Abstract
Background: Atypical hemolytic uremic syndrome (aHUS) is a rare microangiopathic hemolytic anemia characterized by the uncontrolled progression of the alternative complement pathway due to genetic or acquired dysregulation of steady state alternative pathway activity leading to a proinflammatory and prothrombotic condition. Atypical HUS is characterized by intravascular hemolysis, consumptive thrombocytopenia, and microvascular glomerular thrombosis with the formation of thrombi in glomerular capillaries. As the thrombotic microangiopathy is particularly severe in the renal microvasculature, the disease inevitably leads to acute kidney injury with most cases progressing to end-stage-renal-disease. Eculizumab is a complement inhibitor that has been shown to completely and consistently block the activation of the terminal complement cascade thereby preventing the generation of the proinflammatory and prothrombotic molecules C5a and C5b-9. In recent phase 3 clinical studies in patients with the rare hemolytic disease paroxysmal nocturnal hemoglobinuria, chronic eculizumab treatment was shown to be safe, and demonstrated an effective reduction in intravascular hemolysis and a 85% reduction in thrombotic events.
Aim: The safety and efficacy of eculizumab in the management of aHUS. We sought to investigate the potential benefit of the complement inhibitor eculizumab in aHUS, a disease characterized by uncontrolled progression of the alternative complement pathway.
Methods: Two aHUS patients that were unresponsive to plasmapheresis were dosed with 600 mg of eculizumab to inhibit the terminal complement cascade. The patients were monitored closely for adverse events and platelet counts, creatinine and haptoglobin were assessed. PK/PD analyses were performed on one of the two patients.
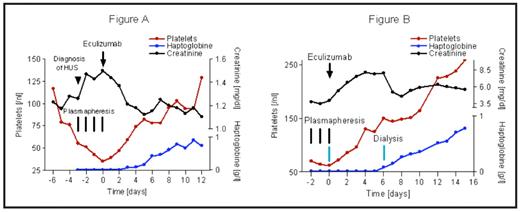
Results: We report aHUS in two patients successfully treated with the complement inhibitor eculizumab. The first was a 37-year old female with recurrence of aHUS after renal transplantation. At the age of 25 years, the patient developed aHUS with end-stage-renal- disease and stayed on dialysis until she received a first cadaveric kidney transplant 5 years later. This first transplant was lost 5 weeks after transplantation due to chronic aHUS. A second attempt of kidney transplantation was undertaken using a calcineurin-inhibitor free immunosuppressive protocol and despite immediate plasmapheresis (4 times), aHUS worsened (platelet count dropping, haptoglobin decreasing, creatinine increasing) indicating a high probability of repeated renal transplant loss. The patient was characterized as having a missense mutation in the gene encoding the complement regulatory protein factor H. The current literature indicates that patients with such mutations have a high incidence of graft rejection (7 of 8 grafts rejected). Eculizumab was therefore administered and resulted in immediate and complete inhibition of terminal complement activation for at least 5 days. During the week following treatment, platelet count increased, hemolysis normalized (as assessed by haptoglobin levels), and transplant function recovered (as assessed by creatinine levels) indicating successful reversal of aHUS (see Figure A).
The second patient was an 18-year old female with first the initial manifestation of aHUS. After a total of 18 plasmaphereses, the young patient was referred to our hospital with aHUS. When symptoms persisted despite another three plasmaphereses in our University hospital, we decided to administer eculizumab. Over the following week, platelet count normalized, hemolysis was reversed, and renal function partially recovered (see Figure B).
Summary: This is the first report evaluating the use of a complement inhibitor as a potential therapy for the treatment of aHUS. These data suggest that eculizumab therapy results in a reduction in thrombotic microangiopathy and hemolysis as evidenced by a reversal of thrombocytopenia, the normalization of hemolytic parameters, and the recovery of kidney transplant function. These data suggest the eculizumab modifies the course of aHUS and warrant further clinical investigation to confirm whether complement inhibition with eculizumab is an effective treatment of this devastating and life-threatening disease.
Disclosures: Rother:Alexion: Employment. Off Label Use: Use of Eculizumab in atypical HUS.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal