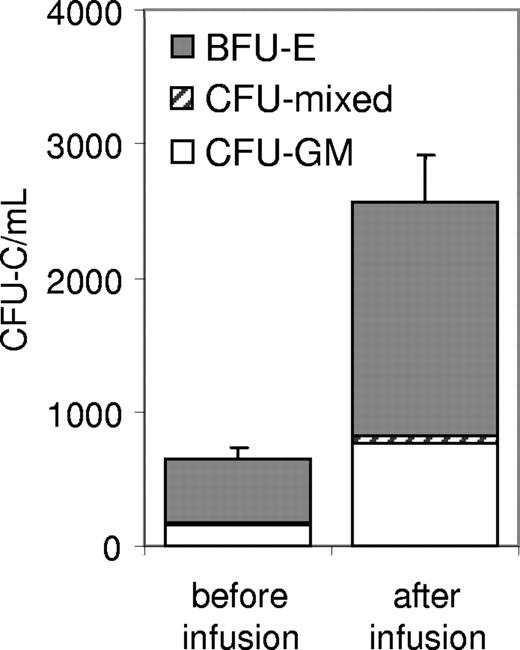
We thank Vuaillat and colleagues for their comments about our paper. In their letter, the authors are somewhat disappointed that several points “remain to be addressed.” Up front, we wish to emphasize that the goal of our studies1 was to test hematopoietic stem/progenitor cell (HSPC) mobilization in humans after a single dose and after chronic treatment with the anti–functional alpha4-integrin antibody natalizumab. Natalizumab-treated multiple sclerosis (MS) patients are a unique population to study these aims. Regarding the disease status of MS patients, we were restricted by the Institutional Review Board to collect only data essential to the goal of our study. However, some of the authors' questions can be answered indirectly: Natalizumab is licensed only for treatment of relapsing-remitting MS; accordingly, all our patients were of this category, none of them in acute relapse. Within this patient group, those 9 that had never received natalizumab (our control group) had normal circulating HSPCs immediately before the first dose of the drug. Whether circulating HSPCs fluctuate with disease activity, as might be suggested by the authors' observations in the murine experimental autoimmune encephalomyelitis (EAE) model, was not addressed by our studies and remains to be seen. Further, in contrast to the authors' statement (“… studies do not provide data on more committed CD34+ cells such as CD34+ myeloid progenitors”), in addition to CD34+ cells, quantitative data for progenitors, functionally assessed by colony-forming units in culture (CFU-C) assays, were reported throughout (Figure 1D-F1 ). The specific lineage affiliation is shown in Figure 1. The authors raise several other issues that, although not directly applicable to our studies, require a comment. In the absence of experimental data, whether HSPC “target the central nervous system (CNS) of MS patients” or whether there is a “link between CD34+ HSPCs and natalizumab-induced multifocal leucoencephalopathy” remains speculative. How John Cunningham (JC) virus is carried to the central nervous system has not been established.2 As JC virus has been demonstrated in the cerebro-spinal fluid in a significant number of MS patients not treated with natalizumab, without causing progressive multifocal leukoencephalopathy (PML), in contrast to normal subjects and patients with other neurologic illnesses,3 an increased number of HSPCs is not necessary for shuttling the virus into the brains of this patient group. On the other hand, clinical experience clearly suggests that severe immunosuppression is needed for development of PML. Therefore, we speculate that the impaired trafficking of and immunosurveillance by mature lymphocytes is a more likely cause of the rare cases of PML in this patient group.
As to the comment that natalizumab “might be useful for patients with poor response to granulocyte-colony–stimulating factor (G-CSF)–based protocols,” as suggested by Zohren et al,4p3893 our paper contains a cautionary note regarding this issue.1p3441
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Halvard B. Bonig, Sandhofstr 1, 60528 Frankfurt, Germany; e-mail: h.boenig@blutspende.de.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal