Abstract
Background: The combination of fludarabine with a myeloablative dose of busulfan (FluBu4) has been shown to be well tolerated with reduced toxicity compared to other myeloablative regimens. Clofarabine is a second generation purine antimetabolite with potent anti-leukemia properties. We replaced fludarabine with clofarabine in a phase I/ II trial of Clofarabine-Busulfan x 4 (CloBu4) in an attempt to develop a pre-transplant conditioning regimen suitable for pts with refractory, non-remission hematologic malignancies at the time of transplant.
Methods: All pts had a hematologic malignancy not in remission at the time of transplant. Busulfan was administered as a single daily dose of 3.2 mg/kg IV x 4d (days -5 to -2) and clofarabine as a single daily dose of 20, 30 or 40 mg/m2 IV x 5d (days -6 to -2) with the specific dose per pt determined by the Time to Event-Continuous Reassessment Method (TITE-CRM). All pts received dexamethasone 12 mg IV on the days of clofarabine to avoid capillary leak syndrome. GVHD prophylaxis was tacrolimus/MMF in19 pts and tacrolimus/methotrexate in 1.
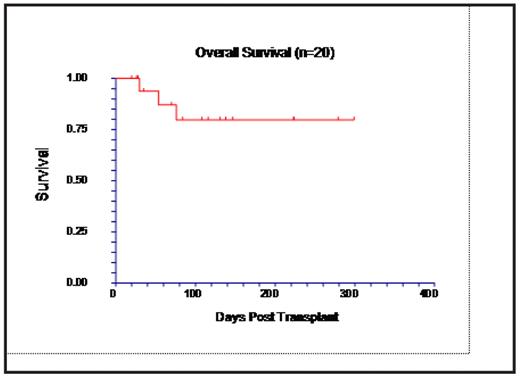
Results: To date, 20 pts are evaluable for toxicity, 19 for engraftment, and 16 for response. Diseases were AML (including 2 myeloid blast crisis of CML) (n=13), ALL (n=2), CLL (n=2), NHL (n=2), and multiple myeloma (n=1). Six pts had received previous stem cell transplantation (SCT), 2 auto SCT (range between SCT 295-1066 d) and 4 allo (range 75–1002 d). The clofarabine dose levels were: 20 mg/m2 (n=6), 30 mg/m2 (n=13) and 40 mg/ m2 (n=1). The median age of patients was 51 years (range 13–68). Donors were siblings (n=8) or unrelated volunteers (n=12). All 19 evaluable pts engrafted at a mean of 11 days for both neutrophils and platelets. Busulfan kinetics were evaluated in 14 patients with an average steady state level of 803 ng/ml (605–1132). Dose increase was required in 2 cases and decrease in 2 cases to maintain target range of 600–900 ng/ml. One pt who developed hypersensitivity to clofarabine with fever and joint pain after the first dose, received an additional 20 mg of dexamethasone with subsequent doses and finished all five doses of clofarabine. Other grade 3–4 toxicities attributable to the conditioning regimen include transient liver enzyme abnormalities (8/20), hypoxia (5/20), hypertension (2/20), seizure (1/20), and ascites (2/20). Neither case of ascites had elevated bilirubin, but one case showed pathologic findings compatible with veno-occlusive disease on liver biopsy. Both of these pts had pre-existing conditions including pretransplant liver damage (1) and enlarged spleen before SCT due to preceding myelofibrosis (1). Acute GVHD developed in 2/20 and proved fatal in one case. Peripheral blood CD3+ cell chimerism was evaluable in 10 pts on day 30; 1 pt had 100% donor CD3+ cells, whereas 9 pts had in mixed chimerism (<5% to 29% recipient cells). By day 100, 5/10 pts achieved complete donor type chimerism, and 5 still had mixed chimerism (<5% to 20% recipient cells). Four pts were too early for disease assessment; 11/16 pts were in CR at day 30, including 9/11 pts with AML. The 10 months OS is 80% (82% for AML) with a median follow-up of 6 months.
Conclusion: Clofarabine is well tolerated when given with full dose busulfan in this group of very high risk pts. All pts engrafted rapidly, and this combination shows promising anti-tumor activity in pts with relapsed/refractory diseases. The trial continues to accrue pts and updated results will be presented at the meeting.
Disclosures: Mineishi:Genzyme: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Research Funding. Erba:Genzyme: Consultancy, Research Funding, Speakers Bureau. Off Label Use: Clofarabine was used for transplant conditioning. .
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal