Abstract
Nuclear Factor of Activated T-cells 1 (NFAT1) is a member of the NFAT family of transcription factors (NFAT1-NFAT5) that has been shown to play an important role in regulating genes related to T-cell expansion, differentiation, and apoptosis. As murine NFAT1 knockout mice exhibit lymphoproliferative disease, we hypothesize that aberrant expression of NFAT1 may impact cell cycle dysregulation underlying T-ALL pathogenesis. Four T-ALL cell lines – including 3 mature T-cell lines: CCRF-CEM (no clear chromosomal abnormalities), Jurkat (karyotype 46, XY, -2, -18, del(2) (p21p23), del(18) (p11.2)), Loucy (translocation t(16;20)(p12;q13), and p53 overexpression), and one immature T-cell line MOLT-4 (hypertetraploid chromosomes and 6q-, t(7;7)) (ATCC Manassas, VA), established from peripheral blood of T-ALL patients were analyzed and compared to normal resting adult CD4+ T-cells. Flow cytometry analysis was performed as previously described including CD34, CD38, HLA-DR, CD3, CD4, CD8, CD2, and CD7 to determine maturation stage; MOLT-4 being the most primitive and Jurkat the most mature.
Methods: Growth curves were determined and proliferation potential was determined using MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. The cell cycle phase distribution was assessed by flow cytometry analysis of Hoechst 33342-stained cells. mRNA expression was examined by quantitative rtPCR using TaqMan probes (ABI) on cDNA derived from TRIzol purified total RNA. For stimulation, we used 2 μM ionomycin treatment for 3 hours. Proteins were prepared as whole cell extract from radioimmunoprecipitation assay (RIPA) buffer lysed cells. Protein expression was evaluated by Western blotting analysis of 20 μg of proteins using anti-NFAT1 antibody (BD Biosciences) and anti-γ-tubulin antibody (Sigma) as a loading control. Transient transfection of GFP vector or plasmid containing the wild-type or constitutively active NFAT1 gene along with the GFP gene was performed by either electroporation (Amaxa) or Lipofectamine 2000 (Invitrogen). GFP positive cells were sorted by FACSAria sorter.
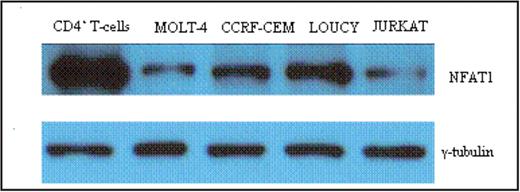
Results: Despite their different maturation states, all cell lines (except Loucy) have similar high growth rates. However, the cell cycle distribution analysis of Hoechst-stained cells revealed a lower proportion of Loucy cells in the G0/G1 phase (35% vs. 48–51% for the other 3 cell lines) and a higher proportion in the G2/M phase (37% vs. 19–24% for the other 3 cell lines). In addition, Loucy cells have a higher rate of apoptosis as measured by Annexin V staining. Analysis of NFAT1 expression demonstrated decreased levels of NFAT1 mRNA (30-70-fold) and protein (2-10-fold) in these cell lines compared to resting adult peripheral blood CD4+ T lymphocytes.
Moreover, ionomycin stimulation of the calcium-calcineurin pathway in these cells revealed aberrant activation of NFAT1. There was no dephosphorylated or activated NFAT1 in MOLT-4 and Jurkat cells; in contrast, dephosphorylated or activated NFAT1 was degraded in CCRF-CEM and Loucy. As NFAT1 is implicated in the regulation of cell cycle and apoptosis, the expression of cell cycle and apoptosis genes was measured by qrtPCR. Consistent with the negative regulatory role of NFAT1 in cell cycle, T-ALL cells expressing low level of NFAT1 showed upregulation of cyclin A2 (20-80-fold), cyclin E2 (5-8-fold) and CDK4 (3-7-fold) as well as downregulation of p21Cip1 (20-470-fold) and p27Kip1 (20-180-fold). In addition, these cells also demonstrated downregulation of the expression of the pro-apoptotic gene Nur77 (2.5-10-fold). Introduction of exogenous NFAT1 gene into Jurkat cells resulted in decreased proliferation rate to 64% and 42%, for wild-type and constitutively active form of NFAT1 gene, respectively, compare to the cells transfected with the empty GFP vector.
Conclusion: Aberrant expression of NFAT1 contributes to leukemogenesis via dysregulation of proliferation and apoptosis. Targeted NFAT1 expression may be an effective therapeutic strategy in T-ALL.
Disclosures: Laughlin: Abraham J & Phyllis Katz Cord Blood Foundation: Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal