Abstract
Allogeneic transplantation of hematopoietic cells is an effective treatment of leukemia, even in advanced stages. Allogeneic lymphocytes produce a strong graft-versus-leukemia (GVL) effect, but the beneficial effect is limited by graft-versus-host disease (GVHD). Depletion of T cells abrogates GVHD and GVL effects. Delayed transfusion of donor lymphocytes into chimeras after T cell–depleted stem cell transplantation produces a GVL effect without necessarily producing GVHD. Chimerism and tolerance provide a platform for immunotherapy using donor lymphocytes. The allogeneic GVL effects vary from one disease to another, the stage of the disease, donor histocompatibility, the degree of chimerism, and additional treatment. Immunosuppressive therapy before donor lymphocyte transfusions may augment the effect as well as concomitant cytokine treatment. Possible target antigens are histocompatibility antigens and tumor-associated antigens. Immune escape of tumor cells and changes in the reactivity of T cells are to be considered. Durable responses may be the result of the elimination of leukemia stem cells or the establishment of a durable immune control on their progeny. Recently, we have learned from adoptive immunotherapy of viral diseases and HLA-haploidentical stem cell transplantation that T-cell memory may be essential for the effective treatment of leukemia and other malignancies.
Introduction
Treatment of leukemia and other malignancies of the hematopoietic system with total body irradiation (TBI) and transplantation of bone marrow from healthy persons was developed from studies on radiation protection in the 1950s. However, it was evident from bioassays of murine leukemia that radiation could not eliminate leukemia in doses tolerated clinically.1 In contrast, TBI followed by allogeneic marrow transplantation could cure leukemia in some murine models.2 The term adoptive immunotherapy by allogeneic marrow transplantation was coined by Mathé3 in the early 1960s when little was known about donor selection, appropriate conditioning treatment, and prevention of graft-versus-host disease (GVHD). Selection of sibling donors by human leukocyte antigen (HLA) typing, the combination of TBI with cyclophosphamide for conditioning treatment, and postgrafting immunosuppressive treatment with methotrexate provided the basis of success in the treatment of acute leukemia with allogeneic marrow transplantation.4,5 In these patients, Weiden et al described the beneficial effect of GVHD on the incidence of leukemia relapse.6 In patients with chronic GVHD, this reduced relapse was even associated with a survival benefit.7 The role of T cells became evident when depletion of T cells from the graft was introduced into the clinical practice for prevention of GVHD.8,9 In chronic myelogenous leukemia (CML), the beneficial effect of T-cell depletion on GVHD was negated because of an increased relapse rate.10 Increased relapse after T-cell depletion was most pronounced in CML, less in acute myeloid leukemia (AML), and lowest in acute lymphoblastic leukemia (ALL).11
The challenge of allogeneic stem cell transplantation for treatment of leukemia and other malignancies of the hematopoietic system is the prevention of GVHD without losing the graft-versus-leukemia (GVL) effect. One possible approach has been is to apply a delayed transfusion of T cells after T cell–depleted marrow transplantation. Previous experiments in stable canine chimeras showed that graft-versus-host tolerance was not abrogated by donor lymphocyte transfusions (DLTs).12 In DLA-identical littermate chimeras, we analyzed at which time after T cell–depleted marrow transplantation we could transfuse donor lymphocytes without producing GVHD. In the first days and weeks, we produced fatal GVHD by DLTs, at 2 months, and later we could transfuse large numbers of donor lymphocytes without producing GVHD. Using small numbers of marrow cells, we could induce mixed chimerism, which was converted to complete chimerism after DLT.13 The results of these animal experiments encouraged us in October 1988 to treat a patient with relapse of CML with DLT after interferon-α (IFN-α) treatment had failed.14 This was the first evidence that lymphocytes could induce lasting remissions without chemotherapy or radiotherapy. Another early case was reported by S. Slavin15 involving a patient treated in January 1987 for early relapse of ALL at one month after transplantation. These early successes stimulated research and wide clinical application of DLT for the treatment of leukemia, lymphoma, and myeloma. Moreover, the possibility of controlling residual disease by DLT has encouraged conditioning regimens of reduced intensity16,17 that are better tolerated by elderly and debilitated patients.
Graft-versus-host and GVL
In patients with immune deficiency, blood transfusions can produce severe myelosuppression18 by a graft-versus-host reaction of donor blood unit T cells against recipient hematopoiesis. A GVL effect can also be obtained by transfusion-induced suppression of the host's hematopoiesis resulting from sharing of histocompatibility antigens with the leukemia. The primary targets of graft-versus-host reactions are cells of the hematopoietic system of the host. In dogs, mixed chimerism was studied as a model for GVL reactions in the form of a graft-versus-host hematopoiesis reaction.13 Similarly, mixed chimerism could be converted into complete chimerism by DLT in human patients.19 Several minor histocompatibility antigens (mHA) with a tissue distribution restricted to hematopoietic cells have been described20-22 that may elicit strong GVL effects.23 However, the gene frequency of minor histocompatibility antigens with restricted tissue distribution allows the use only in a small proportion of patients.
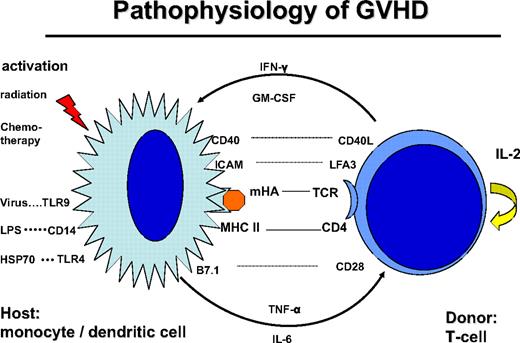
Donor T cells are stimulated by dendritic cells (DCs) of the host, through presentation of mHA (Figure 1). CD4+ helper T cells are stimulated by HLA class II peptide complexes of DCs. Stimulation of T cells requires additional signals via costimulatory molecules, adhesion molecules, and stimulatory cytokines, which may also produce acute GVHD-like changes by themselves.24 This GVHD-like reaction is seen in syngeneic transplants and some autologous transplantations. Normally, it subsides after several days of treatment with steroids. In contrast, in allogeneic transplantations, the reaction is maintained by the T-cell recognition of mHA. Intensive conditioning treatment with radiation and chemotherapy prematurely activates antigen-presenting cells (APCs) and leads to an accelerated stimulation of T helper cells. Similarly, bacterial and viral infections activate APCs via Toll-like receptors (TLRs).25 Indeed, activation of APCs and release of pro-inflammatory cytokines stimulate T helper cells, and their subsequent release of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) results in a vicious circle known as a “cytokine storm.”26 Tempering the cytokine storm by treatment with TNF-α antibody during the conditioning treatment suppressed acute GVHD27 or led to its delay. Similarly, delayed acute GVHD may occur in patients conditioned with reduced intensity to avoid up-regulation of costimulatory molecules on APCs and release of proinflammatory cytokines.
Pathophysiology of GVHD: the donor T cell is encountering a host antigen-presenting cell (APC: monocyte/dendritic cell). The APC is activated by the conditioning treatment with radiation and chemotherapy; kit may also be activated by infections: viral via Toll-like receptor-9 (TLR9), bacterial infection via lipopolysaccharide (LPS), CD14, and TLR4. The interaction is regulated by recognition of mHA in the context with CD4 and HLA class II. The reaction is stimulated by costimulatory molecules CD40-CD40 ligand, B7.1-CD28, and adhesion molecules ICAM and LFA3. Cytokine secretion includes tumor necrosis factor-α and IL-6 by the APC and IFN-γ and GM-CSF by the T cell.
Pathophysiology of GVHD: the donor T cell is encountering a host antigen-presenting cell (APC: monocyte/dendritic cell). The APC is activated by the conditioning treatment with radiation and chemotherapy; kit may also be activated by infections: viral via Toll-like receptor-9 (TLR9), bacterial infection via lipopolysaccharide (LPS), CD14, and TLR4. The interaction is regulated by recognition of mHA in the context with CD4 and HLA class II. The reaction is stimulated by costimulatory molecules CD40-CD40 ligand, B7.1-CD28, and adhesion molecules ICAM and LFA3. Cytokine secretion includes tumor necrosis factor-α and IL-6 by the APC and IFN-γ and GM-CSF by the T cell.
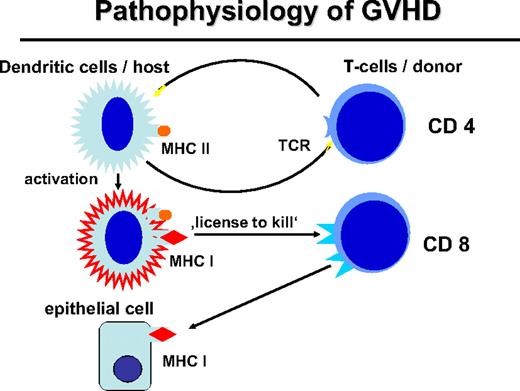
Activation of myeloid DCs not only stimulates helper T cells but also stimulates CD8+ cytotoxic T cells through presentation of HLA class I–restricted peptides (Figure 2). Activated DCs provide a “license to kill” to cytotoxic T cells.28 This license is also required for the GVL reaction29 whereby expression of MHC class II by APCs and stimulation of CD4+ cells are necessary for a CD8-mediated GVL response. The role of MHC class I on host DCs in GVHD was stressed by Shlomchik et al.30 Host DCs deficient for MHC class I were unable to initiate CD8-associated GVHD. DCs of the graft that express MHC class I maximize GVHD through cross-presentation of host antigens; however, they could not optimize the GVL reaction in a murine CML model.31
Pathophysiology of GVHD. The interaction of the donor T cell and the host APC leads to the activation of APC and immunogenic presentation of HLA class I–restricted minor histocompatibility antigens (“license to kill”) to activate CD8 cells of the transplantation that can attack mHA on epithelial tissue or also on hematopoietic tissue.
Pathophysiology of GVHD. The interaction of the donor T cell and the host APC leads to the activation of APC and immunogenic presentation of HLA class I–restricted minor histocompatibility antigens (“license to kill”) to activate CD8 cells of the transplantation that can attack mHA on epithelial tissue or also on hematopoietic tissue.
DCs of leukemia origin have been demonstrated in CML,32-34 and they may present mHA23 and leukemia-specific peptides to donor lymphocytes.35 Most probably, the spontaneous differentiation of CML progenitor cells to DCs is one important factor in the good response of CML to DLT. Similarly, blasts of AML and MDS may differentiate spontaneously to DCs,36 or differentiation can usually be induced by stimulation with GM-CSF alone or in combination with other cytokines.37 In the presence of cytokines, differentiation of leukemia blasts toward DCs in vitro has been demonstrated to be independent of the karyotype. This finding indicates that the prognostic role of the karyotype may not apply to immunotherapy.37,38 In CML, the combination of GM-CSF and IFN-α has been better than the combination of GM-CSF and IL-4 for producing DCs presenting HLA class I–restricted peptides.39
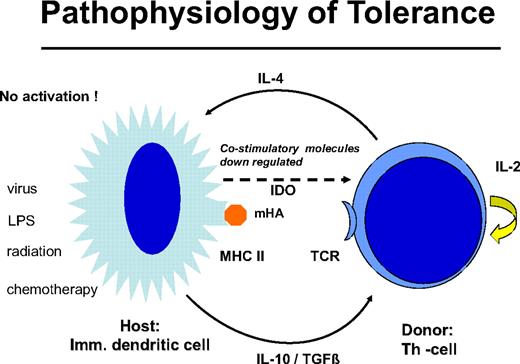
Delayed DLT takes advantage of the transplantation tolerance induced by stem cell transplantation (Figure 3). In long-term canine chimeras, transplantation tolerance could not be abrogated by DLT.12 In these animals, only T cells of donors sensitized against the recipients produced GVHD in some cases. Resistance to the induction of GVHD by DLT may be the result of replacement of host DCs by DCs produced by the graft or to the generation of nonstimulatory, immature DCs of the host that fail to stimulate naive donor T cells. The majority of patients with leukemia are age 40 years and older, and peripheral tolerance is more probable than central tolerance. Host DCs are down-regulated by regulatory T cells of donor origin (Figure 4). Peripheral tolerance is maintained by down-regulation of costimulatory molecules and production of IL-10 and transforming growth factor-β by DCs and IL-4 by T cells. This tolerance can be broken by T-cell depletion before DLT (H. Menzel, H.-J.K., S. Thierfelder, unpublished data, 1994).40,41 CD4,CD25,CD28 positive regulatory T cells of the donor42 as well as residual host T cells able to produce Fas-L, perforin, and IFN-γ have been found to be responsible for the GVHD resistance with delayed DLT.41 Depletion of T cells by prior chemotherapy increases the risk for GVHD caused DLT. In human patients with recurrent acute leukemia, immunosuppressive conditioning with fludarabine and cyclophosphamide before DLT produced significantly more GVHD.43 Patients given T cell–depleted stem cell transplantations have a higher risk of GVHD after DLT than those given unmanipulated transplantations.44 This supports the role of regulatory T cells controlling tolerance via DCs. On the other side, activation of the DCs by conditioning treatment with chemotherapy and radiotherapy before DLT and severe infections may activate the costimulatory mechanisms and produce GVHD after DLT (H.-J.K., C. Schmid, E. Weissinger, E. Holler, unpublished data, 2000).
Graft-versus-host tolerance: donor T cells and “immature” host DCs are nonstimulatory. IL-10 and transforming growth factor-β are produced by the DC and IL-4 by the T cell; no costimulatory molecules are expressed by indoleamin 2,3 deoxygenase is released. This peripheral tolerance is maintained as long as there is no conditioning treatment with radiation or chemotherapy and no infection that may activate the DCs.
Graft-versus-host tolerance: donor T cells and “immature” host DCs are nonstimulatory. IL-10 and transforming growth factor-β are produced by the DC and IL-4 by the T cell; no costimulatory molecules are expressed by indoleamin 2,3 deoxygenase is released. This peripheral tolerance is maintained as long as there is no conditioning treatment with radiation or chemotherapy and no infection that may activate the DCs.
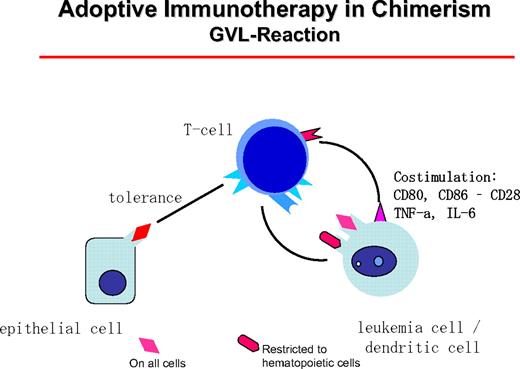
GVL reactions are maintained by host DCs that stimulate donor T cells (Figure 5). T helper cells type I can themselves become cytotoxic or “license” the DCs to activate CD8+ donor cells. In CML patients, clinical responses have been seen in all patient groups with an allogeneic donor. In syngeneic twins, donor lymphocytes did not induce remissions.44 The best candidates for target antigens of the GVL reactions are mHA expressed on hematopoietic cells, such as HA-1, HA-2, HB-1, BCL2A1, and HB-145 (for review, see Bleakley and Riddell22 ). However, donor-recipient combinations with relevant differences for known mHA are a small minority of cases. Of higher clinical relevance are Y chromosome-coded antigens that are recognized by female donor cells. Unfortunately, most Y chromosome-coded proteins have a broad tissue expression, but UTY is only weakly expressed on nonhematopoietic cells and highly expressed on hematopoietic cells.46
The GVL reaction is maintained by the interaction of host DCs of leukemia origin that presents mHA restricted to hematopoietic tissue or mHA with general distribution. These can be presented on epithelial cells at a lower concentration, leaving a hematopoiesis-restricted activity.
The GVL reaction is maintained by the interaction of host DCs of leukemia origin that presents mHA restricted to hematopoietic tissue or mHA with general distribution. These can be presented on epithelial cells at a lower concentration, leaving a hematopoiesis-restricted activity.
Overexpressed differentiation antigens may also serve as targets for the GVL reaction. Examples of such targets are Wilms' tumor 1,47 the peptide PR-148 of proteinase 3 of granulocytes, and receptor for hyaluronic acid–mediated motility.49 Vaccination studies with Wilms' tumor 1 and PR-1 have shown responses in patients with AML.50 Overexpressed proteins are more antigenic when presented by foreign HLA molecules. The allogeneic situation can be simulated by transfection of allogeneic T-cell receptors against these proteins into autologous T cells.51
CML
The best results for DLT have been obtained in the treatment of relapse of CML after transplantation15,44,52-57 (Table 1). In most studies, between 70% and 80% of patients treated for hematologic and cytogenetic relapse obtained a complete cytogenetic response. In patients with cytogenetic or molecular relapse, defined as an increase of the quantitative reverse-transcribed polymerase chain reaction for bcr/abl, remissions could be rescued in 80% by early DLT treatment. Most remissions are durable, and the first patients treated with DLT for CML relapse in 1988 are still in remission. In more advanced stages of relapse, the rate of complete cytogenetic remission is lower and responses are not as durable. In the European Blood and Marrow Transplantation (EBMT) experience,44 responses were observed in all patient groups with allogeneic donors, HLA-identical siblings, HLA-mismatched family members, and unrelated HLA-matched donors. However, no response was observed in 4 patients with syngeneic monozygotic twin donors. The GVL effect appeared to be part of the GVH reaction; there was a correlation of GVL with GVHD, and the best responses were seen in patients with GVHD of grade more than or equal to II. However, 50% of patients had a complete cytogenetic remission without any signs of clinical GVHD. Therefore, there may be a GVL reaction separate from GVHD or a GVH reaction severe enough to manifest with clinical symptoms.
Donor lymphocyte transfusion for treatment of relapse and persistence of malignancy
| Disease/study cohorts . | No. of patients responding/no. treated . | % CCR (y) . | Reference number/comments . | |
|---|---|---|---|---|
| CML molecular/cytogenetic relapse | ||||
| EBMT study | 40/50 | 80% (4 y) | 44 | |
| North American | 3/3 | 56 | ||
| Chronic phase | ||||
| EBMT | 88/114 | 60% (4 y) | 44 | |
| North American | 25/34 | 56 | ||
| Japan | 11/12 | 82% (3 y) | 158 | |
| Transformed phase | ||||
| EBMT | 13/36 | 20% (4 y) | 44 | |
| North American | 5/18 | 56 | ||
| Japan | 3/11 | 0% (3 y) | 158 | |
| AML/MDS | ||||
| EBMT | 15/58 | 15% (4 y) | 44 | |
| North American | 8/44 | 56 | ||
| Prospective US study | 25/51 | 19% (2 y) | 88 | |
| Japan | 13/32 | 7% (2/3 y) | 95 | |
| 33% (2/3 y) | ||||
| Korean | 10/17 | 31% (2 y) | 162/chemotherapy + G-CSF mobilized DLI | |
| Lille, France | 2/14 | 2/14 (4 y) | 92 | |
| ALL | ||||
| EBMT | 3/20 | 0% (4 y) | 44 | |
| North American | 2/11 | ND | 56 | |
| IBMTR | 11/44 | 13% (3 y) | 160 | |
| Japan | 6/23 | 0% (3 y) | 95 | |
| Korean | 7/10 | 10% (2 y) | 161/chemotherapy + G-CSF mobilized DLI | |
| CLL | ||||
| German Multicenter trial on molecular relapse and persistence | 7/9 molecular remission | 7/9 (> 2 y) | 103 | |
| Bristol multicenter | 1/7 | 0 | 19 | |
| DFCI | 6/7 | NK | 102 | |
| NHL | ||||
| North American study | 0/6 | NK | 56 | |
| EBMT study | 10/14 OR | NK | 162 | |
| Progressive and refractory | 6/14 CR | |||
| UC London | LG-NHL 6/10 | NK | 163 | |
| Relapsed and refractory | HG-NHL 3/9 | |||
| Myeloma | ||||
| EBMT study | 5/17 | 45% (2 y) | 44 | |
| Relapse/progression | ||||
| North American Study relapse/progression | 2/4 | 56 | ||
| US multicenter study persistent/progressive | 7/22 | 4/7 (> 1 y) | 113 | |
| Dutch multicenter study relapse and progression | 14/27 OR | 5/27 (> 2.5 y) | 164 | |
| 10/27 CR | ||||
| Preemptive in chemosensitive MMY | 6/20 CR/PR | 30% (2 y) | 165 | |
| → 7/14 CR/PR | ||||
| Relapse/progression | 24/63 | 117 | ||
| 12 CR | ∼ 45% (3 y) | |||
| 12 PR | ||||
| Relapse/progression Johns Hopkins Hospital | 8/16 | 5 > 2 y | 166 | |
| 6 CR 2PR |
| Disease/study cohorts . | No. of patients responding/no. treated . | % CCR (y) . | Reference number/comments . | |
|---|---|---|---|---|
| CML molecular/cytogenetic relapse | ||||
| EBMT study | 40/50 | 80% (4 y) | 44 | |
| North American | 3/3 | 56 | ||
| Chronic phase | ||||
| EBMT | 88/114 | 60% (4 y) | 44 | |
| North American | 25/34 | 56 | ||
| Japan | 11/12 | 82% (3 y) | 158 | |
| Transformed phase | ||||
| EBMT | 13/36 | 20% (4 y) | 44 | |
| North American | 5/18 | 56 | ||
| Japan | 3/11 | 0% (3 y) | 158 | |
| AML/MDS | ||||
| EBMT | 15/58 | 15% (4 y) | 44 | |
| North American | 8/44 | 56 | ||
| Prospective US study | 25/51 | 19% (2 y) | 88 | |
| Japan | 13/32 | 7% (2/3 y) | 95 | |
| 33% (2/3 y) | ||||
| Korean | 10/17 | 31% (2 y) | 162/chemotherapy + G-CSF mobilized DLI | |
| Lille, France | 2/14 | 2/14 (4 y) | 92 | |
| ALL | ||||
| EBMT | 3/20 | 0% (4 y) | 44 | |
| North American | 2/11 | ND | 56 | |
| IBMTR | 11/44 | 13% (3 y) | 160 | |
| Japan | 6/23 | 0% (3 y) | 95 | |
| Korean | 7/10 | 10% (2 y) | 161/chemotherapy + G-CSF mobilized DLI | |
| CLL | ||||
| German Multicenter trial on molecular relapse and persistence | 7/9 molecular remission | 7/9 (> 2 y) | 103 | |
| Bristol multicenter | 1/7 | 0 | 19 | |
| DFCI | 6/7 | NK | 102 | |
| NHL | ||||
| North American study | 0/6 | NK | 56 | |
| EBMT study | 10/14 OR | NK | 162 | |
| Progressive and refractory | 6/14 CR | |||
| UC London | LG-NHL 6/10 | NK | 163 | |
| Relapsed and refractory | HG-NHL 3/9 | |||
| Myeloma | ||||
| EBMT study | 5/17 | 45% (2 y) | 44 | |
| Relapse/progression | ||||
| North American Study relapse/progression | 2/4 | 56 | ||
| US multicenter study persistent/progressive | 7/22 | 4/7 (> 1 y) | 113 | |
| Dutch multicenter study relapse and progression | 14/27 OR | 5/27 (> 2.5 y) | 164 | |
| 10/27 CR | ||||
| Preemptive in chemosensitive MMY | 6/20 CR/PR | 30% (2 y) | 165 | |
| → 7/14 CR/PR | ||||
| Relapse/progression | 24/63 | 117 | ||
| 12 CR | ∼ 45% (3 y) | |||
| 12 PR | ||||
| Relapse/progression Johns Hopkins Hospital | 8/16 | 5 > 2 y | 166 | |
| 6 CR 2PR |
CML indicates chronic myelogenous leukemia; EBMT, European Blood and Marrow Transplantation; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; ND, not done; CLL, chronic lymphocytic leukemia; DFCI, Dana Farber Cancer Institute; NHL, non-Hodgkin lymphoma; LG-NHL, low-grade non-Hodgkin lymphoma; HG-NHL, high-grade non-Hodgkin lymphoma; NK, not known; OR, overall response; CR, complete remission; PR, partial remission; MMY, multiple myeloma; and CCR, continuous complete remission.
It is noteworthy that the GVL effect may not be observed until 4 to 8 weeks after DLT. In some patients, the disease may even progress for a month before counts drop suddenly and remission is induced. Therefore, clinicians must be patient and not give up on DLT at less than 2 months. The time until molecular remission is achieved can be 4 to 6 months, and remissions have been observed more than 1 year after DLT. This delayed type of response could be the result of an ongoing immune reaction of donor T cells against the leukemia or the result of the elimination of an early leukemic stem cell whose progeny survive for months.
Risks of the treatment are the occurrence of GVHD and myelosuppression. The risk of GVHD of moderate and severe degree (≥ II°) was 34%. This risk is lower than that for GVHD after stem cell transplantation, despite the omission of immunosuppressive therapy. Prevention of GVHD has been attempted by depletion of CD8+ T cells.58,59 CD4+ T cells produce a sufficient GVL effect and CD8+ cells can be recruited in vivo.60 Others have transferred a suicide gene into the T cells,61,62 providing an opportunity to ablate T cells if GVHD occurs by treatment with a drug that leads to selective death of transduced T cells. The most widely accepted method for DLT is the transfusion of donor lymphocytes in escalating doses.63 It could be shown that less than or equal to 2 × 107/kg mononuclear blood cells produced less than 5% DLT-related mortality in patients with an HLA-matched donor.64 Recommended initial doses are 106/kg CD3+ T cells in patients with an HLA-identical sibling donor and 0.5 × 106/kg CD3+ T cells in patients with an HLA-matched unrelated donor. Considering the delayed response seen after DLT, escalation of the dose can be delayed for 2 months in patients without rapid progression of the disease. In more progressive forms of relapse, the interval may be shortened to 4 weeks because in most cases GVHD occurs within 4 weeks of DLT, if it occurs.
Myelosuppression was seen in approximately 10% of patients and was most pronounced in patients with hematologic relapse. It may be the consequence of eliminating host-type hematopoiesis without sufficient replacement by donor-type hematopoiesis. Transfusion of donor marrow without conditioning treatment restored hematopoiesis in some patients. In others, stem cell replacement was not effective. In these patients, it has been hypothesized that the stroma may have become deficient in supporting HSCs.
DLT, IFN-α, and GM-CSF
Our preferred method to treat recurrent CML after transplantation is the combination of IFN-α and DLT. Low dose IFN-α (106 U/day) is given daily together with escalating doses of donor lymphocytes, starting with 106/kg CD3+ T cells in HLA-identical sibling transplantations. Escalated doses of 5 × 106/kg and 10 × 106/kg are given at 2-month intervals, according to the clinical course. In patients with unrelated matched donors, the starting dose is 0.5 × 106/kg CD3+ T cells. In patients with HLA-haploidentical transplantations, 105/kg is advocated as the starting dose.65
Patients not responding to the combination of IFN-α and DLT may still respond to the combination of interferon-α, GM-CSF, and DLT.66 However, it is very important that the disease be treated in a chronic phase. Five of 7 patients in chronic phase relapse responded with complete remission, whereas the combination failed in 2 patients and they were subjected to retransplantations without immunosuppression. One responder died of myelosuppression at 4 months. Of 3 patients with blast phase relapse, only one responded after the combination of chemotherapy with IFN-α, GM-CSF, and DLT, but she died of extensive chronic GVHD.
Remissions induced by DLT are mostly durable, with the longest survivors now alive more than 19 years after treatment. Yet relapses occur, as summarized for outcome of patients treated in Great Britain by Cummins et al.67 Although most patients responded again to the treatment with DLT, the role for imatinib in treatment of relapse is uncertain.
Imatinib and DLT
The selective tyrosine kinase inhibitor imatinib has revolutionized the treatment of CML by its high efficiency and low toxicity.68 Therefore, treatment with imatinib was given for relapse of CML in several studies instead of DLT.69,70 The majority of patients with hematologic relapse responded to imatinib with cytogenetic remissions. Several patients developed signs of GVHD and myelosuppression. The majority of patients experienced relapse after discontinuation of imatinib; however, single patients remained in remission. In our own experience, 7 of 10 patients treated with imatinib became negative in reverse-transcribed polymerase chain reaction. Six patients relapsed: 4 on treatment and 2 patients within 2 to 4 months after discontinuation of imatinib. However, 1 patient remained negative for more than 2 years.71
Application of imatinib before transplantation provided excellent results in patients treated for advanced phase CML. Three of 6 patients with blast crisis who responded to imatinib remain in continued complete remission after transplantation.72
The combination of DLT and imatinib has been advocated by some investigators73 for the treatment of recurrent CML after transplantation. This combination induced complete remissions in 11 patients, including 4 patients in advanced phase relapse; 7 patients were still on treatment at the time of reporting, whereas imatinib could be discontinued in 4 patients. Controversial results have been reported on the immunosuppressive effects of imatinib on T cells and APCs.74-79 In contrast, there is little doubt that imatinib spares leukemia stem cells.80,81 In a small study of 12 patients where imatinib was discontinued after 2 to 4 years, 6 patients remained in remission, whereas 6 patients relapsed within 6 months.82 It is possible that prior treatment with IFN-α contributed to the long-lasting success in some patients.
AML/MDS
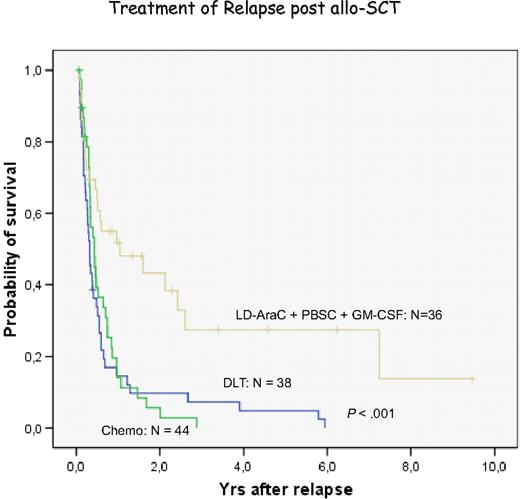
Treatment of relapsed AML and MDS with DLT has been less effective than chronic phase CML.44,56 The pace of the disease may be one factor because AML may outpace a GVL reaction that may need several months for effective control of leukemia. Therefore, most centers have used some form of chemotherapy before DLT. We have chosen low-dose cytarabine for 2 to 4 weeks to avoid a cytokine storm released by intensive therapy. However, some patients are progressive during low-dose cytarabine treatment and require more intensive treatment.83 Another factor for less favorable response to DLT is the immune phenotype of AML blasts. In more than 80% of patients, blasts do not express CD86 costimulatory molecules,38,84,85 potentially allowing blasts to escape immune attack. GM-CSF in combination with IFN-α and other cytokines is highly effective to produce DCs of leukemia origin.38,39,86 Therefore, we used GM-CSF in addition to granulocyte-CSF–mobilized blood cells for the treatment of relapses of AML after transplantation. In a retrospective analysis, we found a better response in patients with this combination than with chemotherapy or DLT alone (Figure 6). These results compare favorably with the results of a retrospective evaluation of the EBMT data.87 Levine et al88 performed a prospective multicenter study of treating AML relapse after allogeneic transplantation with chemotherapy and DLT. The best results were obtained in patients with relapse occurring more than 6 months after transplantation and with a response to chemotherapy (Figure 7). In our own study, several patients are alive after more than 4 years, a late death from leukemia was the result of refractory central nervous system (CNS) leukemia,83 and another patient developed testicular leukemia. These observations point to the fact that GVL does not eliminate leukemia in sanctuary sites; therefore, regular control and prophylactic treatment of the CNS are required even in patients with AML. After allogeneic stem cell transplantation, relapses of AML often occur at extramedullary sites without evidence of blasts in marrow and blood. These relapses rarely respond to DLT.
Evaluation of relapses of AML/MDS at the University of Munich Transplant Center. Retrospective analysis of the treatment with chemotherapy, donor lymphocyte transfusion, and the combination of low-dose cytarabine, mobilized blood stem cells, and GM-CSF.
Evaluation of relapses of AML/MDS at the University of Munich Transplant Center. Retrospective analysis of the treatment with chemotherapy, donor lymphocyte transfusion, and the combination of low-dose cytarabine, mobilized blood stem cells, and GM-CSF.
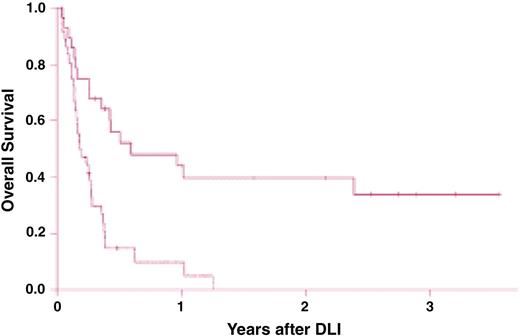
Survival after chemotherapy and donor leukocyte infusions (DLIs) for patients with relapsed acute myelogenous leukemia (AML). Survival is significantly improved in recipients of DLI for relapsed AML more than 6 months after bone marrow transplantation (BMT; solid line) compared with recipients of DLI for relapse less than 6 months from BMT (dashed line; P < .001). Reprinted from Levine et al88 with permission.
Survival after chemotherapy and donor leukocyte infusions (DLIs) for patients with relapsed acute myelogenous leukemia (AML). Survival is significantly improved in recipients of DLI for relapsed AML more than 6 months after bone marrow transplantation (BMT; solid line) compared with recipients of DLI for relapse less than 6 months from BMT (dashed line; P < .001). Reprinted from Levine et al88 with permission.
In a small series, 16 patients from Seattle with myelodysplastic syndromes (MDSs) were treated with DLT; 2 were not evaluable because of chemotherapy-induced remission. Three of 14 patients had a remission, but no patient survived because of GVHD or relapse.89 In a study of 25 patients with relapse of AML (N = 22) and MDS (N = 3), various strategies of DLT, second transplantations and chemotherapy were studied: 2 patients with MDS and one patient with AML survived more than 2 years or 1 year, respectively, after DLT and second transplantation.90 Positive results were also reported in an earlier study by Porter et al91 and more recently by Depil et al.92 The treatment of AML relapse with low-dose cytarabine, mobilized DLT, and GM-CSF is currently studied in a German multicenter trial. The results of our center were confirmed in a preliminary analysis.
Preemptive treatment of AML with DLT
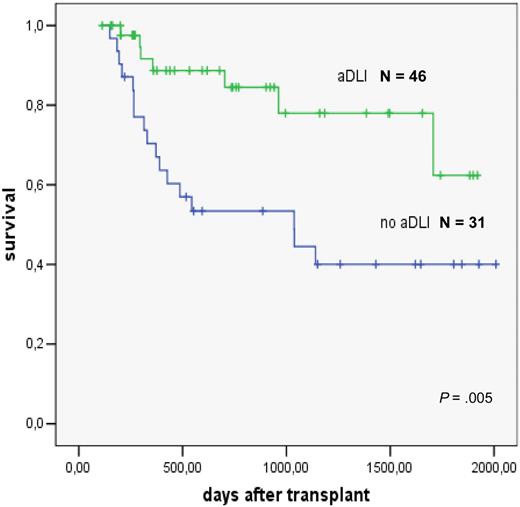
The role of preemptive DLT has been studied in patients with high-risk AML treated with reduced intensity conditioning93 and allogeneic stem cell transplantation. DLT was administered to those patients who had survived 120 days after transplantation and were free of GVHD, immunosuppressive treatment, and infections. Escalating doses of 106/kg, 5 × 106/kg, and 107/kg CD3+ T cells were given at monthly intervals. Forty-six patients were treated. Reasons for not giving the next scheduled DLT were ongoing immunosuppression, presence of GVHD, and early relapse.94 The comparison in a matched-pair analysis showed a significantly better survival of patients given preemptive DLT (Figure 8).
Preemptive donor lymphocyte transfusions in high-risk AML; evaluation in a matched pair analysis. Courtesy of M. Schleuning, EBMT 2007.
Preemptive donor lymphocyte transfusions in high-risk AML; evaluation in a matched pair analysis. Courtesy of M. Schleuning, EBMT 2007.
DLT for ALL
The response of standard B-cell or pre–B-cell ALL to DLT is generally inferior to that of myeloid leukemia,44,56,95 although the first patient treated successfully by DLT for residual leukemia was a child with ALL.15 Hematologic responses to DLT alone are rare96 ; the rare prolonged remissions have been observed primarily in patients treated with intensive chemotherapy or radiotherapy. The response rate of relapsed ALL to chemotherapy and DLT may not be so different from AML, but the duration of remission is generally short. Presumably, the GVL effect has to occur early after transplantation to be effective. In a treatment model of ALL in nonobese diabetic/severe combined immunodeficiency mice, Nijmeijer et al found donor CTL to be turned off after a few weeks resulting in tumor regrowth.97 The GVL effect was strongest if CTL and tumor cells were HLA-incompatible. The limited efficacy of DLT against ALL may be associated with the pre-B lymphocyte phenotype that lacks costimulatory molecules and induces tolerance.98
In contrast, several cases of T-ALL and T-cell lymphoma have been reported to respond to DLT,99,100 with durable responses. The GVL effect of acute GVHD is strong in B- and T-ALL,101 with the effect being stronger in T-ALL, although not significantly. We have observed several patients with durable responses to DLT in T-ALL and mature T-cell lymphoma.
Chronic lymphocytic leukemia and lymphoma
Chronic lymphocytic leukemia is of B-cell origin in most of the cases. Single cases of a strong response to DLT have been reported in patients with recurrent clinical disease after allogeneic stem cell transplantation.102 However, in most cases, the beneficial effect of DLT has been observed at the molecular level,103 stressing the importance of the measurement of minimal residual disease. In 7 of 9 patients, durable molecular remissions were induced by DLT, whereas molecular remissions were seen in only 6 of 26 patients not given DLT, and these were not durable.
The graft-versus-lymphoma effect has been questioned in a statistical analysis of the International Bone Marrow Transplant Registry and EBMT.104 The results of syngeneic and allogeneic transplantation were compared in low-grade and high-grade lymphoma. In low-grade lymphoma, there was no difference in relapse rate in allogeneic and syngeneic transplantations; these patients had a lower relapse rate than in autologous transplantations. In high-grade lymphoma, the differences in relapse rates after allogeneic, syngeneic, and autologous transplantations were not significant.
The evidence for an allogeneic graft-versus-lymphoma effect has been discussed by Grigg and Ritchie.105 There is good evidence for graft-versus-lymphoma effects in mantle cell lymphoma,106 follicular lymphoma, and chronic lymphocytic leukemia.107 Reduced intensity conditioning and preemptive DLT were studied by Peggs et al, revealing strong evidence for a graft-versus-lymphoma effect in lymphoma, including Hodgkin disease.108 High response rates were also reported in low-grade lymphoma, in some cases even without signs of GVHD (Table 2).109
Evidence for a graft-versus-lymphoma effect
| Lymphoma . | No. of patientsin CCR . | Effect . | Reference (year) . |
|---|---|---|---|
| Follicular | 8/13 | DLI | Marks et al19 (2002) |
| Mantle cell | 15/18 | DLI | Khouri et al167 (2003) |
| High grade | 8/9 vs 0/9 | T-cell depletion | Glass et al168 (2004) |
| Hodgkin | 5/7 | DLI | Peggs et al169 (2007) |
| T-NHL | 12/17 | RIC + DLI | Corradini et al99 (2004) |
| CLL | 8/9 | MRD | Ritgen et al103 (2004) |
| Lymphoma . | No. of patientsin CCR . | Effect . | Reference (year) . |
|---|---|---|---|
| Follicular | 8/13 | DLI | Marks et al19 (2002) |
| Mantle cell | 15/18 | DLI | Khouri et al167 (2003) |
| High grade | 8/9 vs 0/9 | T-cell depletion | Glass et al168 (2004) |
| Hodgkin | 5/7 | DLI | Peggs et al169 (2007) |
| T-NHL | 12/17 | RIC + DLI | Corradini et al99 (2004) |
| CLL | 8/9 | MRD | Ritgen et al103 (2004) |
DLI indicates donor lymphocyte transfusion; RIC, reduced intensity conditioning; MRD, minimal residual disease; T-NHL, T-cell non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; and CCR, continuous complete remission.
Multiple myeloma
Multiple myeloma is a disease responsive to the treatment with donor lymphocytes.44,56,110-112 In 20% to 50% of cases, the disease responded to the treatment with donor lymphocytes (Table 1). However, most remissions were not durable,59,113-115 and the strongest prognostic factor predicting response was the occurrence of GVHD.116,117 The best results can be achieved at a stage of minimal residual disease, in patients in remission after chemotherapy.118 Seven of 20 patients with complete remission (N = 4) and partial remission (N = 16) remained in continuous complete remission more than 4 years after partial T cell–depleted stem cell transplantation and preemptive donor lymphocyte transfusions (actuarial 6-year survival, 29%). The response of multiple myeloma to donor lymphocyte transfusion is not predictable and, in advanced disease relapses, often occurs outside the marrow cavity. Myeloma is a tumor with close interaction to the microenvironment that may determine the response to chemotherapy.119 This interaction protects myeloma cells from regular chemotherapy, whereas new drugs may disrupt this protection. It is not known how allogeneic T cells affect this interaction. Recently, evidence has been provided that myeloma relapses from CD20+ and CD27+ memory B cells with distinct properties of stem cells.120 These B lymphoid stem cells may also be the relevant targets of the donor lymphocyte attack. Similar to low-grade lymphoma, long-term remissions are most probably obtained in patients with minimal residual disease.
Solid tumors
The largest experience using adoptive immunotherapy for solid tumors has been obtained in renal cell carcinoma.121 A review of allografts for solid tumors performed at EBMT centers lists renal cell cancer as the most frequent indication (335 patients), followed by breast cancer (143 patients), neuroblastoma (70 patients), and ovarian carcinoma (40 patients).122 The original study by Childs et al121 provided unequivocal evidence for a graft-versus-tumor effect of DLT in renal cell cancer. In several patients with clear cell cancer, complete remissions were achieved and maintained.123 Evidence of a graft-versus-tumor effect has been reported in many other solid tumors.124 In general, less advanced disease, good clinical performance status of the patient, and the occurrence of GVHD were prognostic factors for response.125 Animal studies in mice126 and rats127 indicated that CD8+ T cells were effective in tumor control after T cell–depleted marrow transplantation. In human patients with metastatic breast cancer, DLT after T cell–depleted stem cell transplantation produced a graft-versus-tumor effect.128 Solid tumors may express mHA otherwise restricted to hematopoietic cells.129 In patients with renal cell cancer that responded to allografting, CD8 T cells have recently been found that recognize peptides from endogenous human retrovirus specific for renal cell cancer.123 In solid tumors, the role of stroma can be critical; it maintains viability of tumor stem cells by paracrine cytokine stimulation and fostering interaction. On the other hand, it may be relevant in antigen presentation130 and a target of effector mechanisms.131
Immunotherapy in patients with HLA-haploidentical donors
Transplantations from HLA-haploidentical donors take advantage of the antileukemic activity of NK cells. NK cells are inhibited by cross-reactive groups of self-HLA-antigens via an inhibitory killer immunoglobulin-like receptor.132 In the case of the patient's cells expressing antigens of a different group from the donor, the transplanted NK cells are not inhibited and exert a strong GVL effect. NK cells can eliminate host hematopoietic cells, including leukemia cells, without producing GVHD. The GVL effect of NK cells is most pronounced in AML and is less evident in ALL. Several groups could not show the beneficial effect of NK cells,133 but these groups did not use the extensive T-cell depletion used by the Perugia group.132
In our own group of patients with advanced leukemia, we observed a very good response in ALL patients. However, we did not use T-cell depletion of marrow, and we added CD6-depleted mobilized blood cells on day 6. In the CD6-depleted preparation, we retained CD34+ stem cells, NK cells, and a minority of CD8+ T cells. In this way, a large number of cells could be given without an increased risk of GVHD. In this cohort of patients, we observed NK activity of grafts from donors that were heterozygous for the cross-reactive group C KIR-ligand in recipients homozygous for a cross-reactive group C-KIR ligand. Moreover, we observed a better GVL effect for cells from female donors given to male recipients and with cells from mothers compared with fathers, without an increase of GVHD. The advantage of female and maternal donors may be the result of memory T cells in the transplantation recognizing mHA in the patient.
Lessons from the treatment of viral infections with donor lymphocytes
Most experience in the treatment of viral infections after allogeneic transplantation has been accumulated in cytomegalovirus (CMV)134 and Epstein-Barr virus (EBV) infections.135 Both infections can be life-threatening, and reactivation of EBV may produce highly malignant lymphoblastic lymphoma. Virostatic drugs may control lytic infections of CMV and EBV, but posttransplant lymphoproliferative disease and lymphoma are associated with latent EBV infection. CD20 antibody is able to kill B cells immortalized by EBV, but lasting protection is only provided by T cells. Virus-reactive T cells can be selected from seropositive donors by tetramers136 and immunomagnetic bead selection, less than 104/kg T cells are sufficient to protect the patient. These cells proliferate in the patient within 1 to 2 weeks to protective levels. Similarly, EBV infections have responded to EBV-specific T cells selected from the blood of the stem cell donor by the gamma-IFN capture technique137 (Moosmann et al, in preparation). One requirement for these successful attempts at treating viral infections with these techniques is preexisting seropositivity of the stem cell donor, indicating immunologic memory for the virus.
The role of T-cell memory has been stressed by the lower relapse rate of male patients given female transplantations.138 The lower relapse rate was also evident after adjustment for GVHD. Recently, we have found a significantly lower relapse rate in male patients transplanted with marrow and blood cells from HLA-haploidentical female donors96 (H.-J.K., I. Bigalke, D. Ter Meer, A. Hausmann, C. Falk, B. Simoes, R. Buhmann, unpublished data, 2008). Moreover, recipients of maternal transplantations had less relapse than recipients of paternal transplantations. Memory T cells in the blood of mothers may recognize mHA on the leukemia of their children. Recently, the survival of central memory T cells has been shown to be independent of CD4 help; CMV-reactive CD8+, CD28+, Fas high central memory T cells survive in blood, marrow, and lymph node of macaques.139 Therefore, the ideal effector cell of GVL reactions is the central memory T cell.
Do donor lymphocytes eliminate leukemia stem cells?
Patients with CML surviving after allogeneic transplantation more than 10 years may be cured, but relapses after more than 10 years have been reported in single cases.140 Normal stem cells may survive and host-type hematopoiesis may reappear during severe infections without evidence of relapse.141 The reports on the elimination of leukemia stem cells are controversial. mHA-specific CD8+ T cells eliminate progenitor cells forming colonies in vitro142 and stem cells inducing leukemia in nonobese diabetic/severe combined immunodeficiency mice.143,144 CML patients responding to DLT produce antibodies against stem cell antigens CML 28 and CML 66145 ; in others, antigens on progenitor cells are not recognized.146 In myeloma, progression-free survival for many years after allogeneic transplantation has been observed in some patients who were refractory to high-dose chemotherapy and autologous transplantation. Persistence of monoclonal immunoglobulin at a low level indicated that the tumor was not eradicated, but it was controlled for several years until relapse occurred. Presumably DLT established immune control for a prolonged period of time.
Failures of durable control: immune escape mechanisms
Unfortunately, many patients develop a relapse of their leukemia, lymphoma, or myeloma after allogeneic transplantation and DLT (Table 3). It is not infrequent that these relapses occur at extramedullary sites as chloromas or plasmocytomas. These sites can be immunologically privileged sites, including CNS or gonads or other sites, such as skin, kidneys, and any other site outside of the marrow. Therefore, it is necessary to survey spinal fluid and gonads and to teach the patient self-examination. Intrathecal medication should be given in every patient treated by DLT for relapse of acute leukemia. Unfortunately, extramedullary infiltrates do not respond sufficiently to immunotherapy. There are no ideal methods of treatment; some patients respond to acute GVHD on discontinuation of immunosuppressive therapy. The preferred method is local treatment with radiotherapy in limited infiltrates. More extended infiltrations usually require intensive chemotherapy.
Mechanisms of immune escape of leukemia
| Invasion of immunologically privileged sites: CNS, gonads |
| Down-regulation of HLA class I and class II expressions |
| Deficient processing and presentation of peptides |
| Deficient expression of costimulatory molecules: CD80, CD83, CD86, CD40, LFA-1, ICAM |
| Secretion of inhibitory cytokines by leukemia cells: IL-10, TGF-β |
| Abnormal secretion of and resistance to pro-inflammatory cytokines: TNF-α, IFN-γ |
| Nonfunctional FAS on leukemia cells |
| FAS-L expression by leukemia cells |
| Regulatory T cells and production of indoleamin 2,3-dioxygenase (IDO) by leukemia cells |
| Down-regulation of ζ-chain (lymphoma and CML) and ϵ-chain (CML) of the T-cell receptor |
| Down-regulation of CD28 in AML |
| Down-regulation of IL-2 receptor |
| Low IL-2 |
| Apoptosis of high-avidity T cells |
| Invasion of immunologically privileged sites: CNS, gonads |
| Down-regulation of HLA class I and class II expressions |
| Deficient processing and presentation of peptides |
| Deficient expression of costimulatory molecules: CD80, CD83, CD86, CD40, LFA-1, ICAM |
| Secretion of inhibitory cytokines by leukemia cells: IL-10, TGF-β |
| Abnormal secretion of and resistance to pro-inflammatory cytokines: TNF-α, IFN-γ |
| Nonfunctional FAS on leukemia cells |
| FAS-L expression by leukemia cells |
| Regulatory T cells and production of indoleamin 2,3-dioxygenase (IDO) by leukemia cells |
| Down-regulation of ζ-chain (lymphoma and CML) and ϵ-chain (CML) of the T-cell receptor |
| Down-regulation of CD28 in AML |
| Down-regulation of IL-2 receptor |
| Low IL-2 |
| Apoptosis of high-avidity T cells |
Different mechanisms can contribute to the failure of DLT to eradicate leukemia. Leukemia cells may persist in immunologically privileged sites, such as the CNS and gonads, or in extramedullary sites, such as the skin or the kidney, while still showing a continued remission in marrow. Malignant cells may escape immune detection and elimination if they have an altered expression of target antigens or costimulatory molecules required for efficient recognition. They may also directly down-regulate effector cells through secretion of inhibitory cytokines. Modulation of expression of FAS or FAS-ligand can also contribute to tumor escape from immune control.
There are many ways leukemia and other tumor cells camouflage themselves from the immune system, including changes in antigen presentation,38,84,85,147-149 production of inhibitory cytokines,150-152 and the production of regulatory T cells by indoleamin 2,3 deoxygenase secretion and local tryptophan depletion.153
T-cell effector functions may be influenced by the leukemia cells. High avidity T cells disappear during active disease and reappear in interferon-α responders.154 Defective effector functions of T cells, which were associated with diminished expression of the CD3-ζ and CD3-ϵ chains, have also been described.155,156 Decreased expression of CD3-ζ and CD3-ϵ was more probable on lymphocytes of patients with advanced disease. Diminished expression of CD3-ζ and CD28 was also observed in patients with advanced malignancies, chronic viral infections, or autoimmune diseases, occurring as a consequence of chronic lymphocyte stimulation.157 T cells with these phenotypes were prone to apoptosis, but they could be rescued with IFN-α and IL-2.156
Outlook
In the past decades, the paradigm of stem cell transplantation has changed. Nowadays confidence in the power of the GVL effect has encouraged the use of donor lymphocytes and mobilized donor cells for adoptive immunotherapy in cases of relapse or preemptively in patients with high relapse risk. The conditions for a GVL effect without severe GVHD have become better defined. Furthermore, it has become clear that myeloid diseases have a better response to DLT than lymphoid diseases and that this response can be improved by simultaneous treatment with IFN-α and/or GM-CSF. The better response to DLT from female donors and maternal donors suggests a role of memory T cells that may also control lymphoid leukemia. Obviously, these memory T cells should not produce vigorous GVHD. In the near future, we will learn more about these cells and the antigens they recognize.
To achieve maximum GVL effects, it will also be necessary to perform stem cell transplantation without immune suppression after transplantation. The intensity of conditioning should only be reduced to a level that allows prompt and permanent engraftment of T cell–depleted transplantations. Learning about the immune repertoire of our donors will allow treatment of patients with hematologic malignancies as well as those with other tumors. In this way, we will be able to select our donors not only according to optimum histocompatibility, but also to the relevant T-cell repertoire.
Acknowledgments
The author thanks Dolores Schendel, PhD, Helmholtz Zentrum Muenchen, and Jamie Ferrara, MD, PhD, University of Michigan, for critical review of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft (SFB TRR 36), Deutsche Krebshilfe, Wilhelm Sander Stiftung, Deutsche José Carreras Leukämie Stiftung, and EU-Contract Marie Curie Program.
Authorship
Contribution: H.-J.K. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Hans-Jochem Kolb, Hematopoietic Cell Transplantation, Department of Medicine 3, University of Munich & Helmholtz Zentrum Muenchen-National Research Centre for Environmental Health, Munich, Germany; e-mail: Hans.kolb@med.uni-muenchen.de.