Abstract
Defibrotide (DF) is a mixture of porcine-derived single-stranded phosphodiester oligonucleotides (9-80-mer; average, 50-mer) that has been successfully used to treat severe hepatic veno-occlusive disease (sVOD) with multiorgan failure (MOF) in patients who have received cytotoxic chemotherapy in preparation for bone marrow transplantation. However, its mechanism of action is unknown. Herein, we show that DF and phosphodiester oligonucleotides can bind to heparin-binding proteins (eg, basic fibroblast growth factor [bFGF] but not vascular endothelial growth factor [VEGF] 165) with low nanomolar affinity. This binding occurred in a length- and concentration-dependent manner. DF can mobilize proangiogenic factors such as bFGF from their depot or storage sites on bovine corneal endothelial matrix. However, these molecules do not interfere with high-affinity binding of bFGF to FGFR1 IIIc but can replace heparin as a required cofactor for binding and hence cellular mitogenesis. DF also protects bFGF against digestion by trypsin and chymotrypsin and from air oxidation. In addition, DF binds to collagen I with low nanomolar affinity and can promote human microvascular endothelial cell-1 (HMEC-1) cell mitogenesis and tubular morphogenesis in three-dimensional collagen I gels. Thus, our data suggest that DF may provide a stimulus to the sinusoidal endothelium of a liver that has suffered a severe angiotoxic event, thus helping to ameliorate the clinical sVOD/MOF syndrome.
Introduction
Defibrotide (DF) is a mixture of single-stranded phosphodiester oligonucleotides (length, 9-80-mer; average, 50-mer; average molecular mass, 16.5 ± 2.5 kDa) derived from the controlled depolymerization of porcine intestinal mucosal DNA.1,2 In multiple single-arm, multi-institutional, phase II trials3-5 in patients with severe hepatic veno-occlusive disease (sVOD) and multiorgan failure (MOF) caused by the high-dose cytoreductive conditioning regimens administered in preparation for stem cell or bone marrow transplantation,6,7 DF has consistently demonstrated 100-day survival rates of 35% to 45% versus 10% to 20% for best available therapy (heparin8-10 ). A pivotal, prospective, nonrandomized, institutional, multi-institutional phase III trial of DF versus historical controls is currently ongoing to confirm clinical efficacy.
The mechanism of action of DF is uncertain. DF has only minimal anticoagulant activity, but potent antithrombotic and profibrinolytic effects both in vitro and in vivo can be observed after its administration.2,11,12 In healthy volunteers, the drug increases plasma tissue plasminogen activator activity and decreases plasminogen activator inhibitor (PAI-1) activity, while concurrently decreasing thrombomodulin levels. In addition, DF mobilizes tissue-factor pathway inhibitor (TFPI) from endothelial cells.13 DF also increases prostaglandin E2 plasma concentrations,14 which inhibits platelet aggregation. Moreover, its selectivity for microvascular injury is especially noteworthy.12
Nevertheless, it is uncertain how a procoagulant environment contributes to the clinical syndrome of sVOD. A key pathogenic event early in its development seems to be the disruption of hepatic sinusoidal endothelium by a toxic insult (eg, cytotoxic chemotherapy). Endothelial cells round up, detach, and eventually occlude microvascular lumina.15 Occlusion of vessel lumina is eventually followed by hepatic stellate cell activation and by deposition of collagen in the hepatic venules.6 The entire process has given rise to the alternative name “sinusoidal obstruction syndrome”6 for sVOD. Obstruction of the hepatic veins can lead to liver failure and patient demise.
Whereas DF is composed of an extremely large number of individual oligonucleotide sequences of different lengths, the concentration of any particular sequence is probably no higher than femtomolar. This, in addition to the inefficient penetration of cells by polyanions both in vitro and in vivo, makes it extremely unlikely that the effect of DF on the microvasculature is related to any Watson-Crick base-pairing interactions. However, it has long been known that oligonucleotides, particularly 18 to 28-mers with phosphorothioate backbones, are capable of non–sequence-specific binding with low nanomolar affinity to heparin-binding proteins,16 particularly basic fibroblast growth factor (bFGF), but not vascular endothelial growth factor 165 (VEGF165). The length required for similar high-affinity binding of a phosphodiester oligonucleotide (ie, those found in DF) to a heparin-binding protein is unknown.
Herein, we present evidence to suggest that DF, and phosphodiester oligonucleotides of defined length (N-mers, where N = length) that were synthesized to mimic the properties of DF can bind as polyanionic polyelectrolyte with high affinity to specific heparin-binding proteins, including bFGF2. DF and appropriate length N-mers can mobilize bFGF from its depot or storage sites on extracellular matrix. These molecules do not interfere with high-affinity binding of bFGF to fibroblast growth factor receptor 1c (FGFR1 IIIc), and can replace heparin as a required cofactor. DF also protects bFGF against protease digestion, and from air oxidation. Furthermore, DF binds to collagen I with low nanomolar affinity, and can promote human microvascular endothelial cell-1 (HMEC-1) cell mitogenesis and tubular morphogenesis in three-dimensional (3D) collagen I gels. We speculate that these data, in toto, suggest that the role of DF in ameliorating syndromes such as sVOD and those characterized by microangiopathy may from its ability to promote endothelial repair and revascularization in profoundly injured and disrupted hepatic vasculature.
Methods
Cells
Simian virus 40–transformed HMEC-1 cells were obtained from the Centers for Disease Control and Prevention (Atlanta, GA). They were grown in Molecular Cellular Developmental Biology 131 (MCDB 131) media supplemented with 10% heat-inactivated fetal bovine serum, 10 ng/mL epidermal growth factor (EGF), 1 μg/mL hydrocortisone, 100 U/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate. The stock culture was maintained at 37°C in a humidified 5% CO2 incubator. Human umbilical vein endothelial cells (HUVECs) were obtained from Invitrogen (Carlsbad, CA) and grown per the HUVEC protocol of the ATCC (Manassas, VA). BaF3 cells transfected with FGFR1 IIIc (C11 clone) were a kind gift of Dr D. M. Ornitz (Washington University, St Louis, MO) and were grown in RPMI 1640 medium + 10% newborn bovine serum, 0.5 ng/mL murine-recombinant interleukin-3, 2 mM l-glutamine, penicillin-streptomycin, 50 nmol/L β-mercaptoethanol, and G418 (600 mg/mL). The stock cultures were maintained at 37°C in a humidified 5% CO2 incubator.
Materials
Defibrotide (DF) and DF fractionated by molecular mass with the use of high-performance liquid chromatography were used. DF fractions (average fraction lengths: A2, 16-mer; E2, 32-mer; G2, 45-mer; I2, 62-mer; L2, 71-mer) were provided by Gentium (Como, Italy). Because of the near-Gaussian distribution of its constituent 9 to 80-mers (average length, 50-mer), the DF and DF fraction concentrations are expressed in micromoles per liter rather than micrograms per milliliter without introducing substantial error. N-mers (a series of synthetic phosphodiester oligonucleotides of defined lengths in which each base is present in equal amount at each position), and T-mers (a series of phosphodiester homopolymers of thymidine of defined length) were synthesized and purified via standard procedures (Trilink Biotechnologies, San Diego, CA). Recombinant human bFGF, VEGF165, platelet-derived growth factor, BB form (PDGF BB), and heparin-binding EGF-like growth factor (HB-EGF) were from R&D Systems (Minneapolis, MN). MCDB 131, fetal bovine serum (FBS), and rhodamine-phalloidin were obtained from Invitrogen. 125I-bFGF and 125I-VEGF were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). The IMUBIND Total TFPI enzyme-linked immunosorbent assay (ELISA) kit was from American Diagnostica (Greenwich, CT). The human FGF2 ELISA kit was purchased from R&D Systems. Collagen I was from BD Biosciences (San Jose, CA).
Modification of heparin-binding proteins by ClRNH32P-OdT18
The probe, 4-(N-2-chloroethyl-N-methyl)aminobenzylamine-32P-labeled phosphodiester 18-mer homopolymer of thymidine (ClRNH32P-OdT18) [“the probe”], was synthesized as described by Guvakova et al.16 Determination of Km for the binding of the probe for PDGF BB, VEGF165, and collagen I was by the method of Yakubov et al.17 For Kc determination, either bFGF (50 nmol/L), PDGF BB (500 nmol/L), VEGF165 (150 nmol/L), or collagen I (30 nmol/L) was incubated in 0.1 mol/L Tris-HCl, pH 7.4, containing 10-20 μmol/L ClRNH32P-OdT18. DF and/or N-mer were added at increasing concentrations as competitors of probe binding. After 1 hour at 37°C, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed, and the gel was exposed to X-ray film. These were quantitated by laser densitometry, the IC50 of competition was determined, and the competition constant Kc (approximately equal to Ki) calculated by the Cheng-Prusoff relationship (Kc = IC50/(1 + [ClRNH32P-OdT18]/Kd,18 where Kd is approximately equal to KM).
Displacement of extracellular matrix-bound 125I-bFGF by DF and N-mers
Bovine eyes were obtained from Morris Insel Cohen (Newark, NJ), and extracellular matrix (ECM) was prepared by previous methods.19
For release experiments, ECM was incubated (3 hours, room temperature) with 125I-bFGF (2.5 × 104 cpm/0.3 mL/well) in phosphate-buffered saline (PBS) + 0.02% gelatin. The ECM was incubated with increasing concentrations of either DF, DF fractions, or N-mers at room temperature for 3 hours. The amount of released 125I-bFGF was determined by γ-counting. The remaining ECM was incubated (3 hours, 37°C) with 1 N NaOH and counted. The percentage of released 125I-bFGF was calculated from the total ECM-associated radioactivity. For all experiments, measurements were made in duplicate (each duplicate was repeated thrice) with average differences no greater than 5% between the replicates.
Protection of bFGF by DF against protease digestion
bFGF or VEGF165 (∼0.5 μg in a total volume of 20 μL, pH7.4) with tracer amounts of 125I-bFGF or 125I-VEGF165 in the presence or absence of DF (25 μmol/L) were equilibrated at 37°C for 5 minutes. Trypsin or chymotrypsin was added to a final concentration of 1 μg protease/50 μg bFGF or VEGF165, and digestion was allowed to proceed at 37°C for 4 hours. SDS gel sample buffer was then added, the samples were heated at 100°C for 5 minutes, and SDS-PAGE was performed. The gels were dried and exposed to Kodak X-ray film until bands were visualized, which were quantitated by laser densitometry. To air-oxidize bFGF, 20 ng/mL in complete media were incubated at 37°C in room air for 3 to 4 hours. Under these conditions, as described under “Results,” all bFGF activity is lost.
bFGF-induced mitogenesis in C11 cells
C11 (FGFR1 IIIc–overexpressing) cells were washed twice with RPMI 1640 media lacking interleukin-3 (IL-3) and plated at 2.2 × 104 cells per well in 48-well plates. bFGF (1 nmol/L), DF (25 μmol/L), N-mers, and/or heparin (1 μg/mL) were added in a total volume of 200 μL. The cells were then incubated for 2 to 3 days, fixed, and stained with sulforhodamine blue (SRB). The absorbance at a λ of 530 nm was taken as proportional to cell number.
Three-dimensional collagen I gels
Collagen I gels were formed by mixing complete M199 media, 1 N NaOH (0.023 × volume of collagen I), and collagen I at a final concentration of 0.6 mg/mL (4°C). HMEC-1 cells were seeded onto the collagen I gels (105 cells/well) in complete media. Three hours later, the attached cells were overlaid with a second layer of collagen I, and 0.5 mL of complete media with or without DF was added above the second layer. DF was added every 3 days.
After 6 days, cells were fixed with 4% paraformaldehyde + 0.25% glutaraldehyde in PBS at room temperature overnight, and permeabilized with 0.2% Triton X-100 for 30 minutes. Cells were stained with rhodamine phalloidin (160 nmol/L). Tubular morphogenesis was monitored by confocal microscopy, and quantitated (n = 3) with the use of ImageJ software (http://rsb.info.nih.gov/ij/). To liberate cells, the 3D collagen gels were treated with 0.1% collagenase type I in PBS for 30 to 40 minutes at 37°C, washed with PBS, and centrifuged. Cells were counted by trypan blue exclusion (n = 4). In some experiments, cells were treated with air pre-exposed bFGF (37°C, 3.5 hours, with or without 25 μmol/L DF). The final concentration of bFGF was 20 ng/mL. The final DF concentration was 0.2 μmol/L, which is far too low to induce either tubular morphogenesis or cellular mitogenesis.
Radial invasion of matrix by aggregated cells
A modification of the method of Vernon and Sage was employed that eliminates the use of nylon gaskets.20 Dispersed HMEC-1 cells (180 ×104 cells/mL in complete media) were cultured in 40-μL drops suspended upside down from 12-well plate lids lined with Parafilm M. After 2 days in culture, aggregates of cohesive cells (one aggregate per drop) were transferred into wells (12-well plates) that had been pre-filled with 0.5 mL of a 0.6 mg/mL collagen I gel. A second layer of collagen gel was added, and 1 mL of complete media with or without bFGF (20 ng/mL), DF (25 μmol/L), or both was added above the second layer. The cells were cultured for 2 to 5 days at 37°C in a humidified 5% CO2 incubator and photographed daily. The average distances of migration of the HMEC-1 cells into the surrounding collagen, calculated from 9 to 12 replicates, were averaged to yield the final values of radial invasion.
Confocal microscopy
Images were collected with a laser scanning system (Radiance 2000; Bio-Rad Laboratories, Hercules, CA) coupled to a confocal microscope (Zeiss, Thornwood, NY) with a Kr/Ar laser for excitation at 568 nm, narrow-band filters for emission, and Nikon 20× PlanApo optics on an Eclipse 200 laser-safe microscope. Images were analyzed by the use of ImageJ software.21
Statistics
Unless otherwise mentioned, experiments were performed at least in triplicate, and data are presented as the average (± SD). P values were determined by a 2-sided Student t test with unequal variance; P values less than .05 were considered significant.
Results
DF interacts with heparin-binding proteins in a length-dependent manner
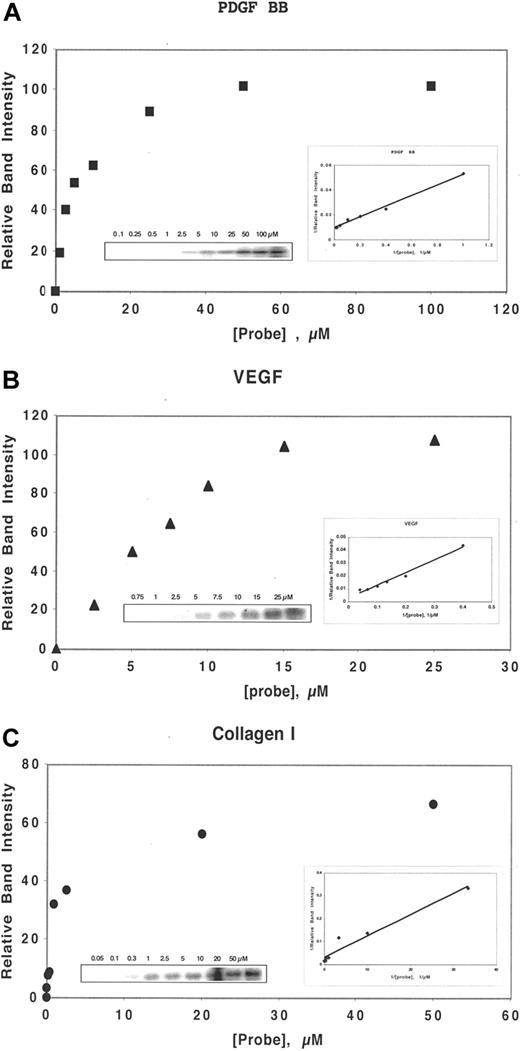
By a competition assay, we determined the Kc of binding, as defined by the Cheng-Prusoff relationship18 , of DF and N-mers to several heparin-binding proteins, including bFGF, VEGF165, PDGF-BB, and collagen I. Determination of Kc of competition requires the measurement of the Kd of binding to that protein of the probe, ClRNH32P-OdT18. For bFGF, KM = 0.5 μmol/L.16 For the other proteins above, the association with the probe exhibits approximate saturation binding, described by a single-site binding equation of the Michaelis-Menten type (Figure 1A-C). Double-reciprocal Lineweaver-Burke plots of the Michaelis-Menten-type data provided −1/KM. These plots were linear for each protein (Figure 1A-C), and the values of KM for VEGF165 (Figure 1B), PDGF-BB (Figure 1A), and collagen I (Figure 1C) were 34, 5, and 0.6 μmol/L, respectively.
DF interacts with heparin-binding proteins in a concentration-dependent manner. Either PDGF BB (50 nmol/L, A), VEGF (150 nmol/L, B), or collagen I (30 nmol/L, C) was incubated in 0.1 mol/L Tris-HCL, pH 7.4, with ClRNH32P-OdT18 at the stated concentrations for 1 hour at 37°C. The sample buffer was then added, and the mixture was boiled and subjected to 12% PAGE. Unreacted oligomer ran off the bottom of the gel and is not shown. The gel bands in left insert were quantitated by laser densitometry. Shown is a plot of relative band intensity versus probe oligodeoxynucleotide concentration. Right insert: Double-reciprocal plot of these data. The value of apparent Kd (−1/x intercept) for PDGF BB, VEGF, and collagen I were 5, 34, and 0.6 μmol/L, respectively.
DF interacts with heparin-binding proteins in a concentration-dependent manner. Either PDGF BB (50 nmol/L, A), VEGF (150 nmol/L, B), or collagen I (30 nmol/L, C) was incubated in 0.1 mol/L Tris-HCL, pH 7.4, with ClRNH32P-OdT18 at the stated concentrations for 1 hour at 37°C. The sample buffer was then added, and the mixture was boiled and subjected to 12% PAGE. Unreacted oligomer ran off the bottom of the gel and is not shown. The gel bands in left insert were quantitated by laser densitometry. Shown is a plot of relative band intensity versus probe oligodeoxynucleotide concentration. Right insert: Double-reciprocal plot of these data. The value of apparent Kd (−1/x intercept) for PDGF BB, VEGF, and collagen I were 5, 34, and 0.6 μmol/L, respectively.
We then determined the effect of length (Figure 2) on the protein-specific values of the competition constant Kc by synthesizing a series of phosphodiester oligonucleotides of defined length (N-mers), in which each nucleotide base is equally present at each position, producing a true random oligomer. In contrast, the sequences of DF, because of their genomic source, are not truly random. Also synthesized were a series of homopolymers of thymidine (T25-T200); these polypyrimidine sequences minimize contributions to protein-binding from purine base-stacking. In Figure 2, competition for probe binding to collagen I (Figure 2A), bFGF (Figure 2B), PDGF-BB (Figure 2C), and VEGF165 (Figure 2D) is shown. The IC50 values of competition were determined, and the values of Kc for bFGF are given in the corresponding figures. For both series of competitors (T25-T200, N30-N80) the value of Kc is length-dependent; for the N30-N80mer series, these values substantially increased when the length of the oligonucleotide was less than 35 to 40-mer. This is also true of the T25-T200mer series, but these homopolypyrimidines have a greatly reduced affinity for either bFGF or PDGF-BB versus the N-mer competitors of probe binding of identical length.
DF, DF fractions, N-mers, and T-mers interact with heparin-binding proteins in a length-dependent manner. Comparison of the Kc values for DF, molecular weight fractions of DF, N-mer, and Tmer competitors of probe ClRNH32P-OdT18 binding to collagen I (A), bFGF (B), PDGF (C), and VEGF (D). All oligonucleotides were used as competitors of ClRNH32P-OdT18 (8 μmol/L) binding to collagen I (30 nmol/L, A), bFGF (50 nmol/L, B), PDGF (50 nmol/L, C), or VEGF (150 nmol/L, D) as described under “Methods.” The value of Kc for ClRNH32P-OdT18 was determined by the Cheng-Prusoff equation.18
DF, DF fractions, N-mers, and T-mers interact with heparin-binding proteins in a length-dependent manner. Comparison of the Kc values for DF, molecular weight fractions of DF, N-mer, and Tmer competitors of probe ClRNH32P-OdT18 binding to collagen I (A), bFGF (B), PDGF (C), and VEGF (D). All oligonucleotides were used as competitors of ClRNH32P-OdT18 (8 μmol/L) binding to collagen I (30 nmol/L, A), bFGF (50 nmol/L, B), PDGF (50 nmol/L, C), or VEGF (150 nmol/L, D) as described under “Methods.” The value of Kc for ClRNH32P-OdT18 was determined by the Cheng-Prusoff equation.18
Collagen I, a basic protein because of its lysine content, interacted with strikingly high affinity in its monomeric form with the probe oligonucleotide (KM = 0.6 μmol/L; R2 = 0.95). Competition of probe binding by DF gave a Kc value of 0.7 nmol/L (R2 = 0.99). Competition by N-mers of probe binding to collagen I also proceeded in a length-dependent fashion, with Kc values of 2.7, 0.6, and 0.05 nmol/L for 35-, 50-, and 80-mer, respectively (Figure 2A). For the A2, E2, and I2 DF-fractions (average length, 31-, 41-, and 72-mer, respectively), the values of Kc were 2, 0.8, and 0.2 nmol/L, consistent with the data obtained with the N-mers of defined length. To our knowledge, these overall values of Kc represent the highest affinity interactions for any oligonucleotide-protein binding yet measured and must be reflective of strong electrostatic interactions between polyanionic oligonucleotides and basic collagen I.
In contrast, the Kc of DF and N-mer competition for probe binding to VEGF165 (for DF, Kc = 1200 nmol/L, R2 = 0.93; Figure 2D) is much higher than that of collagen I and even of bFGF. Relatively low-affinity interactions were also observed between laminin and DF (Kc = 270 nmol/L; R2 = 0.95; not shown), and the N-mer and T25-T200 series of oligomers. Both HB-EGF and tumor necrosis factor-α also interacted relatively weakly with DF (Kc = 650 and 120 μmol/L, respectively; R2 = 0.99 and 0.96, respectively).
DF and N-mers mobilize, in a length-dependent manner, tissue factor pathway inhibitor bound to cell surface glycosaminoglycans
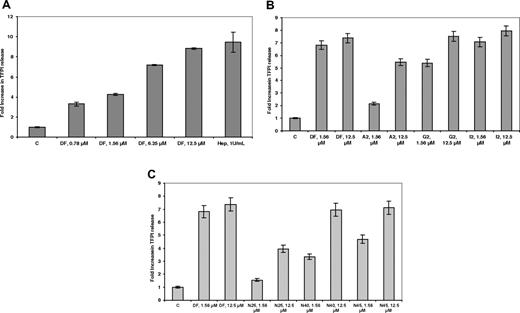
It has previously been demonstrated13 that DF can cause the release of the antithrombotic protein TFPI from its glycosaminoglycan-binding sites on endothelial cells. As shown in Figure 3A, after treatment of HMEC-1 cells with increasing concentrations of DF, and reaching a plateau at 25 μmol/L, an approximately 8-fold increase in TFPI concentration was found in the media after 20 to 30 minutes (n = 3) and was comparable with what was observed for heparin. The release was highly length-dependent for the DF-fractions (Figure 3B), reaching a plateau with the G2 fraction at 12.5 μmol/L and for the I2 fraction (average length, 72-mer) at 1.56 μmol/L. For the N-mer series (Figure 3C), release was maximal at N40 (12.5 μmol/L), consistent with what was observed for the E2 fraction.
DF, DF fractions, and N-mers mobilize TFPI bound to cell-surface glycosaminoglycans in a concentration- and length-dependent manner. HMEC-1 cells were treated with increasing concentrations of DF (A), DF fractions (B), and N-mers (C) for 24 hours. Each data point is the average of triplicate wells plus or minus SD.
DF, DF fractions, and N-mers mobilize TFPI bound to cell-surface glycosaminoglycans in a concentration- and length-dependent manner. HMEC-1 cells were treated with increasing concentrations of DF (A), DF fractions (B), and N-mers (C) for 24 hours. Each data point is the average of triplicate wells plus or minus SD.
DF releases 125I-bFGF from low-affinity binding sites on extracellular matrix
bFGF binds to low-affinity (Kd = 1-10 nmol/L) heparin binding sites on extracellular matrix22-24 and can be isolated in large quantities from polyanion-treated matrix.25,26 Mobilization of this bound bFGF is believed to promote endothelial proliferation.27-30
Dishes coated with bovine corneal endothelial matrix were allowed to adsorb 125I-bFGF and were then treated with DF, DF fractions, or N-mers. The maximal release was compared with release by a 28-mer phosphorothioate homopolymer of cytidine (SdC28) and is arbitrarily assigned a value of 1. The release of ECM-bound 125I-bFGF by SdC28 and 2 mol/L NaCl were equivalent. As shown in Figure 4A,B, release was dependent on the concentration and DF fraction or N-mer length, although in the latter case release equivalent to that of DF is attained only at N-mer lengths of 80. In contrast, there was little or no release of ECM-bound 125I-VEGF165 by DF at any concentration evaluated (up to 100 μmol/L; not shown). These data are consistent with our previous experiments demonstrating the low affinity of DF for VEGF165.
DF, DF fractions, and N-mers release 125I-bFGF from low-affinity binding sites on ECM. ECM-coated wells were incubated (3 hours, room temperature) with 125I-bFGF (2.5 × 104/well). The ECM was washed 3 times and incubated (3 hours, room temperature) with increasing concentrations of DF (A and C), DF fractions (A), or N-mers (B). Released radioactivity is expressed as the percentage of total ECM-bound 125I-bFGF and is compared with release by 15 μmol/L SdC28. Each assay was conducted in duplicate, with the differences in duplicate measurements of less than 10%. Identical experiments were repeated 3 times. Maximum release of 125I-bFGF by 2 mol/L NaCl was used as the positive control.
DF, DF fractions, and N-mers release 125I-bFGF from low-affinity binding sites on ECM. ECM-coated wells were incubated (3 hours, room temperature) with 125I-bFGF (2.5 × 104/well). The ECM was washed 3 times and incubated (3 hours, room temperature) with increasing concentrations of DF (A and C), DF fractions (A), or N-mers (B). Released radioactivity is expressed as the percentage of total ECM-bound 125I-bFGF and is compared with release by 15 μmol/L SdC28. Each assay was conducted in duplicate, with the differences in duplicate measurements of less than 10%. Identical experiments were repeated 3 times. Maximum release of 125I-bFGF by 2 mol/L NaCl was used as the positive control.
Because added 125I-bFGF might only be adsorbed to the surface of the ECM, we determined whether 125I-bFGF bound to low-affinity binding sites within the ECM slab could also be mobilized by DF. Accordingly, after growth factor adsorption, 0.5 g of minced ECM was treated either with increasing concentrations of DF or with 2 mol/L NaCl for 17 hours (Figure 4C). The maximum release of 125I-bFGF by 2M NaCl, as measured by ELISA, was 48 pg/mL. The release of 125I-bFGF by DF was concentration-dependent and was equivalent at 25 μmol/L to the release produced by 2M NaCl.
DF and N-mers potentiate the binding of bFGF to FGFR1 IIIc (C11 clone)
We postulated that DF and N-mers could substitute for heparin in its ability to potentiate the proliferative effects of bFGF on endothelial cells. BAF3 cells transfected with FGFR1 (to which bFGF binds with high affinity31 ) have an absolute requirement for heparin or heparin-like activity (which can sometimes be found in serum) to proliferate in response to bFGF.32,33 The mitogenic response (evaluated by SRB staining) of these cells was evaluated in the presence of 1 nmol/L bFGF and 5 μmol/L DF. Proliferation of the transfectants was increased by 3.8-fold (± 0.9-fold) compared with the non–DF-treated cells (n = 3; P = .035) and was approximately double that seen with treatment by bFGF alone (Figure 5A). No increase in proliferation was produced by heparin (1 μg/mL) or DF alone (5 μmol/L). N-mers also supported bFGF-induced C11 cell proliferation, but the length dependence was not as dramatic as previously observed (Figure 5B). These experiments conclusively demonstrate that DF and N-mers can substitute for heparin in promoting the proliferation of bFGF-dependent cells. Moreover, they also show that neither DF nor N-mers, despite their interactions with low-affinity binding sites as shown, interfere with the binding of bFGF to high-affinity (Kd = 20-200 pmol/L34 ) cell surface receptors.
DF and N-mers substitute for heparin, which is required for the biologic activity of bFGF. C11 cells were washed twice with media without IL-3, and seeded (2.2 × 104 cells/well in 48-well plates) in media containing 10% FBS but not IL-3. The cells were then treated with 1 nmol/L bFGF + DF (25 μmol/L, A) or 1 nmol/L bFGF + N-mers (1 μmol/L, B). After 2 days, proliferation was evaluated by SRB staining. Data are presented as the average plus or minus SD, n = 3. bFGF + Hep versus bFGF, P less than .05; bFGF + DF, N50, N60, or N80 versus bFGF, P less than .05; bFGF + N25, N30 versus bFGF, P = not significant. DF or Hep versus C, P = not significant. C = control, Hep = Heparin.
DF and N-mers substitute for heparin, which is required for the biologic activity of bFGF. C11 cells were washed twice with media without IL-3, and seeded (2.2 × 104 cells/well in 48-well plates) in media containing 10% FBS but not IL-3. The cells were then treated with 1 nmol/L bFGF + DF (25 μmol/L, A) or 1 nmol/L bFGF + N-mers (1 μmol/L, B). After 2 days, proliferation was evaluated by SRB staining. Data are presented as the average plus or minus SD, n = 3. bFGF + Hep versus bFGF, P less than .05; bFGF + DF, N50, N60, or N80 versus bFGF, P less than .05; bFGF + N25, N30 versus bFGF, P = not significant. DF or Hep versus C, P = not significant. C = control, Hep = Heparin.
DF protects bFGF from enzymatic digestion and air oxidation
Hepatic cell necrosis can occur during sVOD,1,10 and necrotic cells may release proteases. DF (25 μmol/L) can partially protect 125I-bFGF from digestion by 2 proteases, trypsin and chymotrypsin (37°C, 2 hours; Figure 6A,B). However, VDGF165 was not protected by DF from enzymatic digestion (Figure 6C), consistent with our measured low affinity of this protein for DF. Further, bFGF is inactivated relatively rapidly after exposure to air at 37°C, possibly because of oxidation at cysteine thiol. We incubated bFGF in complete media at 37°C for 3 hours with or without 10 μmol/L DF and then used this mixture, in the presence of heparin, to treat the FGFR1 IIIc–transfected BAF3 cells. When bFGF was not preincubated with DF, it was susceptible to air oxidation, and there was no increase in the proliferation of the FGFR1 IIIc–transfectants in the presence of heparin versus control cells not treated with bFGF. In contrast, in the presence of bFGF that had been pre-treated with DF, bFGF-dependent proliferation was increased to normal levels, 3- to 4-fold versus control (Figure 6D).
Preincubation with DF protects bFGF from degradation by trypsin and chymotrypsin. bFGF (A,B) or VEGF165 (C; ∼0.5 μg in 20 μL, pH 7.4) with tracer amounts of 125I-bFGF or 125I-VEGF165 were exposed to 2% trypsin (w/w) in the absence or presence of 25 μmol/L DF. Trypsin digestion was carried out at 37°C for 4 hours, and the digestion products were mixed with sample buffer, boiled for 5 minutes, and analyzed by 12% SDS-PAGE. Gel bands were quantitated by laser densitometry. Data are presented as the average plus or minus SD, n = 3. *P (vs no oligo) less than .04; **P (vs no oligo) less than .02. (D) DF protects bFGF from inactivation at 37°C. bFGF was incubated at 37°C for 3.5 hours with or without 25 μmol/L DF, added to the C11 FGFR1 IIIc–overexpressing cells (2.2 × 104 cells/well in 48-well plate) in media containing DF (25 μmol/L) but without IL-3. Cell proliferation was measured after 3 days by SRB. bFGF (1 nmol/L) without preincubation at 37°C for 3.5 hours was used as a positive control. Data are presented as the average plus or minus SD, n = 3. *P (vs bFGF + preincubation) less than .03.
Preincubation with DF protects bFGF from degradation by trypsin and chymotrypsin. bFGF (A,B) or VEGF165 (C; ∼0.5 μg in 20 μL, pH 7.4) with tracer amounts of 125I-bFGF or 125I-VEGF165 were exposed to 2% trypsin (w/w) in the absence or presence of 25 μmol/L DF. Trypsin digestion was carried out at 37°C for 4 hours, and the digestion products were mixed with sample buffer, boiled for 5 minutes, and analyzed by 12% SDS-PAGE. Gel bands were quantitated by laser densitometry. Data are presented as the average plus or minus SD, n = 3. *P (vs no oligo) less than .04; **P (vs no oligo) less than .02. (D) DF protects bFGF from inactivation at 37°C. bFGF was incubated at 37°C for 3.5 hours with or without 25 μmol/L DF, added to the C11 FGFR1 IIIc–overexpressing cells (2.2 × 104 cells/well in 48-well plate) in media containing DF (25 μmol/L) but without IL-3. Cell proliferation was measured after 3 days by SRB. bFGF (1 nmol/L) without preincubation at 37°C for 3.5 hours was used as a positive control. Data are presented as the average plus or minus SD, n = 3. *P (vs bFGF + preincubation) less than .03.
DF promotes the growth of HUVECs
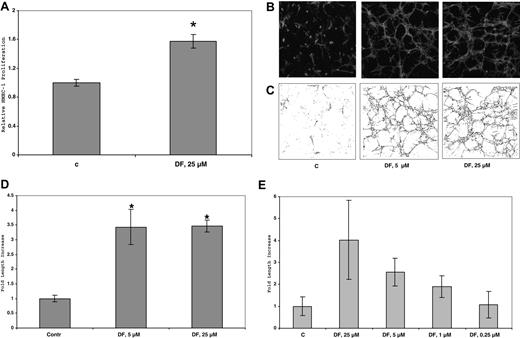
In our hands, HUVECs grow very poorly, either on plastic or on plastic but underneath collagen I gels (2 mg/mL). These cells do not grow at all in 3D collagen I gels. However, in the presence of DF (100 μmol/L; cells underneath gel), cellular proliferation after collagenase digestion and counting by trypan blue exclusion (n = 4, P vs untreated for all experiments) increased by 2.0-fold (± 0.2-fold; 2.2 versus 1.1 × 104 cells/well, P < 10−3). This increase is almost as dramatic as what was observed with heparin, the standard growth additive for HUVEC cells (2.7 ± 0.2-fold; P < 10−4). On plastic alone, the increase in cellular mitogenesis by DF (100 μmol/L) was only 1.6-fold (± 0.03-fold; P < 10−7) and by heparin was 2.7 (± 0.05-fold; P < 10−6).
DF stimulates tubular morphogenesis of HMEC-1 cells in 3D collagen I gels
Treatment of HMEC-1 cells on plastic plates or in two-dimensional (2D) collagen I gels with up to 50 μmol/L DF for 6 days produced only minimal morphologic effects or changes in cell proliferation. In contrast, in 3D collagen gels treated with 25 μmol/L DF every 3 days and incubated in complete media, the number of HMEC-1 cells increased by 1.6-fold (± 0.2-fold; 1.1–1.9 × 104 cells/well, n = 3; P < 10−2) after 6 days versus control, non–DF-treated cells. (Figure 7A). (In similarly treated cells in the absence of collagen I, the increase in proliferation is only approximately 20%). Furthermore, by this time, the DF-treated cells, but not the untreated cells, had undergone dramatic tubular morphogenesis (Figure 7B). The extent of tubular structure formation in the rhodamine-phalloidin–stained cells after fixation was quantitated by using confocal microscopy and ImageJ software. Initial analysis provided a “streak” pattern, which was then skeletonized (Figure 7C) and quantitated (Figure 7D). Under these conditions in 3D collagen I gels, DF (25 or 5 μmol/L) induced an increase of approximately 6- to 7-fold in the tubular morphogenesis of HMEC-1 cells after skeletonization versus the non–DF-treated cells. These effects of DF are concentration dependent (Figure 7E), essentially disappearing at concentrations of DF below 0.25 μmol/L.
DF increases HMEC-1 proliferation in 3D-collagen I gels. Cells (45 × 104 cells/well in 6-well plates) were seeded in collagen I (0.6 mg/mL) and treated with DF (25 μmol/L, every 3 days). After 6 days, the cells were counted. Each data point is the average of triplicate wells plus or minus SD. Relative HMEC-1 proliferation = 1.6-fol (± 0.08-fold). P less than 10−2 DF (25 μmol/L) versus C (A). (B) Confocal micrograms of HMEC-1 cells in 3D collagen I gels after 6 days in culture. HMEC-1 cells (105 cells/well) were seeded in collagen I (0.6 mg/mL) and treated with DF (5 or 25 μmol/L, every 3 days). At the end of treatment (6 days), the cells were fixed with 4% paraformaldehyde + 0.25% glutaraldehyde, permeabilized with 0.2% Triton X-100, and stained with rhodamine phalloidin (160 nmol/L). (C) Skeletonization by Image J of the images in panel A. (D) Quantitation of skeletonization by Image J of the confocal micrograms of HMEC-1 cells in 3D collagen I gels in (B) after 6 days in culture. Each experiment was performed in triplicate. Fold increase of skeletonized line = 3.5 (± 0.6; 5 μmol/L DF), and 3.5 (± 0.2; 25 μmol/L DF). *P (vs C) less than .02 (5 μmol/L); P (vs C) less than 10−4 (25 μmol/L DF). (E) Quantitation of skeletonization by Image J of the confocal micrograms of HMEC-1 cells in 3D collagen I gels treated with DF at multiple concentrations (0.25-25 μmol/L, every 3 days) after 6 days in culture. Each experiment was performed in triplicate.
DF increases HMEC-1 proliferation in 3D-collagen I gels. Cells (45 × 104 cells/well in 6-well plates) were seeded in collagen I (0.6 mg/mL) and treated with DF (25 μmol/L, every 3 days). After 6 days, the cells were counted. Each data point is the average of triplicate wells plus or minus SD. Relative HMEC-1 proliferation = 1.6-fol (± 0.08-fold). P less than 10−2 DF (25 μmol/L) versus C (A). (B) Confocal micrograms of HMEC-1 cells in 3D collagen I gels after 6 days in culture. HMEC-1 cells (105 cells/well) were seeded in collagen I (0.6 mg/mL) and treated with DF (5 or 25 μmol/L, every 3 days). At the end of treatment (6 days), the cells were fixed with 4% paraformaldehyde + 0.25% glutaraldehyde, permeabilized with 0.2% Triton X-100, and stained with rhodamine phalloidin (160 nmol/L). (C) Skeletonization by Image J of the images in panel A. (D) Quantitation of skeletonization by Image J of the confocal micrograms of HMEC-1 cells in 3D collagen I gels in (B) after 6 days in culture. Each experiment was performed in triplicate. Fold increase of skeletonized line = 3.5 (± 0.6; 5 μmol/L DF), and 3.5 (± 0.2; 25 μmol/L DF). *P (vs C) less than .02 (5 μmol/L); P (vs C) less than 10−4 (25 μmol/L DF). (E) Quantitation of skeletonization by Image J of the confocal micrograms of HMEC-1 cells in 3D collagen I gels treated with DF at multiple concentrations (0.25-25 μmol/L, every 3 days) after 6 days in culture. Each experiment was performed in triplicate.
bFGF, when protected by DF, promotes HMEC-1 mitogenesis in 3D collagen I gels but does not promote radial cellular outgrowth
As described previously, when bFGF was incubated in air at 37°C for 3 to 4 hours, its ability to induce the mitogenesis of FGFR1 IIIc–transfected BAF-3 cells (C11 cells) was completely lost. Likewise, when HMEC-1 cells in 3D collagen I gels were incubated with bFGF (20 ng/mL) incubated similarly, mitogenesis was also blocked. In contrast, when the HMEC-1 cells in collagen I were treated with preincubated bFGF to which DF (25 μmol/L) had been added, the bFGF-induced mitogenesis was completely restored.
In contrast, DF does not protect bFGF with respect to induction of radial invasion of matrix (in this case 3D collagen I gels). In the radial invasion of matrix by aggregated cells (RIMAC) model, bFGF (20 ng/mL) induced substantial HMEC-1 radial invasion (2.5-fold versus control; average, 0.75 mm versus 0.3 mm; n = 6) in 2-3 days. Neither DF alone (25 μmol/L) nor bFGF preincubated in air (37°C, 3.5 hours) could induce radial invasion. We had anticipated that bFGF similarly preincubated with 25 μmol/L DF would protect its ability to induce radial invasion of HMEC-1 cells in 3D collagen I gels. However, this was not the case, and minimal to no increase in radial invasion (vs no added bFGF) was observed.
Discussion
The dogma that phosphodiester (PO) oligonucleotides cannot be used as pharmacologically active agents is challenged by the clinical activity of DF in sVOD/MOF. This entity results from a highly angiotoxic event, the destruction by cytotoxic agents of sinusoidal endothelial cells.6 The exact mechanism of action of DF in ameliorating sinusoidal endothelial injury is unknown, but it may enhance endothelial survival in response to the angiotoxic insult. Here, we have demonstrated that DF binds with high affinity to bFGF and mobilizes bFGF from its low-affinity binding sites on ECM. These nonspecific effects with a mixture of phosphodiester oligomers are virtually identical to what has been observed16 with phosphorothioate (PS) oligonucleotides, although a PS oligomer of only approximately half the length of a PO is required to achieve an equivalent Kc for bFGF binding. Thus, for both PO and PS oligomers, increasing length and hence charge density dramatically affects the Kc of its binding to bFGF. Increasing the average oligomer length in both DF and the N-mers also may at least partially compensate for the relatively rapid rate of in vivo nuclease digestion and clearance of PO oligomers. This high rate of clearance, among other factors, requires DF to be administered at high doses (25 mg/kg per day) to patients with sVOD, relative to the 5 mg/kg per day dose of Genasense 18-mer anti-Bcl-2 oligomer administered to patients with melanoma.35
In addition, DF stabilizes bFGF with respect to oxidative and protease degradation and potentiates the binding of bFGF to FGFR1 IIIc. The protection of bFGF from air oxidation promotes endothelial cell mitogenesis, but the ability of bFGF to promote radial invasion in the RIMAC assay was not protected. Because DF binds to bFGF at the heparin-binding site, this observation may suggest that a different site on the bFGF molecule, which may be involved in the signaling that leads to radial migration, is not protected by DF.
bFGF has long been known to be promote microvessel formation,36-38 and addition of bFGF to endothelial cells39 or stromal cells40 in culture results in the expression of the highly pro-angiogenic VEGF. Thus, agents that can potentiate the effects of bFGF will stimulate angiogenesis.41
We also show that DF dramatically increases the tubular morphogenesis of HMEC-1 cells when embedded in 3D collagen I gels. As described by Liu and Senger,42 when endothelial precursor cells contact collagen I-containing matrix in the embryo, they align into solid precapillary-cord–like structures that are interconnected, forming a polygonal network. This morphogenetic program can be mimicked in mature endothelial cells after degradation of basement membrane and subsequent contact with collagen I. Collagen I then drives the morphogenesis of new vessel sprouts. This process seems to depend at least in part on an interaction of the β1 integrin with collagen I and on the subsequent up-regulation of both phospho-Src expression and the activity of rho, a GTPase. It is perhaps relevant that strongly positive collagen I immunohistochemical staining can be found in the liver as an almost continuous lining along the wall of the sinusoids.43 Disruption of the sinusoidal basement membrane can occur in sVOD via dissection of red blood cells into the space of Disse.15 It is intriguing to conjecture that this may provide access for the surviving endothelial cells to the collagen I that seems to be necessary for their regrowth.
Our data suggest the speculative notion that the mechanism of action of DF (which, like all other oligonucleotides, will probably be concentrated in the liver44 ) in sVOD may, in part, be related to its ability to aid in the partial revascularization of an injured, hypoxic hepatic parenchyma. This perhaps might be due to the direct potentiation of bFGF-dependent angiogenesis, coupled with indirect effects on VEGF-induced angiogenesis. Although some of the angiogenesis-altering properties of DF have also been observed with a small number of other polyanions (such as heparin and fucosylated chondroitin sulfate or other natural products,41,45 none so far have the clinical promise of DF in sVOD. This is probably due to the minimal anticoagulant effects and limited clinical toxicity of DF.
Observed changes in the in vivo expression of PAI-1 protein after DF administration are consistent with our speculative mechanism. PAI-1 serum protein levels frequently increase more than 10-fold in sVOD compared with pre-treatment levels.46 PAI-1 expression is lowered not only in healthy volunteers receiving DF, but in patients recovering from sVOD/MOF5 as well. Because PAI-1 is induced by hypoxia47 under the control of HIF-1,48 decreases in PAI-1 plasma levels in recovering patients may be indicative of the relief of hepatic hypoxia via induction of liver revascularization by DF, an observation clinically corroborated by the reduction plasma PAI-1 in patients with sVOD/MOF responding to DF therapy.
How could the binding of DF to collagen I produce the observed alterations in tubular morphogenesis and radial cellular migration? The major collagen I receptors are the α2β1 and the α1β1 integrins,49-52 although the latter seems to be confined mainly to inflammatory cells. Some integrins (eg, αMβ253 ) are known to bind oligonucleotides with high affinity. Others (αVβ354 and α6β155 ) require a glycosaminoglycan (GAG) cofactor for ligand binding, and it is likely that DF can be a GAG substitute. These facts suggest that DF perhaps may promote either α2 or β1 integrin interactions with collagen I in a manner similar to that in which DF potentiates bFGF binding to FGFR1 IIIc. Furthermore, the consensus binding peptide (GFOGER) of collagen I to the α2β1 integrin contains highly basic arginine.56 The formation of triple-helical collagen I may bring these arginines into close apposition, thus facilitating acidic DF binding via ionic interactions and perhaps ultimately bridging integrin and collagen I in a ternary complex. However, given the high lysine content of collagen, which certainly contributes to its high picomolar affinity for DF and N-mers, many potential interactions between DF and N-mers, collagen I, and integrin receptors are possible.
In conclusion, the evidence we have presented demonstrates that DF and N-mers can, as a function of length, be angiogenesis-altering molecules, specifically interacting with and potentiating the FGFR1 IIIc binding of bFGF and promoting tubular morphogenesis in 3D collagen I matrices. The angiogenesis-altering properties of DF suggest the possibility that if a patient with sVOD can be supported through the clinical sequelae of hepatic failure, treatment with DF may promote and shorten the time required for hepatic revascularization and hence, ultimate clinical recovery.
Finally, DF is pharmacologically an extremely complicated agent, and its effects may be highly dependent on the extracellular microenvironment. For example, when HMEC-1 cells were plated on a 2D Matrigel matrix, tubular morphogenesis in the presence of DF decreased dramatically,57 in contrast to the data presented here. Therefore, the appropriate caution must be exercised if attempts at extrapolation of these results obtained with DF to other systems (eg, the interaction of multiple myeloma cells with their microenvironment58 ) are attempted.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by funding from Gentium S.p.A. (Como, Italy; C.S.), and from the Rick Corman Foundation (P.R. and C.S.).
Authorship
Contribution: C.S. designed and analyzed experiments and drafted manuscript; L.B. analyzed data and performed experiments; S.W., A.V., J.Z., J.S., and K.A. performed experiments; P.R. analyzed data and drafted the manuscript; C.E. provided vital new reagents and analyzed data; and M.I. provided vital new reagents and analyzed data.
Conflict-of-interest disclosure: C.S. received research funding from, is a member of the Scientific Advisory Board of, and is a consultant for Gentium, but has no ownership interests. L.B. received research funding from Gentium but has no ownership interests. C.E. and M.I. are employees of Gentium. P.R. is a member of the Scientific Advisory Board of and is a consultant for Gentium but has no ownership interests. The remaining authors declare no competing financial interests.
Correspondence: C. A. Stein, Montefiore Medical Center, 111 East 210 Street, Bronx, NY 10467; e-mail: cstein@montefiore.org.