Abstract
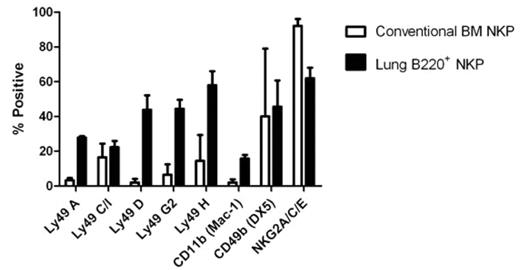
Natural Killer (NK) cells are important effector cells in innate immunity, and play a vital role in antiviral defense, tumor surveillance and modulation of the adaptive immune response. NK cells were originally believed to arise from a lin−NK1.1−CD122+ bone marrow (BM) progenitor. However, recent findings have identified NK progenitors (NKP) and distinct NK differentiation pathways in the thymus, lymph node, spleen and liver. The physiological role of these extra-BM developmental pathways remains to be determined. We hypothesized that these alternative pathways on NK development would have an impact on the generation of the phenotypic heterogeneity observed in cell-surface receptor expression and functional NK cell subsets. Here, we demonstrate the identification of a small population of cells in the lung of C57Bl/6 mice (0.02% of lung leukocytes) that have a lin−NK1.1−CD122+B220+ cell surface phenotype. These cells also show potent in vitro NK cell activity when cultured on OP-9 stromal cells with IL-7, mSCF, Flt3L and IL-15, as well as in vivo NK cell potential upon adoptive transplant into RAG-2−/− IL2Rγ−/− and NOD/SCID IL2Rγ−/− hosts. Mature NK cells (CD3−NK1.1+) derived in vitro from conventional BM NKP and lung B220+ NKP were characterized for cell-surface receptor expression after 16–18 days (figure 1, mean with SEM). Clear differences in activating and inhibitory NK cell-surface marker expression were observed between NK cells derived in vitro from conventional BM NKP and lung B220+ NKP (Ly49D p<0.05, Ly49G2 p<0.05, NKG2A/C/E p<0.05). These findings suggest that B220+ NKP may generate phenotypically and functionally distinct NK cell types.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal