Abstract
Introduction: The ability to define high-risk cohorts of chronic lymphocytic leukemia (CLL) patients has led to clinical trials exploring the utility of early aggressive interventions. Recent work, such as that by the Spanish GELCAB group, demonstrates that molecular remission is an appropriate therapeutic goal for high risk CLL. However, molecular remission may not be evident in some patients until 9 to 12 months post-hematopoietic cell transplant (HCT), even with highly sensitive minimal residual disease (MRD) testing methods. To facilitate clinical management of these patients during the first year after HCT, we evaluated HCT and CLL risk factors for predictive associations to molecular remission as determined by MRD monitoring in 19 patients undergoing nonmyeloablative allogeneic HCT.
Methods: Nineteen high-risk CLL patients received a nonmyeloablative transplant preparative regimen consisting of total lymphoid irradiation and anti-thymocyte globulin, followed by rituximab 375mg/m2 on days 56, 63, 70, and 77 after transplant. Median follow-up was 611 days, with a range of 1–3 years.
Peripheral blood was collected pre-HCT, and post-HCT monthly for six months, then every 3 months thereafter.
Serial assessment of MRD by allele-specific oligonucleotide quantitative PCR (ASOQ-PCR) was performed (177 analyses), using a TaqMan probe specific for the unique CDR3 region of the clonal IgH gene rearrangement. Molecular remission was defined as two consecutive measurements of 2 30 copies/ug WBC-DNA (1.8E-4 cells) at a minimum interval of 30 days.
Molecular remission, as determined by the MRD analyses, was compared by univariate analyses with CLL and HCT risk factors. The CLL risk factors evaluated were VH-mutation status, high risk cytogenetics (deletions or mutations of p53 and/or ATM), and fludarabine refractoriness. Allogeneic HCT risk factors evaluated were lymph node >5cm, elapsed time from diagnosis to HCT, disease status before HCT, MRD level before HCT, number of prior treatment regimens, related vs. unrelated donor, female donor to male recipient and time to establish full donor chimerism. Data for the latter was obtained by serial PB analyses of T-cell (CD3+) and NK-cell (CD56+) donor chimerism (119 analyses) by DNA fragment analysis of serial tandem repeat (STR) polymorphisms. Samples were collected in parallel with the MRD specimens. Complete donor chimerism (CDC) was defined as ≥95% donor for both T- and NK-cells. Because of the therapeutic use of rituximab in this trial, B-cell (CD19+) chimerism was not included in our analysis.
Results: Twelve of 19 patients (63%) achieved molecular remission by one year. Univariate analyses of found no statistically significant association between this “MR” group –those that attain molecular remission at one year -- and any of the factors studied except complete T/NK donor chimerism. Table 1 shows that CDC precedes and predicts molecular remission.
Table 1. Time to complete donor chimerism in patients that achieve molecular remission by one year post HCT (“MR group”)
| days post-HCT . | Percent of MR group in CDC . | p-value . |
|---|---|---|
| d+90 | 58% (7/12) | 0.0113 |
| d+120 | 75% (9/12) | 0.0037 |
| d+150 | 83% (10/12) | 0.0013 |
| d+180 | 92% (11/12) | 0.0002 |
| d+180 | 92% (11/12) | 0.0002 |
| d+365 | 100% (12/12) |
| days post-HCT . | Percent of MR group in CDC . | p-value . |
|---|---|---|
| d+90 | 58% (7/12) | 0.0113 |
| d+120 | 75% (9/12) | 0.0037 |
| d+150 | 83% (10/12) | 0.0013 |
| d+180 | 92% (11/12) | 0.0002 |
| d+180 | 92% (11/12) | 0.0002 |
| d+365 | 100% (12/12) |
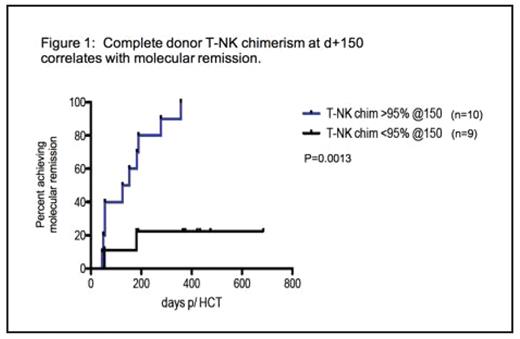
Figure 1 is an example of the univariate analyses comparing chimerism data with time to molecular remission, here showing the percent of patients achieving durable molecular remission for two groups of patients – those showing CDC by d+150 post HCT and those whose chimerism is <95% at that time.
Conclusion: Extensive ASO-Q-PCR MRD and donor-chimeric status monitoring was performed in this study, demonstrating that complete donor chimerism (CD3 and CD56) in nonmyeloablative allogeneic HCT for CLL is strong predictor for achieving molecular remission by one year post transplantation.
Complete donor T-NK chimenism at d+ 150 correlates with molecular remission.
Complete donor T-NK chimenism at d+ 150 correlates with molecular remission.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal