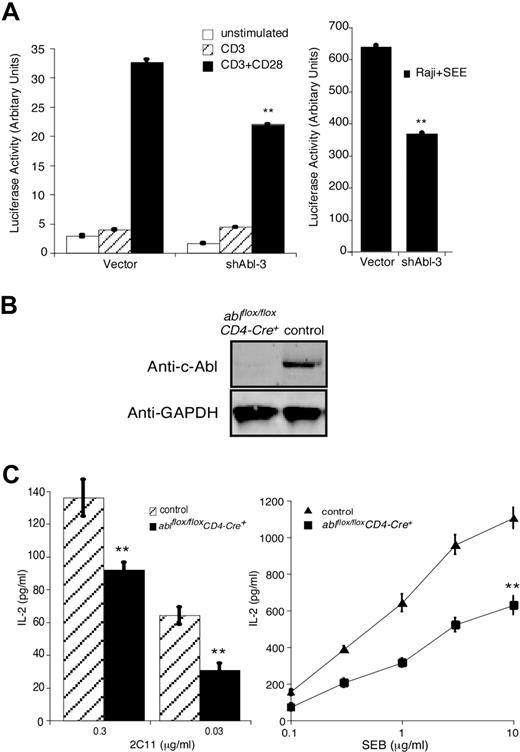
Since HS1 is required for IL-2 promoter activation in response to TCR engagement,4 we assessed IL-2 promoter activation in Jurkat T cells cotransfected with shAbl-3 and a luciferase reporter linked to the IL-2 promoter. Abl suppression reduced IL-2 promoter activation in T cells stimulated with either anti-CD3/anti-CD28 antibodies or with Raji B cells pulsed with SEE (Figure 3A). These results are in agreement with the findings of Zipfel et al in imatinib mesylate–treated T cells.26 As an alternate approach, ablflox/flox mice were bred to CD4-Cre+ transgenic mice39 to generate mice lacking c-Abl in the mature T-cell compartment. T cells from these ablflox/floxCD4-Cre+ mice develop normally and exhibit normal levels of TCR and activation/memory markers (data not shown). They do not express c-Abl protein (Figure 3B). When these c-Abl–deficient primary T cells were stimulated with anti-CD3 or SEB-pulsed APCs, they produced significantly less IL-2 than T cells from control littermate mice (Figure 3C).
c-Abl is required for IL-2 production in T cells. (A) Jurkat T cells were transfected with vector or shAbl-3 together with a luciferase reporter gene under the control of the IL-2 promoter. After 48 hours, cells were stimulated with the indicated antibodies (left) or with Raji B cells pulsed with SEE (right) for 6 hours, and IL-2 promoter activity was assayed. (B) T cells purified from lymph nodes and spleens of ablflox/floxCD4-Cre+ and littermate control mice (ablflox/floxCD4-Cre−) were lysed and blotted with anti–c-Abl. GAPDH expression from the same blot is shown as a loading control. (C) T cells prepared as in panel B were incubated with APCs plus the indicated doses of 2C11 or SEB for 24 hours. IL-2 levels in the supernatant were measured by ELISA. All data represent the average plus or minus the standard deviation of quadruplicate samples from 1 experiment (representative of 3 independent experiments). **P < .01.
c-Abl is required for IL-2 production in T cells. (A) Jurkat T cells were transfected with vector or shAbl-3 together with a luciferase reporter gene under the control of the IL-2 promoter. After 48 hours, cells were stimulated with the indicated antibodies (left) or with Raji B cells pulsed with SEE (right) for 6 hours, and IL-2 promoter activity was assayed. (B) T cells purified from lymph nodes and spleens of ablflox/floxCD4-Cre+ and littermate control mice (ablflox/floxCD4-Cre−) were lysed and blotted with anti–c-Abl. GAPDH expression from the same blot is shown as a loading control. (C) T cells prepared as in panel B were incubated with APCs plus the indicated doses of 2C11 or SEB for 24 hours. IL-2 levels in the supernatant were measured by ELISA. All data represent the average plus or minus the standard deviation of quadruplicate samples from 1 experiment (representative of 3 independent experiments). **P < .01.
c-Abl regulates actin responses during T-cell activation
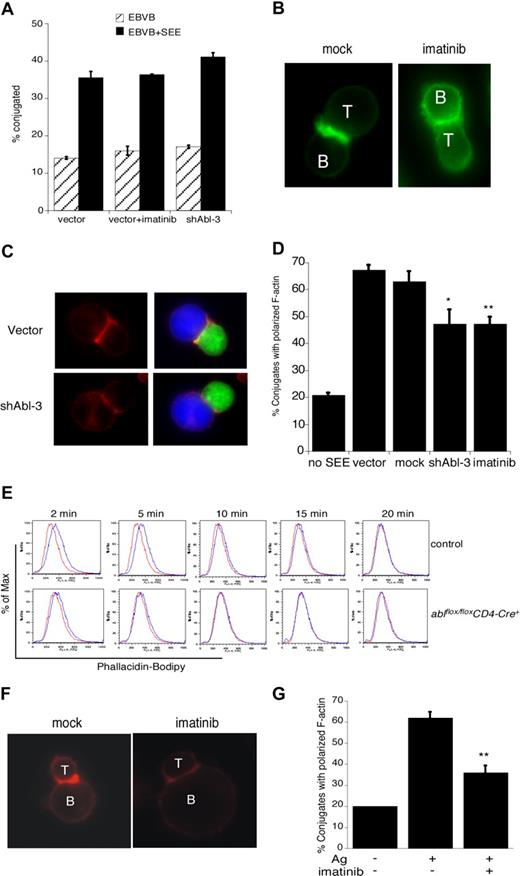
Although it is well established that c-Abl regulates actin dynamics in nonhematopoietic cells, its actin regulatory role in T cells has not been investigated. We and others have shown that disruption of some actin regulatory pathways (eg, loss of Vav1 or WAVE2) leads to defects in T-cell conjugate formation, while disruption of other pathways (eg, loss of WASp or HS1) does not.4,40,41 To ask if c-Abl is required for APC binding, Jurkat T cells were treated with imatinib mesylate or transfected with shAbl-3 to suppress c-Abl expression, and their ability to form conjugates with SEE-pulsed B cells was assessed by flow cytometry. As shown in Figure 4A, conjugate formation proceeded with normal efficiency. Thus, Abl, like HS1 and WASp, is dispensable for conjugate formation in this assay.
c-Abl is required for actin polymerization in response to TCR engagement. (A) Jurkat T cells were transfected with control vector or shAbl-3. At 48 hours after transfection, cells were treated with or without imatinib mesylate for 1 hour, and conjugate formation with SEE-pulsed (or unpulsed) Epstein-Barr virus (EBV) B cells was analyzed by flow cytometry. (B) Jurkat T cells were mock treated or treated with 10 μM imatinib mesylate for 1 hour and allowed to conjugate with SEE-pulsed Raji B cells for 15 minutes. Conjugates were fixed, and F-actin was labeled with bodipy-phallicidin (green). (C) Jurkat T cells were transfected with control vector or shAbl-3 and allowed to conjugate with SEE-pulsed Raji B cells as in panel B. F-actin was labeled with rhodamine-phalloidin (red). B cells are labeled blue with CMAC; T cells expressing the suppression vectors are green due to GFP expression. (D) Conjugates prepared as in panels B and C were scored for the frequency of F-actin at the IS. Data are averages from 3 independent experiments, each with 50 randomly selected conjugates. **P < .01; *P < .05. (E) Purified primary lymph node CD4+ T cells from ablflox/floxCD4-Cre+ mice and control littermates were stimulated with 10 μg/mL anti-CD3 for the indicated times. Cells were fixed, labeled with bodipy-phallicidin, and analyzed by flow cytometry. Red indicates unstimulated; blue, anti-CD3–stimulated. Representative data from one of 3 independent experiments performed in duplicate is shown. (F) Purified CD4+ T cells from AND TCR transgenic mice were blasted and treated with 10 μM imatinib mesylate for 1 hour. Cells were conjugated with CH12 B cells prepulsed with or without 10 μM moth cytochrome c 88-103 peptide. Conjugates were fixed, and F-actin was stained with rhodamine-phalloidin. (G) Conjugates prepared as in panel F were scored for the frequency of F-actin at the IS. Data are averages plus or minus 1 SD from 3 independent experiments, each with 50 randomly selected conjugates; **P < .01.
c-Abl is required for actin polymerization in response to TCR engagement. (A) Jurkat T cells were transfected with control vector or shAbl-3. At 48 hours after transfection, cells were treated with or without imatinib mesylate for 1 hour, and conjugate formation with SEE-pulsed (or unpulsed) Epstein-Barr virus (EBV) B cells was analyzed by flow cytometry. (B) Jurkat T cells were mock treated or treated with 10 μM imatinib mesylate for 1 hour and allowed to conjugate with SEE-pulsed Raji B cells for 15 minutes. Conjugates were fixed, and F-actin was labeled with bodipy-phallicidin (green). (C) Jurkat T cells were transfected with control vector or shAbl-3 and allowed to conjugate with SEE-pulsed Raji B cells as in panel B. F-actin was labeled with rhodamine-phalloidin (red). B cells are labeled blue with CMAC; T cells expressing the suppression vectors are green due to GFP expression. (D) Conjugates prepared as in panels B and C were scored for the frequency of F-actin at the IS. Data are averages from 3 independent experiments, each with 50 randomly selected conjugates. **P < .01; *P < .05. (E) Purified primary lymph node CD4+ T cells from ablflox/floxCD4-Cre+ mice and control littermates were stimulated with 10 μg/mL anti-CD3 for the indicated times. Cells were fixed, labeled with bodipy-phallicidin, and analyzed by flow cytometry. Red indicates unstimulated; blue, anti-CD3–stimulated. Representative data from one of 3 independent experiments performed in duplicate is shown. (F) Purified CD4+ T cells from AND TCR transgenic mice were blasted and treated with 10 μM imatinib mesylate for 1 hour. Cells were conjugated with CH12 B cells prepulsed with or without 10 μM moth cytochrome c 88-103 peptide. Conjugates were fixed, and F-actin was stained with rhodamine-phalloidin. (G) Conjugates prepared as in panel F were scored for the frequency of F-actin at the IS. Data are averages plus or minus 1 SD from 3 independent experiments, each with 50 randomly selected conjugates; **P < .01.
To ask if c-Abl is required for actin responses at the IS, conjugates were fixed and F-actin was labeled with bodipy-phallicidin. Conjugates formed using mock-treated T cells exhibited a bright band of F-actin at the IS (Figure 4B). This response was inhibited by treatment with imatinib mesylate. Similar defects were observed in Jurkat T cells expressing the c-Abl suppression vector (Figure 4C). Analysis of the frequency of conjugates showing a productive F-actin response revealed that inhibition of Abl kinase activity or suppression of c-Abl expression significantly diminished F-actin responses (Figure 4D).
Since it is well known that T-cell tumor lines can exhibit signaling abnormalities, it was important to ask if c-Abl is also required for actin responses in primary T cells. We therefore used a flow cytometric assay to assess activation-induced changes in F-actin content of naive mouse CD4+ T cells. T cells from control mice exhibited a transient increase in F-actin content between 2 and 10 minutes after CD3 engagement (Figure 4E). In T cells from ablflox/floxCD4-Cre+ mice, however, the magnitude of the response was significantly dampened and its duration shortened. To assess the role of c-Abl in antigen-specific actin responses, T cells from AND TCR transgenic mice were incubated in the presence or absence of imatinib mesylate. Conjugates were then formed with CH12 B cells in the presence or absence of MCC88-103 peptide, and labeled with rhodamine-phalloidin. As in Jurkat T cells, Abl inhibition in primary T cells resulted in defects in actin polymerization at the IS (Figure 4F,G). Taken together, these results show that c-Abl kinase function is required for actin polymerization in response to TCR engagement.
In addition to its role in signaling at the IS, the actin cytoskeleton is crucial for chemokine-directed T-cell migration. To ask if c-Abl is required for chemotaxis, we assessed the ability of Abl-suppressed Jurkat T cells to migrate toward a gradient of CXCL12. As shown in Figure 5A, Abl suppression dramatically reduced T-cell chemotaxis. Similar results were obtained with naive mouse primary T cells responding to the chemokines CCL21 and CXCL12, which signal through CCR7 and CXCR4, respectively (Figure 5B).42 We conclude that c-Abl is also required for T-cell migration.
c-Abl is required for chemokine-induced T-cell migration. (A) Jurkat T cells were transfected with control vector or shAbl-3, and migration in response to CXCL12 stimulation was tested using a Transwell assay. (B) Lymph node CD4+ T cells were prepared from ablflox/floxCD4-Cre+ mice and control littermates, and migration in response to CXCL12 or CCL21 was tested using a Transwell assay. In each case, representative data (averages of quadruplicate samples plus or minus 1 SD) from one of 3 independent experiments are shown. **P < .01; *P < .05.
c-Abl is required for chemokine-induced T-cell migration. (A) Jurkat T cells were transfected with control vector or shAbl-3, and migration in response to CXCL12 stimulation was tested using a Transwell assay. (B) Lymph node CD4+ T cells were prepared from ablflox/floxCD4-Cre+ mice and control littermates, and migration in response to CXCL12 or CCL21 was tested using a Transwell assay. In each case, representative data (averages of quadruplicate samples plus or minus 1 SD) from one of 3 independent experiments are shown. **P < .01; *P < .05.
c-Abl is required for TCR-induced lamellipodial protrusion
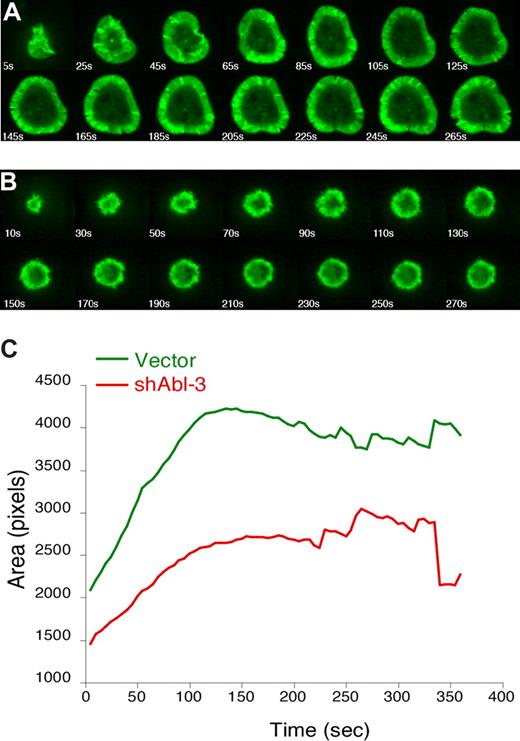
Endpoint analysis of fixed conjugates reveals net effects on F-actin responses, but does not address how these effects occur. For example, T cells deficient for either WAVE2 or HS1 exhibit similar defects in F-actin at the IS in fixed conjugates, but video analysis reveals that WAVE2-deficient cells fail to generate actin-rich structures, whereas loss of HS1 leads to enhanced disassembly of these structures.4,41 To visualize actin dynamics in the absence of c-Abl, Jurkat T cells stably expressing GFP-actin were transfected with shAbl-3 or control vector and monitored by video microscopy while spreading on anti-CD3 coated coverslips. Control T cells (Figure 6A; Videos S1,S2) exhibited normal spreading, characterized by the formation of a round contact site and a broad, regular, actin-rich lamellipodium. However, Abl-suppressed T cells spread inefficiently and exhibited profound defects in the extent and persistence of lamellipodial structures (Figure 6B; Videos S3,S4). Measurement of spreading (calculated as the average area of contact with the coverslip for the population of cells at each 10-second time point) shows that Abl-suppressed T cells fail to undergo the increase in footprint area characteristic of control cells (Figure 6C). The difference in spreading was statistically significant (P = .005 at the 130-second time point). This demonstrates that c-Abl is required for the organization of actin-rich protrusions in response to TCR stimulation. In this respect, the phenotype of Abl-deficient T cells is more profound than that of HS1-deficient cells, suggesting that c-Abl also affects actin responses via additional, HS1-independent mechanisms.
c-Abl is required to organize TCR-induced lamellipodial protrusions. Jurkat T cells stably expressing GFP-actin were transfected with control vector (A) or shAbl-3 (B). Actin dynamics were monitored by video confocal microscopy as the cells spread on anti-TCR–coated coverslips. Selected time-lapse image projections acquired at the indicated times after contact with the coverslip are shown. (C) The contact area of each cell at each time point was determined, and the average was calculated for each cell population at each 5-second time point (40 cells for control vector, and 26 cells for shAbl-3–transfected cells). The difference in area was statistically significant (P = .005 at 130 seconds).
c-Abl is required to organize TCR-induced lamellipodial protrusions. Jurkat T cells stably expressing GFP-actin were transfected with control vector (A) or shAbl-3 (B). Actin dynamics were monitored by video confocal microscopy as the cells spread on anti-TCR–coated coverslips. Selected time-lapse image projections acquired at the indicated times after contact with the coverslip are shown. (C) The contact area of each cell at each time point was determined, and the average was calculated for each cell population at each 5-second time point (40 cells for control vector, and 26 cells for shAbl-3–transfected cells). The difference in area was statistically significant (P = .005 at 130 seconds).
c-Abl is required for appropriate recruitment of WAVE2 to the IS independent of Rac1 activation
The generation of lamellipodia in T cells depends on activation of the Arp2/3 complex by WAVE2, which is itself activated by binding to the GTP-bound form of Rac1.41,43 We therefore asked if c-Abl activity is required for efficient Rac1 activation in response to TCR engagement. In parallel, we tested activation of Cdc42, a related GTPase important for activation of WASP, which also been implicated in driving actin responses at the IS.44,45 Jurkat T cells treated with imatinib mesylate were stimulated with anti-CD3 and the levels of activated Rac1 and Cdc42 were assessed.34 As shown in Figure 7A, within 0.5 to 2 minutes after TCR stimulation, control T cells exhibited activation of both Cdc42 and Rac1. Neither the magnitude nor the kinetics of activation was affected by treatment with imatinib mesylate. We conclude that Abl kinase activity is not required for Rac1 or Cdc42 activation in response to TCR engagement.
c-Abl is required for WAVE2 localization independent of Rac1/Cdc42 activation. (A) Jurkat T cells were treated with or without 10 μM of imatinib mesylate for 1 hour, and cells were stimulated with C305 for indicated times, lysed, and affinity precipitated with GST-Pak-CRIB, followed by immunoblotting with antibodies to Rac1 and Cdc42. (B) Jurkat T cells transfected with control vector or shAbl-3 (green) were conjugated with SEE pulsed Raji B cells (blue). Conjugates were fixed and labeled for WAVE2 (red).
c-Abl is required for WAVE2 localization independent of Rac1/Cdc42 activation. (A) Jurkat T cells were treated with or without 10 μM of imatinib mesylate for 1 hour, and cells were stimulated with C305 for indicated times, lysed, and affinity precipitated with GST-Pak-CRIB, followed by immunoblotting with antibodies to Rac1 and Cdc42. (B) Jurkat T cells transfected with control vector or shAbl-3 (green) were conjugated with SEE pulsed Raji B cells (blue). Conjugates were fixed and labeled for WAVE2 (red).
Although Rac1 activation is likely to be necessary for WAVE2 activation at the IS, other factors are also required. WAVE2 functions in T cells as part of a larger protein complex,41 and the mechanisms that regulate WAVE2 localization and activation at the IS are not well defined. c-Abl has been implicated as a regulator of WAVE function because it binds to Abi1/2, components of the WAVE complex, and can phosphorylate these proteins as well as WAVE itself.46,47 We therefore asked if c-Abl is required for localization of WAVE2 to the IS, an event which is required for proper WAVE function.48 Jurkat T cells transfected with shAbl-3 or control vector were conjugated to Raji B cells, and localization of WAVE2 was assessed. As reported previously,41,48 conjugates formed using control T cells exhibited WAVE2 labeling along the length of the IS (Figure 7B), In conjugates formed with Abl-suppressed T cells, WAVE2 recruitment to the IS was still detectable, but the pattern of localization was highly abnormal. Rather than running continuously along the length of the cell-cell contact zone, WAVE2 was restricted to short regions of the interface or was punctate in distribution. This result indicates that c-Abl plays a role in organizing the WAVE complex at the IS.
Discussion
c-Abl is well established as an actin regulatory protein in nonhematopoietic cells.17,21 c-Abl binds to F-actin and localizes to actin-rich structures such as focal adhesions, lamellipodia, filopodia, membrane ruffles, and neuronal extensions and synapses.17 Moreover, c-Abl interacts with numerous actin-regulatory proteins and promotes cytoskeletal rearrangements, leading to changes in cell shape and motility.21 The role of c-Abl in hematopoietic cells is much less well understood, partly because the literature has focused on leukemogenesis associated with overexpression of constitutively active Abl translocation mutants, and partly because mice with germ-line mutations in c-Abl exhibit serious developmental abnormalities. Recent work from the Pendergast lab shows that Abl family kinases are activated upon TCR engagement, and are required for TCR signaling events leading to efficient IL-2 promoter activation.23,26 Our results support these findings, and additionally show that c-Abl is required for actin responses during both TCR and chemokine signaling. We find that T cells lacking c-Abl or cells in which Abl kinase activity is inhibited fail to efficiently polymerize actin and extend actin-rich lamellipodia in response to TCR engagement. The effects of c-Abl suppression on actin responses at the IS are similar to those of inhibiting kinase activity, suggesting that the kinase activity of c-Abl, rather than its ability to bind F-actin, is most important in this context.
In T cells, we have identified 2 actin regulatory proteins that interact functionally with c-Abl, HS1 and WAVE2. We show that optimal TCR-induced tyrosine phosphorylation of HS1 depends on the expression of active c-Abl kinase. HS1 contains a reasonably good consensus Abl phosphorylation site at Y360. The homologous tyrosine in cortactin (Y421) is among the sites identified by Boyle et al as substrates for Abl/Arg phosphorylation.30 Thus, HS1 may be directly phosphorylated by c-Abl at this site. We have shown previously that HS1 is phosphorylated at 2 distinct tyrosines, Y378 and Y397, in a ZAP-70–dependent manner.4 Zipfel et al have reported that Abl functions upstream of ZAP-70 in the T-cell activation pathway, raising the possibility that Abl promotes HS1 phosphorylation indirectly, via ZAP-70.26 However, since we observe defects in HS1 phosphorylation in Abl-suppressed cells, where ZAP-70 phosphorylation is intact, we favor the idea that c-Abl and ZAP-70 both phosphorylate HS1. One interesting possibility is that ZAP-70 and c-Abl might respond to distinct stimuli. For example, Abl family kinases are known in other cell types to respond to integrin ligation.17,49 If ZAP-70 phosphorylates HS1 primarily in response to TCR ligation, while Abl phosphorylates HS1 in response to integrin binding, this could explain why HS1 phosphorylation is more sustained in response to activation by APCs.4 Another intriguing possibility, not mutually exclusive with the first, is that ZAP-70 and Abl phosphorylate HS1 in a contingent fashion, as proposed for Syk and Src kinases.10 Indeed, the 2 phosphorylation sites phosphorylated by Syk kinases in vitro, Y378 and Y397,4 lie within consensus binding sites for the Abl SH2 domain,50 and we found that the SH2 domains of Abl family kinases bind to phospho-HS1. This interaction could relieve the autoinhibited conformation of Abl, bringing the kinase domain into contiguity with Y360. Additional study will be required to resolve the mechanisms by which c-Abl and ZAP-70 function together regulate HS1 phosphorylation.
Loss of c-Abl has a more profound effect on lamellipodial spreading than loss of HS1, indicating that c-Abl functions upstream of additional actin regulatory proteins.17 Indeed, it is known from studies in nonhematopoietic cells that c-Abl interacts with numerous actin regulatory proteins, including WAVE family members.51 WAVE2, the predominant WAVE isoform in T cells, is required for actin polymerization at the IS.41,48 WAVE proteins are present as part of a larger protein complex, which includes the Abl-interacting proteins Abi1 or Abi2, and c-Abl associates with the WAVE complex by binding to these proteins.41,46,47 Moreover, in nonhematopoietic cells, c-Abl can directly phosphorylate WAVE2 at Y150, leading to enhanced WAVE2-dependent actin responses.46,47 However, we cannot detect WAVE tyrosine phosphorylation in T cells (data not shown), which is consistent with a report from Nolz et al.41 Abl can also phosphorylate Abi1,52-54 and a recent study in B cells shows that Bcr-Abl–dependent Abi-1 phosphorylation induces Abi/WAVE2 membrane localization.55 This finding is in keeping with our observation that WAVE2 localization to the IS is defective in the absence of c-Abl. At present, we cannot exclude the possibility that the defects in WAVE2 localization are secondary to other defects in IS structure, but it will be interesting to ask if c-Abl–dependent phosphorylation of Abi1/2 is important for recruitment of WAVE2 to the IS.
In addition to its role in controlling actin responses at the IS, we show that c-Abl contributes to the control of chemokine-induced T-cell migration. Studies in nonhematopoietic cells point to a role for c-Abl in controlling actin dynamics during cell migration56-60 ; however, the mechanisms of Abl function in migration are unclear. In an effort to reconcile studies showing that c-Abl inhibits overall cell migration with other work showing that c-Abl promotes the formation of actin-rich projections, it has been proposed that c-Abl functions to prolong the exploratory phase of migration.17 Another possibility is that c-Abl's role differs in primary cells versus transformed cell lines. We found that loss of c-Abl expression inhibited chemokine-induced migration of both primary mouse T cells and Jurkat tumor cells. Additional study will be required to understand the mechanisms by which c-Abl promotes T-cell migration and to identify the relevant actin-regulatory proteins. WAVE2 and HS1 are likely downstream targets of Abl function in this pathway, but the role of these proteins in T-cell chemotaxis has not yet been tested. It is also likely that Abl controls T-cell migration by regulating integrin-dependent adhesion, via its known interactions with CrkL.
While this manuscript was under review, a new study from the Pendergast lab showed that in addition to c-Abl, Arg plays an important role in T-cell function.23 Parallel analysis of proliferative responses in T cells lacking one or both proteins indicate that these kinases play at least partially overlapping roles during T-cell activation. Like c-Abl, Arg functions to control actin responses in other cell types.30,37,38 Thus, it will be important to ask whether Arg, like c-Abl, participates in controlling the T-cell cytoskeletal response, and whether loss of both family members leads to more profound cytoskeletal defects than loss of c-Abl alone.
Finally, our findings have important clinical implications for treatment of patients with CML. Imatinib mesylate, a potent inhibitor of Abl/Arg kinases, selectively induces apoptosis of Bcr-Abl+ cells, and is remarkably successful in treating patients with CML. However, an increase in the incidence of some viral infections has been reported in patients with CML treated with imatinib.27 Recent studies show that imatinib mesylate can inhibit T-cell proliferation, IL-2 production, TCR-induced tyrosine phosphorylation, and cell-cycle progression.26,61-63 Our data indicate that imatinib mesylate also impairs T-cell actin responses at the IS, as well as T-cell migration; however, potential effects on cellular immunity still need to be followed clinically in patients treated with this agent.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Esteban Carrizosa and Meredith Shaffer for technical assistance, and Morris Birnbaum and Edward Williamson for assistance with spinning disk confocal microscopy. We thank Martin Carroll, Deborah Klos, and Daniel Billadeau for critical reading of the manuscript.
This work was supported by the Lupus Research Institute and National Institutes of Health grant RO1 AI065644.
National Institutes of Health
Authorship
Contribution: Y.H. designed, performed, and analyzed experiments and drafted the manuscript; E.O.C. and R.S.D. performed and analyzed experiments; S.L. generated key reagents; A.J.K. contributed vital reagents and intellectual input; and J.K.B. designed experiments and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janis K. Burkhardt, Department of Pathology and Laboratory Medicine, Children's Hospital of Pennsylvania, University of Pennsylvania School of Medicine, 816D Abramson Research Center, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: jburkhar@mail.med.upenn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal